Review Article Open Access
Integrated Processing of Rice Hulls for Production of Polyfunctional Materials
Corby G Anderson1*, SV Yefremova2 and YuI Sukharnikov2
1Kroll Institute for Extractive Metallurgy, Colorado School of Mines, Golden, Colorado, USA
2The National Center for Mineral Raw Materials Complex Processing, 67, Zhandosov Street, 050036, Almaty, Ka-zakhstan, USA
- *Corresponding Author:
- Corby G Anderson
Professor, Kroll Institute for Extractive Metallurgy
Colorado School of Mines, Golden, Colorado, USA
E-mail: cganders@mines.edu
Received Date: August 02, 2013; Accepted Date: September 04, 2013; Published Date: September 11, 2013
Citation: Anderson CG, Yefremova SV and Sukharnikov Y (2013) Integrated Processing of Rice Hulls for Production of Polyfunctional Materials. J Powder Metall Min 2:112. doi: 10.4172/2168-9806.1000112
Copyright: © 2013 Anderson, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Powder Metallurgy & Mining
Abstract
The following method has been proposed for processing the rice hulls with producing materials of polyfunctional purpose that includes the washing, drying, and pyrolysis of the raw materials, and condensation of the vapor-gas mixture. The solid product – nanocomposite produced by densely packed carbon nanoparticles (~500 Å) and silicon dioxide (100÷200Å), which were present in the amorphous form in number of ~52.0% and ~35.0% respectively, proved to be advanced filler for elastomers, sorbent for recovery of precious and rare metals, and fodder supplement for the farm poultry. The organic product (aqueous solution of carboxylic acids (22%), phenols (14%), ketones (12%), cyclic aliphatic hydrocarbons (4.5%), heterocyclic compounds (4%), and spirits and esters (4.5%)) is a high-selective collector to the lead minerals when beneficiating complex rebellious ores, plant growth stimulant used on the non-productive soils and an antiseptic agent. The mixture of non-condensable gases (percent by volume: áÃÂ4~ 40÷45;á2ÃÂ4~ 4÷6; áÞ 2 ~ 20÷22; áÞ ~ 22÷24; ÃÂ2~ 4÷6) can be used for producing carbon black or as a high-calorific fuel
Keywords
Rice hulls; Nanocomposite; Carbon nanoparticles; Silicon dioxide; Filler for elastomers; Sorbent
Introduction
The recovery of the rice hulls (RH) is a topical and complex problem, which the scientists of many countries of the world are working at over the period of many years. The list of the proven trends in the RH application is so great that it would seem that this problem should not exist in principle. But on the strength of a number of factors neither of the proposed technologies had the commercial implementation and acuteness of the assigned task continues aggravating.
The method of the thermal processing is the most prevalent method for the problem solving. Some alternative versions of the rice hulls thermal destruction have been known [1-3], which, unfortunately, have a number of disadvantages. The major of them is lack of utilization of the non-condensable gases, which are discharged into the atmosphere polluting the environment or are used as a fuel that precludes a potentiality to obtain valuable chemical by-products.
Details of Study
The objective of this study was determined as follows: to develop the method for the rice hulls processing, which is free of the above said disadvantages, and to determine potential fields for the obtained products application. The tests object was the rice hulls of the Republic of Kazakhstan.
The rice hulls consisting of cellulose (33%), hemicellulose (18%), lignin (26%), moisture (1%), resinous matter (2%), neutrals (5%) and mineral substances (SiO2 ~ 14%, the sum of CaO, MgO, K2O, Na2O, Fe2O3, Al2O3 and others - 1%) were subject to thermal destruction at 650-750°C without free access of air in the rotary kiln that provided the uniform heating for the rice hulls. Pyrolysis of the material under review resulted in formation of the solid residue and pyrolysis gases, which represented a mixture of condensable vapor and non-condensable gases. The solid product was continuously discharged into the receiving bin. The formed vapor-gas mixture was forwarded to the condensation system. The condensable substances were accumulated in the special collector [4].
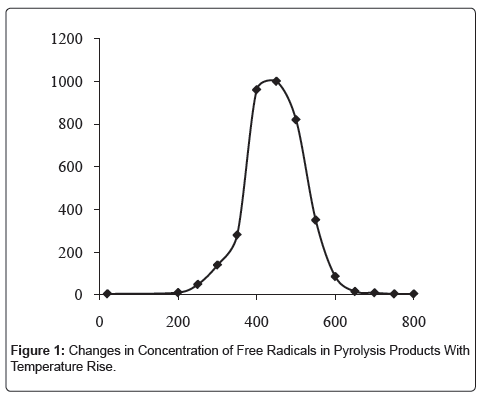
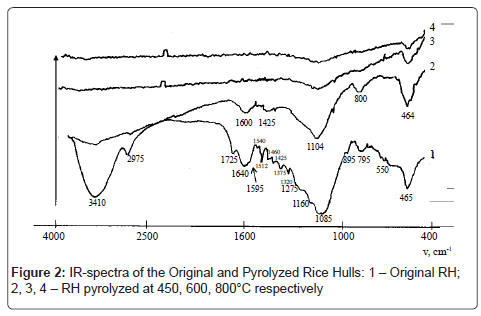
By the thermal analysis findings, it has been specified that the maximum rate of the RH decomposition occurs in the temperature range 230-270°C. At the temperature of 300°C the formation of some advanced materials is developing, which is accompanied by high-heat evolution. The reactions of diminution and elimination of the loosely bound functional groups, as evidenced by rise in concentration of freeradical states (N) in the developed carbonizates, at 450°C are alternating with reactions of condensation of the solid-phase aromatic rings with formation of polycycles, i.e. graphite-alike grids, since N exactly at this temperature, reaching the peak, then decreases (Figure 1), and in the respective IR-spectra the valence-vibrations band is indicated of the conjugated C=C-bonds in the range of 1600 cm-1 (Figure 2).
The nanostructure of the carbon phase (G) was presented by crystallites with the cross sectional dimension L002 ~20-30 Å, which were composed as analogous graphite grids with the spacing C-C 1.42 Å. But as compared with the graphite structure, this nanostructure is less perfect [5].
By X-ray phase analysis method, in the composition of the rice hulls carbonizates besides the graphite phase (G) the presence of two hydrocarbon phases (Table 1) was detected. One of them is the polynaphthenic phase (N), which is diagnosable by the main reflection with d ~ 4.7 Å, represents the clathrate structure that comprises the condensed naphthene cycles separated by methylene bridges, which contain the alkane chains. The structure of the second hydrocarbon phase (H) with d ~ 8 Å is not identified in default of this phase in clear form (Table 1).
| Pyrolysis temperature,°C | Phase content, % | ||
|---|---|---|---|
| G | N | H | |
| 500 | 45 | 45 | 10 |
| 650 | 55 | 35 | 10 |
| 800 | 60 | 30 | 10 |
| 1000 | 100 | N/A | N/A |
Table 1: Phase Composition of the Carbon-Containing Component in the Products from the Rice Hulls Processing.
The rice hulls pyrolysis conditions exert also influence on X-ray characteristics of G in the carbon residues (Table 2): with the temperature rise the interlayer space d002 decreases, the average thickness of the formation members (height of crystallites) L002 and graphitization level c are increasing (it is not practical to calculate the mean diameter of the bedded flat fragments of molecules La due to low intensity of reflections in the area of 21-22°θ). These parameters behavior is indicative of the fact that the structural transformations of the materials under review are mainly developing for account of the intrablock and interlayer orientation.
| Pyrolysis temperature,°C | Parameters for G | ||
|---|---|---|---|
| d002, Å | L002, Å | 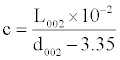 |
|
| 500 | 3.80 | 20 | 0.45 |
| 650 | 3.80 | 23 | 0.50 |
| 800 | 3.75 | 26 | 0.65 |
| 1000 | 3.75 | 30 | 0.75 |
Table 2: The X-ray Indices of the Graphite-alike Phase of Carbonizates from the Rice Hulls.
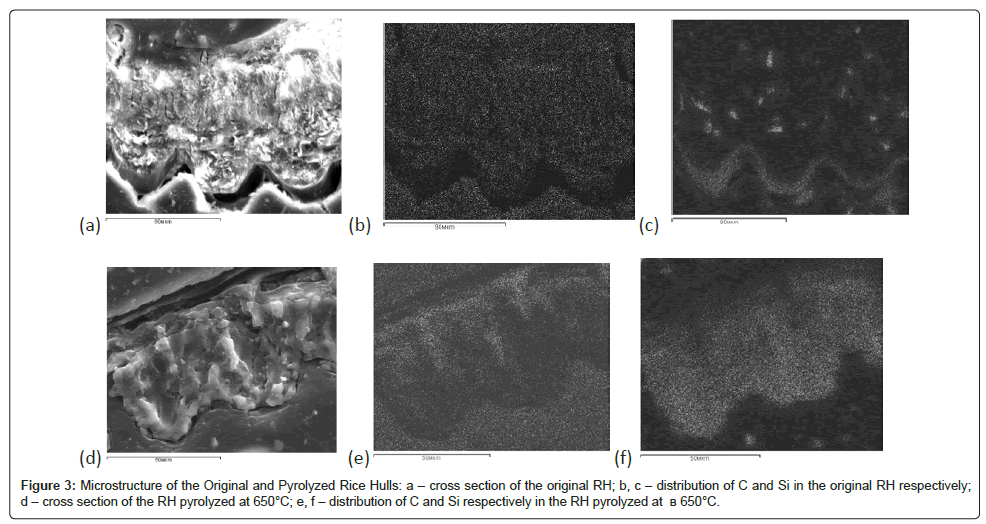
By the Raster Electron Microscopy data (Figure 3), the rice hulls maintain their topology even at relatively high temperature action (up to 800°C) most probably due to high content of silicon, which as seen from the cluster of the light points in Figure 3c is mainly found in the outer and inner tissues of epidermis and also localized in definite places of the inner cells. However in this case the chemical elements redistribution takes place. As a result of the thermal destruction of the rice hulls organic component, a peculiar carbon skeleton is formed (Figure 3e), which is uniformly filled with the silicon-containing phase (Figure 3f) [6].
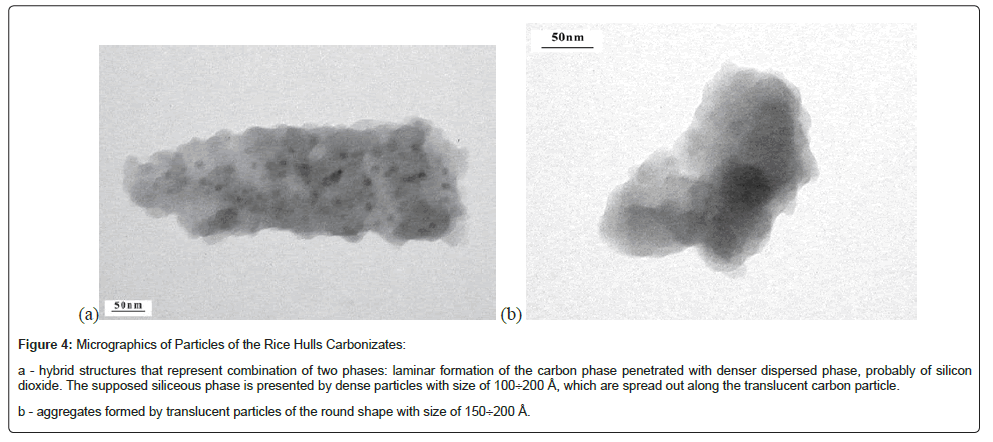
The Transmission Electron Microscopy results indicated that carbonizates of the rice hulls were formed by particles with different morphology among which the laminar type and translucency appear to be the common features (Figure 4).
Figure 4: Micrographics of Particles of the Rice Hulls Carbonizates:
a - hybrid structures that represent combination of two phases: laminar formation of the carbon phase penetrated with denser dispersed phase, probably of silicon dioxide. The supposed siliceous phase is presented by dense particles with size of 100 ÷ 200 Å, which are spread out along the translucent carbon particle.
b - aggregates formed by translucent particles of the round shape with size of 150 ÷ 200 Å.
In virtue of the fact that SiO2 in the rice hulls is in close connection with the organic components, in consequence of the plant tissue thermal destruction it forms a non-separate structure, and on account of high interparticle interaction with carbon it forms a nanocomposite assembly. Therefore, one may draw a conclusion that the solid product from thermal processing of the rice hulls represents nothing else than a nanocomposite, i.e. a macroscopic object formed by densely packed nanoparticles of carbon and silicon dioxide, which are present practically in equal quantities (50-55 and 40-45% by mass respectively) and uniformly distributed between them.
Due to the fact that the technical carbon is not produced in Kazakhstan, while it is the second ingredient after the natural rubber that defines the service performance of the rubber articles, and organization of such production facility seems unpractical considering necessity in combustion of expensive and exhaustible hydrocarbon materials (oil, gas and coal), in the process of which some carcinogenic emissions are originated, it was of some interest to evaluate the siliconcarbon material as a filler for elastomers.
The physical/chemical characteristics of the silicon-carbon (SC) are given in Table 3.
| Name of characteristic | SC |
| Iodine value, g/kg | 54-58 |
| Absorption of dibutyl phthalate, cm3/100 | 100-110 |
| pH of the aqueous slurry | 7-9 |
| Mass fraction of losses at 105°C, % | 2 |
| Bulk density of the granular carbon, kg/m3 | 418 |
Table 3: The Physical/Chemical Characteristics of the Silicon-Carbon Material.
The silicon-carbon product of two types: the product that contained the form of the original rice hulls with the grain size up to 5 mm (granular product) and the product crushed in the rotary jet-type mill up to 90% size grade -15 μm (powdered product) - were tested as a substitute for various grades of technical carbon and silica white when producing general rubber goods. As an illustration, in Table 4 some properties are presented in comparison with the standard properties of the experimental vulcanizates, which were produced using the siliconcarbon filler according to the mix formulation indicated in (Table 5).
| Ingredients | Mix ingredients weight, kg | ||||
|---|---|---|---|---|---|
| Standard 1 | Sample 1 | Sample 2 | Sample 3 | Sample 4 | |
| Natural rubber SKS-30 ARKM-15 | 0.500 | 0.500 | 0.500 | 0.500 | 0.500 |
| Zinc white | 0.040 | 0.040 | 0.040 | 0.040 | 0.040 |
| Technical stearine | 0.095 | 0.0095 | 0.095 | 0.095 | 0.095 |
| Captax | 0.010 | 0.010 | 0.010 | 0.010 | 0.010 |
| White kaolin | 0.330 | 0.330 | 0.330 | 0.330 | 0.330 |
| Carbon black BS-100 | 0.160 | - | - | 0.160 | 0.160 |
| Technical carbon P 803 | 0.395 | 0.200 | 0.200 | 0.075 | 0.075 |
| SC granular | - | 0.355 | - | 0.320 | - |
| SC powdered | - | - | 0.355 | - | 0.320 |
| Plasticine | 0.031 | 0.031 | 0.031 | 0.031 | 0.031 |
| Rubrax | 0.050 | - | - | - | - |
| Sulfur | 0.016 | 0.016 | 0.016 | 0.016 | 0.016 |
| Thiuram | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 |
| Total | 1.635 | 1.585 | 1.585 | 1.585 | 1.585 |
Table 4: The Mix Formulation of the Rubber Compound for Producing the General Rubber Goods.
| Properties | Vulcanizate sample | ||||
|---|---|---|---|---|---|
| Standard 1 | Sample 1 | Sample 2 | Sample 3 | Sample 4 | |
| Rupture strength, kgf/cm2 | 10 | 12 | 12.1 | 28 | 35.5 |
| Ultimate elongation at rupture, % | 120 | 78 | 85 | 100 | 82 |
| Relative residual elongation after rupture, % | - | 5 | 4 | 6 | 4 |
| Hardness | 67-80 | 77 | 76 | 68 | 77 |
Table 5: Physical/Mechanical Properties of General Rubber Goods.
In the course of the rubber compounds batching, the SC is easily dispersed in the natural rubber matrix. Due to this and also to the common presence, and more importantly, as stated above, to the uniform distribution of carbon- and silicon dioxide particles in the mass of each other, which were developed in the active amorphous form by pyrolysis, and due to the material’s ability to be easily pulverized, application of the SC when producing the rubber technical goods, in case of partial and/or complete substitution of the technical carbon and/or silica white, and/or their mix conduces to improvement of technological properties of the rubber mixes and finished vulcanizates (increase in elasticity, improvement of the adhesive behavior and enhancement of the strength properties).
Deployment of the silicon-carbon filler in combination with technical carbon and silica white allows producing the rubber technical articles with the specified properties. In this case the complete and even partial substitution of the standard reinforcing fillers ensures lowering the production costs of the rubber goods and tire industry articles due to relatively low (as compared with technical carbon and silica white) cost of the silicon-carbon produced from the cheap local anthropogenic raw material. Substitution of the traditional fillers with the silicon-carbon material is specified by the requirements imposed to the finished articles, and it has no provision for any changes in the production line and technological process. With a view of producing the rubbers with the required properties, it is permitted to adjust formulation of the rubber compounding.
The silicon-carbon of the rice hulls was tested with a view to use it as sorbent in various engineering processes.
It has been approved that the macroporous silicon-carbon from the rice hulls as sorbent is high active in the process of the waste-water treatment from petroleum- and oil contamination: the static volume capacity (SVC) = 50 mg/g when recovering 98% from solution that contains petroleum products and oils in the quantity of 200 mg/dm3; the dynamic volume capacity (DVC) = 80 mg/g when recovering 99.5%. In the process of the precious metals recovery from the production solutions, by its absorption characteristics on gold (SVC = 2.4 mg/g when recovering 91.4% from the solution that contained 8.1 mg/dm3 of Au+ ions) the activated silicon-carbon is equal to the available industrial sorbents: resin AM-2B and coking coals. When recovering rhenium from the production sulfurous solutions (pH=2), in one sorptiondesorption cycle the silicon-carbon provides increase in concentration of rhenium by 2.4 times that opens prospects for its application in the sorption technology for rhenium recovery with a view to prepare the end product - ammonium perrhenate [7].
The veterinary-toxicological evaluation of the silicon-carbon from the rice hulls (we named this product “Risostim”) on the laboratory animals and by the bioindication method indicated that this product was non-toxic, and it could be used as fodder supplement and various fillers for biopreparations in veterinary. It was recommended to use “Risostim” in the ration for the laying hens and chicken broilers in concentration from 1 to 6%. In the course of the scientific-economic experiment it was specified that feeding with the mixed fodder with 3%-content of “Risostim” increased (by 8.6%) the egg-laying capacity of the laying hens and decreased the egg-breakage (by 31%), the hens mortality (by 28%) and sanitary slaughtering (by13%) [8].
As investigations have shown, the organic condensate from the rice hulls pyrolysis has a wide range of its application. This organic condensate represents the water solution of carboxylic acids (22%), phenols (14%), ketones (12%), cyclic aliphatic hydrocarbons (4.5%), heyerocyclic compounds (4%), spirits and esters (4.5%). Owing to content in its composition of ~6% dihydroxy-benzenes (62% of which shall be pyrocatechol with its derivatives) that are used in production of high-selective ion-exchange resins, some polymeric composites with the globular structure were obtained on its basis, which had relatively high sorption capacity in relation to a number of the water contaminants (ions of As3+, Sb3+ and others), and they could be used for realization of high-selective and high-speed processes of sorption and ion exchange in various branches of industry.
In addition, the pyrolyzate is a highly selective collector in relation to lead minerals in beneficiating complex rebellious lead-zinc ores [9].
The pyrolyzate is active as high-efficiency plant growth stimulant. The presowing treatment of the seeds with 0.5%-solution of pyrolyzate secures increase in productivity of the Lucerne green material by 22.4% and barley corn by 26.3% when growing them on the meadow brown-, alkali-saline-, strongly saline-, medium loamy soils against the background of the zonal agrotechnology, and the Indian corn by 28,6% when growing it on the light-chestnut-, irrigated-, weakly eroded-, medium loamy soils against the background of mineral fertilizers (N92P92).
In addition, the rice hulls pyrolyzate can be used as antiseptic; 50%-solution of it exerts the more intense inhibitory action on the tuberculosis cell culture growth Mycobacterium bovis St. 8 as compared with the industrial preparation “Glutar”.
The thermal decomposition of the non-condensable gases without free air delivery, owing to the presence of the saturated and unsaturated hydrocarbons in their composition (CH4 ~ 40÷45 volume %; C2H4 ~ 4÷6 volume %; CO2 ~ 20÷22 volume %; CO ~ 22÷24 volume %; and H2 ~ 4÷6 volume %), allows the additionally getting of the valuable carbon product: carbon black and high-calorific gaseous fuel.
Thus, the developed method of processing ensures the maximum possible recovery of the rice hulls with production of the silicon-carbon material and valuable by-products (organic condensate, carbon black and fuel gases), enhancement of the ecological cleanness of the process, and it opens up new potentialities for producing a number of materials of different purpose on the basis of the cheap raw material. This study has been performed under the Project of the International Science and Technology Center (the financing party - USA).
References
- US Patent No. 4,591,492. The Method for Crystalline SiC Production Using the Rice Hulls Preliminary Treated by the Acid.
- David ER (1981) Production of Silicon Carbide from Rice Hulls and Silicon Dioxide. US Patent No 4: 248-844.
- Zelenukhova LN, Sergienko VI (2004) The Method for Producing of Silicon Dioxide from the Rice Processing Waste and the Device for this Method Implementation. Patent RU 2: 233-795.
- Sukharnikov Yu I, Yefremova SV, Corby G. Anderson, Zharmenov AA, Bounchouk LV, et al. (2006) The Method for Thermal Processing of the Rice Hulls and Their Lignin Residues. RK Pre-Patent 17: 867.
- Yefremova SV, Korolev Yu M, Sukharnikov Yu I (2008) X-Ray Characteristic of the Silicon-Carbon Nanocomposites from the Rice Hulls and Their Derivatives. Doklady Chemistry 419: 78-81.
- Yefremova SV, Sukharnikov Yu I (2007) Study of Nanostructure and Phase Composition of Silicon-Carbon Fillers Produced from the Rice Hulls for Elastomers. Annales Universitatis Mariae Curie-Sklodowska. Sectio AA. Chemia 194-209.
- Korabayev AS, Bounchouk LV, Yefremova SV, Sukharnikov Yu I, Kobzhasov AR (2008) Bulletin of the KazNTU 5: 137-141.
- Sukharnikov Yu I, Sarsembayeva NB, Yefremova SV, Zharmenov AA, Bounchouk LV, et al. (2008) The Fodder Additive Risostim 1: 132.
- Yefremova SV, Sukharnikov Yu I, Yeremin Yu P, Corby G. Anderson (2006) Assessment of the Flotation Activity of Pyrolyzate from the Rice Hulls When Beneficiating Complex Rebellious Ores. The Abishev’s Readings “The Liquid at the Phase Boundary – Theory and Practice”: Proceedings of the International Scientific and Practical Conference Dedicated to the 70thAnniversary of Mr. Abishev, Zhantore Nurlanovich, Laureate of the RK State Prize, Corresponding Member of the NAS RK 84-87.
Relevant Topics
- Additive Manufacturing
- Coal Mining
- Colloid Chemistry
- Composite Materials Fabrication
- Compressive Strength
- Extractive Metallurgy
- Fracture Toughness
- Geological Materials
- Hydrometallurgy
- Industrial Engineering
- Materials Chemistry
- Materials Processing and Manufacturing
- Metal Casting Technology
- Metallic Materials
- Metallurgical Engineering
- Metallurgy
- Mineral Processing
- Nanomaterial
- Resource Extraction
- Rock Mechanics
- Surface Mining
Recommended Journals
Article Tools
Article Usage
- Total views: 13257
- [From(publication date):
September-2013 - Dec 24, 2024] - Breakdown by view type
- HTML page views : 8910
- PDF downloads : 4347