Interaction between Risk Factors and Gastric Cancer: A Population-Based Case-Control Study in a High Risk Chinese Area
Received: 20-Oct-2018 / Accepted Date: 02-Nov-2018 / Published Date: 05-Nov-2018 DOI: 10.4172/2161-1165.1000359
Abstract
Introduction: An extremely high prevalence of gastric cancer (GC) was observed in Xianyou County, the Southeastern of China, yet it remains unclear how interaction of environmental factors affects the GC risk in this area.
Methods: A population-based case-control study was conducted during March 2013 and January 2017 in Xianyou County. All newly diagnosed patients with GC were compared with healthy controls matched (1:1) by sex, age (± 3 years) and place of residence.
Results: A total of 622 cases of GC and 622 matched healthy controls were included. Participants who consumed pickled vegetables and had a preference for hard solid food had the highest risk of GC (adjusted OR=12.42, 95% CI: 7.56-20.41) compared to individuals who did not have such food patterns. Increased GC risk was also observed among those who consumed pickled vegetables but not drink tea (adjusted OR=6.88, 95% CI: 3.74-12.66), compared to those drinking tea but did not consume pickled vegetables. Furthermore, participants who did not consume tea but were exposed to pesticides during farm work also had 2.50 times (95% CI: 1.30-4.80) higher risk of GC risk compared to those had tea consumption but were not exposed to pesticides. No statistically significant interactions were observed for pesticide exposure, smoking, beef or pork consumption, fruit consumption, chronic atrophic gastritis and family history of any cancer in relation to GC.
Conclusion: Strong synergistic effects in raising the risk of GC were seen between pickled vegetables consumption and hard solid food preference, as well as between pickled vegetables consumption and not drinking tea.
Keywords: Combined effect; Oncology; Stomach cancer
Introduction
Gastric (GC) is one of the most common malignant tumors in the world, although the incidence of GC showed a significant decreasing trend in the past few decades [1]. The overall five-year survival of patients with advanced GC was generally less than 20%, while the five-year survival for early GC is more than 90% [2]. Therefore, prevention and early detection of GC is valuable to improve the prognosis of GC, especially in high-incidence areas such as China, with an estimated 679,100 new cases and 498,000 deaths in 2015 [3]. The etiology of GC has been widely studied, but is still not fully understood. The most strongly established risk factor for GC, Helicobacter pylori infection, cannot fully explain the heterogeneity in its distribution [2] since only a small proportion of people who carry H. pylori in their stomachs develop GC [4], suggesting that other sociodemographic and environmental factors may be important. Xianyou County locates on the southeast coast of China. According to our earlier study during 2011 and 2012, GC was the leading cause of death in this area [5]. The mortality rate of GC was 49.47/100,000, which was two times higher than the Chinese national average (21.9/100,000) [5], while no notable differences in H. pylori detection were noted. Environmental factors were suggested to be associated with a high incidence of GC in Xianyou County, but no study has been conducted to investigate the potential associations and interactions.
Similar to many other high-risk areas of China, pickled vegetables are commonly consumed in Xianyou County as an important part of the family diet. Pickled vegetables are produced by salting Chinese cabbage, carrot/turnip, sweet potato leaves or other vegetables to draw out excess water. These foods may contain carcinogenic N-nitroso compounds, in addition to a high level of salt. A recent meta-analysis has shown that high consumption of pickled vegetables in high-risk areas of China indeed increased the GC risk (OR=1.86, 95% CI 1.61– 2.15) [6]. In addition, residents in Xianyou County also had a preference for hard solid food such as fried food, nuts, and cereals [7]. Preference on hard solid food has been frequently shown to increase the risk of GC in high-risk area of China. Two meta-analyses reported that intake of hard.
Solid food was associated with increased risk of GC based on data from several Chinese populations (OR=1.94, 95% CI: 1.39-2.72;OR=2.74, 95% CI: 1.72-4.35) [8,9]. In contrast, consumption of fresh vegetables and fruits could protect against GC, as also shown in China [10-15]. As in most parts in China, green tea is also commonly consumed in Xianyou County. Although worldwide epidemiological studies examined the association between tea consumption and risk of GC, tea consumption has only been shown to be protective in Chinese populations with a significant risk reduction of 39% (summary RR=0.61, 95% CI=0.47-0.81) [16]. Other risk factors such as family history of any cancer, chronic atrophic gastritis and tobacco smoking were also widely reported to increase the risk of GC [2].
Although all above environmental factors have been independently associated with the risk of GC in some Chinese populations, no previous study has explored interactions between these environmental risk factors in a high-risk area in China. A recent 10-year follow-up cohort study found that a combination of H. pylori infection and atrophic gastritis had increased risk of 32.4-fold compared to H. pylori and atrophic gastritis negative patients [17]. A synergy effect was also observed between Cag A (+) and smoking with nearly 9-fold increased GC risk (OR=8.7, 95% CI=5.1-11.9) [18]. Therefore, the aim of this study was to explore the effects of different risk factors for GC and to identify potential interactions in a large case-control study in Xianyou County, Southeastern China.
Material and Methods
Study design and population
A population-based case-control study was conducted in Xianyou County between March 2013 and January 2017. All newly diagnosed cases of GC in individuals who were aged ≥ 18 years and have lived in Xianyou for at least 10 years were eligible and recruited in Xianyou County Hospital. Patients who had a history of cancer, mental illness, and those unable to complete the questionnaire were excluded. Controls were 1:1 matched to cases on sex, age (± 3 years), and place of residence. They were required to live in the same town or a town with a similar incidence of GC, and were required to be living in Xianyou County for at least 10 years and have no prior history of GC. Matched controls were randomly selected by the Health department of Xianyou County government, who was responsible to send study invitation letters to selected controls. Data was collected from all consenting controls at the Xianyou County Hospital. The study was approved by the ethics committees in Xianyou County Hospital and Fujian Medical University. Written consents were obtained from all participants at study enrollment.
Data collection
All cases and controls were personally interviewed by trained research nurses from Xianyou hospital. A structured questionnaire consisting of 337 items under 7 categories was used for the data collection. The questionnaires included following categories: 1) general demographic information (i.e., age, height, weight, marital status, education level, income); 2) occupational history (i.e., profession, pesticide exposure at work); 3) smoking habits, use of alcohol, and tea consumption; 4) dietary habits; 5) availability of drinking water and living conditions; 6) medical history (i.e., personal disease records, medication, and family history of any cancer) and 7) mental health. For cases, cancer related diagnoses and treatment (i.e., surgery, chemotherapy, and radiotherapy) were obtained from medical records.
More specific, dietary questionnaire included questions to assess the habitual intake of 17 food items including meat (beef/pork), egg, fish/ shrimp, mussels, kelp (i.e. brown algae seaweeds), dairy products, soy products, fresh fruit, fresh vegetables, fungus, fried food, garlic, onion, pickled vegetables, pickled sea food, salted eggs and pickled plums. The frequency of intake of each food item was assessed on the basis of open numerical answers, that is, number of times of consumption per day, week, or month. Furthermore, dietary questionnaire also investigated participants’ preferences on food temperature (hot or cold), eating speed (very fast, fast, medium, very slow, and slow) and food texture preference (hard solid or soft). It should also be noted that participants were asked if they have changed their dietary habits in the past 5 years. If the dietary habits have not changed in the past 5 years, participants were required to answer the dietary questionnaire mainly based on their dietary habits during the past 1-2 years. If the dietary habits have changed during the past 5 years (to exclude reverse causality), participants were requested to report their dietary habits one year before the dietary habit change. If dietary habits changed more than 5 years ago, participants were asked to report their dietary habits during the recent 1-2 years.
Smoking was defined as consuming at least one cigarette per day during the last 6 months or longer. The habit of tea drinking was defined as consuming at least one cup of tea per week during the last 6 months.
Statistical analysis
To effectively identify the interaction effect of potential risk factors on development of GC, we only evaluated the risk factors that showed statistically significant associations with risk of GC in multivariate stepwise logistic regression (p<0.05). All potential risk factors including body mass index (BMI, <18.5, 18.5-24.9, ≥ 25 kg/m2), education level (≤ 6, 7-9, ≥ 10 years), profession (farmer or others), monthly income 10 years ago (<300, 300-599, 600 Chinese Yuan/ month), smoking (yes/no), drinking tea (yes/no), fruit consumption (none, <300, 300 g/week), vegetables consumption (none, <2100, 2100 g/week), pickled vegetables (none, <300, 300 g/week), beef or pork consumption (none, <1200, 1200 g/week), food preference (hard solid or soft), pesticide exposure during farm work (yes/no), chronic atrophic gastritis (yes/no), family history of any cancer (yes/no), and H. pyloric infection (yes/no) were entered into the stepwise logistic regression. H. pyloric was detected by rapid urease tests based on the blood samples collected at study enrolment. A total of nine factors that had a p value <0.05 were eventually selected in the current study for the interaction analysis, including pesticide exposure, smoking, drinking tea, fruit consumption, pickled vegetables consumption, beef or pork consumption, food preference (hard solid, soft), chronic atrophic gastritis and family history of any cancer. Random effect forest plots (software R 3.3.3) were used to select the most significant risk factors for GC based on the importance scores of Mean Decrease Accuracy (MDA), and Mean Decrease Gini (MDG). Both higher values of MDA and MDG imply a stronger predictive effect on the risk of gastric cancer in this study population. But MDA is usually considered as an more important indicator than MDG in deciding the most predictive variables. Out-of-bag error, which is a method of measuring the prediction error of random effect forests, was also calculated to determine the test error of random forest. Classification Regression Tree (CRT) modelling was applied to build a decision tree and identify variables that might have joint interaction effect. The study variable with the highest score of MDA was selected as the main effect factor in decision tree. To assess the multiplicative interaction effect between the selected risk factors on the risk of GC, logistic regression was used to estimate odd ratios (OR) and 95% confidence intervals (CI).The regression analysis calculated both crude OR and adjusted OR, which included adjustment for the following variables: BMI, education level, profession (farmer, others), monthly income 10 years ago, smoking (yes, no), drinking tea (yes, no), fruit consumption, vegetables consumption, pickled vegetables, beef or pork consumption, food preference, pesticide exposure, chronic atrophic gastritis, p<0.05 was regarded as having statistical significance. SPSS version 19.0 and R version 3.3.3 were used for the analyses.
Results
Baseline
A total of 622 cases of GC were included in the final analysis, after excluding 25 cases that were too sick to answer the questionnaire or refused to participate, resulting in a 96% response ratio. The number of sex and age matched controls was 622 (response ratio 100%). The characteristics of the study participants are presented in Table 1. The distributions of BMI, education level and monthly income 10 years ago were significantly different between cases and control groups (all p<0.05). More than 70% of study participants were farmers (73.0% in cases, 70% in controls). Controls had higher education levels and monthly incomes compared to cases (both p<0.05). Among cases, 78.4% had education less than 7 years, in contract to 69.9% in controls. Nearly half of controls (43.7%) had a low monthly income (less than 300 RMB), compared to 20.9% of the controls. Fewer controls were smokers and controls had a higher consumption of tea, and beef/pork compared to cases (all p<0.05). Among cases, 74.8% had a preference for hard solid food, in contrast to 42.9% of controls. Cases had a higher exposure to pesticide, chronic atrophic gastritis, family history of any cancer and H. pylori infection compared to controls (all p<0.05). There are missing value for H. pylori infection in both cases (22.8%) and controls (14.6%).
| Characteristics Total |
Case (%) | Control (%) | p value |
|---|---|---|---|
| Sex | |||
| Male | 453(72.8) | 453(72.8) | |
| Female | 169(27.2) | 169(27.2) | |
| Age (years) | 0.28 | ||
| ≤ 45 | 13(2.1) | 11(1.8) | |
| 46-55 | 53(8.5) | 73(11.7) | |
| 56-65 | 245(39.4) | 221(35.5) | |
| 66-75 | 208(33.4) | 220(35.4) | |
| ≥ 76 | 103(16.6) | 97(15.6) | |
| Body mass index (kg/m2) | <0.001 | ||
| <18.5 | 110(17.7) | 55(8.8) | |
| 18.5-24.9 | 472(75.9) | 456(73.4) | |
| ≥ 25 | 40(6.4) | 111(17.8) | |
| Education (years) | 0.002 | ||
| ≤ 7 | 488(78.4) | 435(69.9) | |
| 7-9 | 85(13.7) | 112(18.0) | |
| ≥ 10 | 49(7.9) | 75(12.1) | |
| Profession | 0.31 | ||
| Farmer | 454(73.0) | 438(70.4) | |
| Others | 168(27.0) | 184(29.6) | |
| Monthly income 10 years ago (Chinese Yuan) | <0.001 | ||
| <300 | 272(43.7) | 130(20.9) | |
| 300-600 | 198(31.9) | 273(43.9) | |
| >600 | 152(24.4) | 219(35.2) | |
| Smoking | 0.04 | ||
| Yes | 331(53.2) | 294(47.3) | |
| No | 291(46.8) | 328(52.7) | |
| Drinking tea | <0.001 | ||
| Yes | 77(12.4) | 171(27.5) | |
| No | 545(87.6) | 451(72.5) | |
| Fruit consumption (g/week) | 0.31 | ||
| None | 410(65.9) | 210(33.8) | |
| <300 | 107(17.2) | 124(19.9) | |
| ≥ 300 | 105(16.9) | 288(46.3) | |
| Vegetables consumption (g/week) | 0.001 | ||
| None | 33(5.3) | 12(1.9) | |
| <2100 | 220(35.4) | 263(42.3) | |
| ≥ 2100 | 369(59.3) | 347(55.8) | |
| Pickled vegetables consumption (g/week) | 0.31 | ||
| None | 218(35.0) | 357(57.4) | |
| <300 | 179(28.8) | 142(22.8) | |
| ≥ 300 | 225(36.2) | 123(19.8) | |
| Beef or pork consumption (g/year) | <0.001 | ||
| None | 516(83.0) | 356(57.3) | |
| <1200 | 59(9.5) | 99(15.9) | |
| ≥ 1200 | 47(7.5) | 167(26.8) | |
| Food preference | <0.001 | ||
| Soft | 157(25.2) | 355(57.1) | |
| Hard | 465(74.8) | 267(42.9) | |
| Pesticide exposure | 0.008 | ||
| Yes | 415(66.7) | 370(59.5) | |
| No | 207(33.3) | 252(40.5) | |
| Chronic atrophic gastritis | <0.001 | ||
| Yes | 69(11.1) | 29(4.7) | |
| No | 553(88.9) | 593(95.3) | |
| Family history of cancer | <0.001 | ||
| Yes | 102(16.4) | 16(2.6) | |
| No | 520(83.6) | 606(97.4) | |
| H.pylori infection | 0.006 | ||
| Positive | 238(49.6) | 218(41.1) | |
| Negative | 242(50.4) | 313(58.9) |
Table 1: Baseline characteristics of gastric cancer and control participants in a Chinese population-based case-control study.
Risk factors and GC
In multivariate logistic regression analysis, nine risk factors were associated with GC (Table 2). The highest adjusted OR for GC was observed in patients with hard solid food preference (OR=1.78, 95% CI 1.45-2.12) compared to participants without such preference. Family history of any cancer also increased the risk of GC (OR=1.75, 95% CI 1.36-2.25). Increased risk was also observed for those with a high level of pickled vegetables consumption (OR=1.44, 95% CI 1.15-1.81; OR=1.42, 95% CI 1.14-1.77). Drinking tea, fruit consumption and beef or pork consumption were all inversely associated with GC.
| Crude OR(95% CI) | p value | Adjusted OR*(95% CI) | p value | |
|---|---|---|---|---|
| Smoking | ||||
| No | 1 | 1 | ||
| Yes | 1.13(0.96-1.32) | 0.138 | 1.22(1.01-1.47) | 0.048 |
| Drinking tea | ||||
| No | 1 | 1 | ||
| Yes | 0.57(0.45-0.72) | <0.001 | 0.60(0.45-0.81) | 0.001 |
| Pickled vegetables consumption(g/week) | ||||
| None | 1 | 1 | ||
| <300 | 1.47(1.21-1.79) | <0.001 | 1.44(1.15-1.81) | 0.001 |
| ≥ 300 | 1.71(1.42-2.06) | <0.001 | 1.42(1.14-1.77) | 0.002 |
| Fruit consumption | ||||
| No | 1 | 1 | ||
| <300 | 0.70(0.57-0.87) | 0.001 | 0.72(0.56-0.93) | 0.01 |
| ≥ 300 | 0.40(0.33-0.50) | <0.001 | 0.53(0.41-0.69) | <0.001 |
| Beef or pork consumption | ||||
| No | 1 | 1 | ||
| <1200 | 0.63(0.48-0.83) | 0.001 | 0.75(0.54-1.03) | 0.078 |
| ≥ 1200 | 0.37(0.28-0.50) | <0.001 | 0.52(0.36-0.74) | 0.001 |
| Food preference | ||||
| Soft | 1 | 1 | ||
| Hard solid | 2.07(1.73-2.48) | <0.001 | 1.78(1.45-2.12) | <0.001 |
| Pesticide exposure | ||||
| No | 1 | 1 | ||
| Yes | 1.87(1.12-2.39) | 0.03 | 1.54(1.04-2.29) | 0.04 |
| Chronic atrophic gastritis | ||||
| No | 1 | 1 | ||
| Yes | 1.46(1.14-1.87) | 0.003 | 1.40(1.04-1.88) | 0.03 |
| Family history of cancer | ||||
| No | 1 | 1 | ||
| Yes | 1.87(1.51-2.31) | <0.001 | 1.75(1.36-2.25) | <0.001 |
*Adjusted for body mass index(<18.5, 18.5-24.9, ≥ 25 kg/m2), education level(<7, 7-9, ≥ 10 years), profession(farmer, others), monthly income 10 years ago(<300, 300-599, ≥ 600 Chinese Yuan), smoking(yes, no), drinking tea(yes, no), fruit consumption(none, <300, ≥ 300 g/week), vegetables consumption(none, <2100, ≥ 2100 g/week), pickled vegetables(none, <300, ≥ 300 g/week), beef or pork consumption(none, <1200, ≥ 1200 g/week), food preference(soft, hard solid), pesticide exposure(yes, no), chronic atrophic gastritis(yes, no), family history of cancer(yes, no) and H. pyloric infection(yes, no).
Table 2: Logistic regression between risk factors and gastric cancer, expressed as odd ratio (OR) and 95% confidence interval (CI) in a Chinese population-based case-control study.
Random forest and decision tree analysis
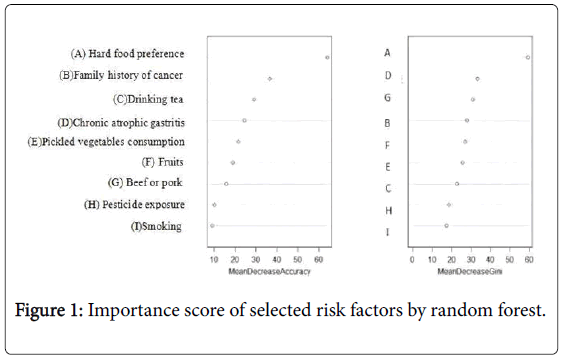
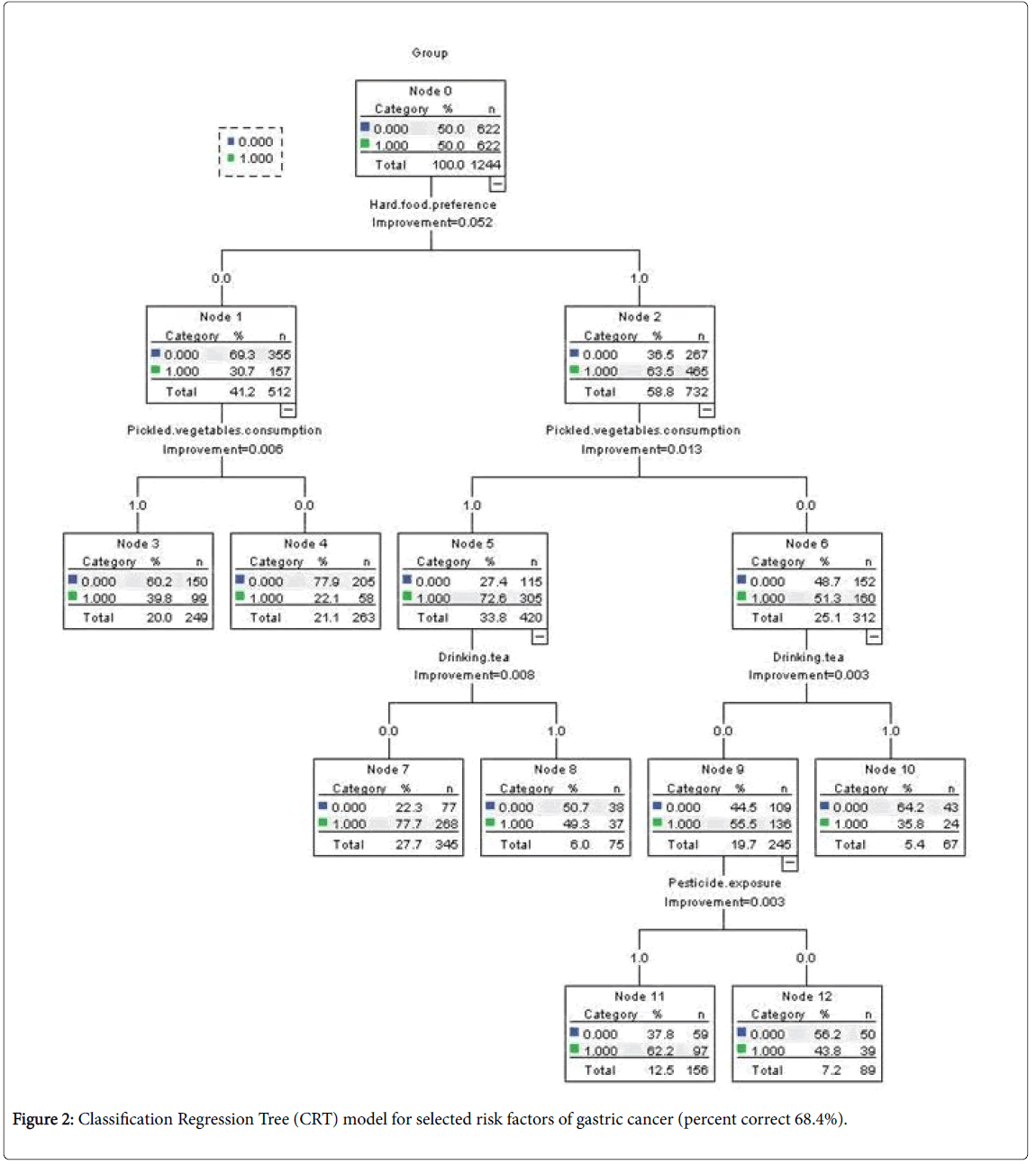
Hard solid food preference was observed with the highest MDA importance score (MDA=64.07), followed by family history of any cancer (MDA=36.61), and drinking tea (MDA=29.03) (Table 3). The highest MDG importance scores were also observed for hard solid food preference (MDG=59.16). The distribution of MDA and MDG among selected factors was also illustrated in Figure 1. Hard solid food preference was therefore set as the principal factor in Classification Regression Tree (CRT) model (Figure 2). Three associations were finally selected for the interaction analysis: 1) Pickled vegetables consumption and hard solid food preference; 2) Pickled vegetables consumption and drinking tea; 3) Drinking tea and pesticide exposure.
| Variables | Mean Decrease Accuracy* | Mean Decrease Gini* |
|---|---|---|
| Hard solid food preference | 64.07 | 59.16 |
| Family history of cancer | 36.61 | 27.95 |
| Drinking tea | 29.03 | 22.81 |
| Chronic atrophic gastritis | 24.52 | 33.28 |
| Pickle vegetables consumption | 21.65 | 25.67 |
| Fruits consumption | 18.71 | 27.01 |
| Beef and pork consumption | 15.79 | 30.94 |
| Pesticide exposure | 10.08 | 18.49 |
| Smoking | 9 | 17.27 |
*Higher values of Mean Decrease Accuracy and Mean Decrease Gini imply higher importance in predicting the risk of gastric cancer.
Table 3: Importance scores for selected risk factors of gastric cancer.
Interaction analysis
Risk of GC was moderately elevated among individuals consuming pickled vegetables without having a hard solid food preference (OR=3.05, 95% CI: 1.83-5.11) and those who had a hard solid food preference but did not consume pickled vegetables (OR=4.97, 95% CI: 3.01-8.20) (Table 4). The highest risk of GC was found in individuals consuming pickled vegetables who also had preference (OR=12.42, 95% CI: 7.56-20.41). Participants with the dietary habit of pickled vegetables who did not drink tea had an OR as high as 6.88 (95% CI: 3.74-12.66) compared to those who did not consume pickled vegetables but drank tea. Finally, a 2.50-fold increased risk of GC (95% CI: 1.30-4.80) was observed in participants exposed to pesticides, not drinking tea, in comparison to those drinking tea without pesticide exposure.
| Variables | Case(N) | Control (N) | Crude OR (95% CI) | p value | Adjusted OR (95% CI)* | p value | |
|---|---|---|---|---|---|---|---|
| Pickled vegetables consumption | Hard food preference | ||||||
| No | No | 58 | 205 | 1 | 1 | ||
| Yes | No | 99 | 150 | 2.33(1.59-3.43) | <0.001 | 3.05(1.83-5.11) | <0.001 |
| No | Yes | 160 | 152 | 3.72(2.58-5.37) | <0.001 | 4.97(3.01-8.20) | <0.001 |
| Yes | Yes | 305 | 115 | 9.37(6.53-13.46) | <0.001 | 12.42(7.56-20.41) | <0.001 |
| Pickled vegetables consumption | Drinking tea | ||||||
| No | Yes | 32 | 99 | 1 | 1 | ||
| No | No | 186 | 258 | 2.23(1.44-3.47) | <0.001 | 2.21(1.20-4.05) | 0.011 |
| Yes | Yes | 45 | 72 | 1.93(1.12-3.34) | 0.018 | 1.5(0.72-3.13) | 0.282 |
| Yes | No | 359 | 193 | 5.76(3.72-8.89) | <0.001 | 6.88(3.74-12.66) | <0.001 |
| Drinking tea | Pesticide exposure | ||||||
| Yes | No | 39 | 76 | 1 | 1 | ||
| Yes | Yes | 38 | 95 | 0.78(0.46-1.34) | 0.365 | 0.44(0.20-0.97) | 0.043 |
| No | No | 168 | 176 | 1.86(1.20-2.89) | 0.006 | 1.58(0.83-3.02) | 0.163 |
| No | Yes | 377 | 275 | 2.67(1.76-4.05) | <0.001 | 2.5(1.30-4.80) | 0.006 |
*Adjusted for body mass index(<18.5, 18.5-25, ≥ 25 kg/m2) , education level(<7, 7-10, ≥ 10 years), profession(farmer, others), monthly income 10 years ago(<300, 300-600, ≥ 600 RMB), smoking( yes, no), drinking tea(yes, no), fruit consumption(none, <300, ≥ 300 g/week), vegetables consumption(none, <2100, ≥ 2100 g/week), pickled vegetables(none, <300, ≥ 300 g/week), beef or pork consumption(none, <1200, ≥ 1200 g/week), food preference(soft, hard), pesticide exposure(yes, no), chronic atrophic gastritis(yes, no), family history of cancer(yes, no) and H. pyloric infection(yes, no).
Table 4: Multiplicative interaction between studied risk factors of gastric cancer, presented as odds ratios (OR) and 95% confidence intervals (CI).
Discussion
In the present study based on a population with a majority of lowereducated farmers in a high-risk gastric cancer are in China, hard solid food preference, family history of any cancer, drinking tea, chronic atrophic gastritis, pickled vegetables consumption, beef and pork consumption, pesticide exposure and smoking were identified as important environmental risk factors for GC. More importantly, strong synergistic effects in increasing risks of GC were observed between picked vegetables consumption and hard solid food preference, as well as pickled vegetables consumption and not drinking tea. No statistically significant interactions were observed for pesticide exposure, smoking, beef and pork consumption, fruit consumption, chronic atrophic gastritis and family history of any cancers in relation to GC.
Some methodological issues of our study deserve attention. The strengths include the large scale population-based design individually matching for age, sex and region, the standardized tumor classification, the personal interviews with all study participants and the availability of H. pylori infection for the majority of the participants. Our study has some limitations that should be described. A concern is the possibility of exposure misclassification using a questionnaire that has not been validated. Another major concern is that the pickled vegetables exposure was based on diet during the past 30 years, resulting in bias from misclassification of the true exposure.
Our study is the first to observe a 12-fold increased risk of GC when both hard solid food preference and pickled vegetables consumption were present. The biologically plausible reason for increased risk by hard solid food preference was probably due to the chronic injury to gastric mucosa, causing susceptibility to gastritis, increasing DNA synthesis and cell proliferation. Injuries of the gastric mucosa might also enhance the absorption of carcinogenic compounds such as nitrosamine. In high-risk areas of China, pickled food, particularly pickled vegetables, were eaten daily for 9 to12 months a year and constituted an important part of the family diet [19]. In our study, nearly 54% of the study subjects in Xianyou County reported that they ever consumed pickled vegetables. High concentration of N-nitroso compounds in picked vegetables has been proven to be strongly carcinogenic for several sites of cancer [20]. Moreover, salty processed food such as pickled vegetables could also increase risk of GC through directly damage of to the gastric mucosa resulting in gastritis [21]. Tea drinking was found to be protective against GC in the current study. It shall be noted that green tea is the most common type of tea in this study area, yet no distinction was made in the questionnaire. The inverse association between green tea consumption and risk of GC has been frequently reported in Chinese population as early as year 1996 and in the recent decades [22-25]. Experimental studies demonstrated that the poly- phenols in green tea had antioxidant effects and could suppress the occurrence and development of cancer [26,27]. Furthermore, our study found that participants who had dietary habits of pickled vegetables without drinking tea had a nearly 7-fold increased risk of GC, compared to those did without intake of pickled vegetables who regurly drank tea. This result indicates that tea consumption might protect against nitrosamine-induced GC. Tea could eradicate nitrosamine and suppress synthesis of N-nitroso compounds in prostate cancer cells [28]. Furthermore, a Japanese study also reported that green tea polyphenol could suppress 0.05% Nbutyl- N-(4-hydroxybutyl) nitrosamine-induced bladder cancer, which was mainly due to the inhibition of cytoplasmic human antigen (Hu)R, cyclooxygenase (COX)-2, and hemeoxygenase (HO)-1 expression by green tea polyphenol [29]. Having a family history of any cancer was also found to be a significant risk factor of GC in the current study. Having a first-degree relative with GC is a consistent risk factor for GC, although the magnitude of the OR associated with a positive family history varied depending on the ethnic group and geographic region, ranging from 2 to 10 [30,31]. Positive family history could be a risk factor as a result of a shared environment, for example, passing of H. pylori from parents to children, or because of shared genetic factors [32]. In conclusion, this study was the first to show strong synergic effects on increasing GC risk between pickled vegetables consumption and hard solid food preference, as well as between pickled vegetables and dietary habit of not drinking tea. Primary prevention of GC shall be addressed to these groups of high-risk populations. Further studies are needed to clarify the potential mechanism for these interaction effects in relation to the development of GC.
Acknowledgment
We appreciate all participants and investigators from the Xianyou Hospital.
Funding
This work was supported by the Fujian Innovative Medicine (Grant No. 2016-CX-41); Natural Science Foundation of Fujian Province, China (Grant No. 2015J01673; 2017J01811) and Xianyou County Government of Putian, Fujian, China (Grant No. 2 2013B008).
Role of the Funding Source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Authors' Contribution
The authors’ contributions to this study were as follow: study design, CW, WY, SG and BL; data collection, CW, WY, SG, and BL; statistical analysis MJ, YL; interpretation of results and manuscript writing by YL, MJ, CW, WY, SG, SL, NB and BL. None of authors had a personal or financial conflict of interest.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Forman D, Burley VJ (2006) Gastric cancer: global pattern of the disease and an overview of environmental risk factors. Best Pract Res Clin Gastroenterol 20: 633-649.
- Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F (2014) Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 23: 700-713.
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, et al. (2016) Cancer statistics in China, 2015. CA: a cancer journal for clinicians 66: 115-132.
- Â Peek RM, Jr., Blaser MJ (2002) Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer 2: 28-37.
- Li D, Wu C, Cai Y, Liu B (2017) Association of NFKB1 and NFKBIA gene polymorphisms with susceptibility of gastric cancer. Tumour Biol 39: 1010428317717107.
- Ren JS, Kamangar F, Forman D, Islami F (2012) Pickled food and risk of gastric cancer--a systematic review and meta-analysis of English and Chinese literature. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 21: 905-915.
- Lin Z, Lin P, Zhang Z, Liu X, Ye W (2012) The Influence of Hypertension on the Elderly by the Diet in Putian. Journal of Liaoning Medical University 33: 164-166.
- Zhang H (2008) Meta-analysis of the relationship between dietary habits and gastric cancer of Chinese residents. Modern Preventive Medicine 35: 216-219.
- Zhou X, Wang Q, Zhang C, Lu X (2006) Assocition between dietary factors and stomach cancer among Chinese people: a Meta analysis. Chinese Journal of Clinical Rehabilitation 10: 1-4.
- Correa P, Fontham E, Pickle LW, Chen V, Lin YP, et al. (1985) Dietary determinants of gastric cancer in south Louisiana inhabitants. J Natl Cancer Inst 75: 645-654.
- Nomura A, Grove JS, Stemmermann GN, Severson RK (1990) A prospective study of stomach cancer and its relation to diet, cigarettes, and alcohol consumption. Cancer Res 50: 627-631.
- Boeing H, Frentzel-Beyme R, Berger M, Berndt V, Gores W, et al. (1991) Case-control study on stomach cancer in Germany. Int J Cancer 47: 858-864.
- Buiatti E, Palli D, Bianchi S, Decarli A, Amadori D, et al. (1991) A case-control study of gastric cancer and diet in Italy. III. Risk patterns by histologic type. Int J Cancer 48: 369-374.
- Gonzalez CA, Sanz JM, Marcos G, Pita S, Brullet E, et al. (1991) Dietary factors and stomach cancer in Spain: a multi-centre case-control study. Int J Cancer 49: 513-519.
- Galanis DJ, Kolonel LN, Lee J, Nomura A (1998) Intakes of selected foods and beverages and the incidence of gastric cancer among the Japanese residents of Hawaii: a prospective study. International journal of epidemiology 27: 173-180.
- Kang H, Rha SY, Oh KW, Nam CM (2010) Green tea consumption and stomach cancer risk: a meta-analysis. Epidemiol Health 32: e2010001.
- Chen XZ, Schottker B, Castro FA, Chen H, Zhang Y, et al. (2016) Association of Helicobacter pylori infection and chronic atrophic gastritis with risk of colonic, pancreatic and gastric cancer: A ten-year follow-up of the ESTHER cohort study. Oncotarget 7: 17182-17193.
- Wang XQ, Yan H, Terry PD, Wang JS, Cheng L, et al. (2011) Interactions between CagA and smoking in gastric cancer. World journal of gastroenterology 17: 3330-3334.
- Yang CS (1980) Research on esophageal cancer in China: a review. Cancer Res 40: 2633-2644.
- Scanlan RA (1983) Formation and occurrence of nitrosamines in food. Cancer Res 43: 2435s-40s.
- Kim J, Park S, Nam BH (2010) Gastric cancer and salt preference: a population-based cohort study in Korea. Am J Clin Nutr 91: 1289-1293.
- Wang Y, Duan H, Yang H (2015) A case-control study of stomach cancer in relation to Camellia sinensis in China. Surg Oncol 24: 67-70.
- Setiawan VW, Zhang ZF, Yu GP, Lu QY, Li YL, et al. (2001) Protective effect of green tea on the risks of chronic gastritis and stomach cancer. Int J Cancer 92: 600-604.
- Ji BT, Chow WH, Yang G, McLaughlin JK, Gao RN, et al. (1996) The influence of cigarette smoking, alcohol, and green tea consumption on the risk of carcinoma of the cardia and distal stomach in Shanghai, China. Cancer 77: 2449-2457.
- Nechuta S, Shu XO, Li HL, Yang G, Ji BT, et al. (2012) Prospective cohort study of tea consumption and risk of digestive system cancers: results from the Shanghai Women's Health Study. Am J Clin Nutr 96: 1056-1063.
- Wang D, Wang Y, Wan X, Yang CS, Zhang J (2015) Green tea polyphenol (-)-epigallocatechin-3-gallate triggered hepatotoxicity in mice: responses of major antioxidant enzymes and the Nrf2 rescue pathway. Toxicol Appl Pharmacol. 283: 65-74.
- Srivastava AK, Bhatnagar P, Singh M, Mishra S, Kumar P, et al. (2013) Synthesis of PLGA nanoparticles of tea polyphenols and their strong in vivo protective effect against chemically induced DNA damage. Int J Nanomedicine 8: 1451-1462.
- Â Gong J, Xue W, Li Y, Ou K, Cao S, et al. (2017) Vine tea suppresses synthesis of N-nitroso compounds and induces PC-3 cell apoptosis of prostate cancer. Chinese Traditional Patent Medicine 39: 2160-2164.
- Matsuo T, Miyata Y, Asai A, Sagara Y, Furusato B, et al. (2017) Green Tea Polyphenol Induces Changes in Cancer-Related Factors in an Animal Model of Bladder Cancer. PloS one 12: e0171091.
- Bernini M, Barbi S, Roviello F, Scarpa A, Moore P, et al. (2006) Family history of gastric cancer: a correlation between epidemiologic findings and clinical data. Gastric cancer 9: 9-13.
- La Vecchia C, Negri E, Franceschi S, Gentile A (1992) Family history and the risk of stomach and colorectal cancer. Cancer 70: 50-55.
- Yaghoobi M, Bijarchi R, Narod SA (2010) Family history and the risk of gastric cancer. Br J Cancer 102: 237-242.
Citation: Lin Y, Jiang M, Wu C, Yan W, Guo S, et al. (2018) Interaction between Risk Factors and Gastric Cancer: A Population-Based Case- Control Study in a High Risk Chinese Area. Epidemiology (Sunnyvale) 8: 359 DOI: 10.4172/2161-1165.1000359
Copyright: © 2018 Lin Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3630
- [From(publication date): 0-2018 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 2830
- PDF downloads: 800