Research Article Open Access
Intake of food pellets containing pre-germinated brown rice alleviates cognitive deficits caused by β-amyloid peptide25-35 in mice: Implication of lipid peroxidation
| Takayoshi Mamiya1*, Makoto Ukai1, Keiko Morikawa2 and Mitsuo Kise2 | ||
| 1Department of Chemical Pharmacology, Graduate School of Pharmaceutical Sciences, Meijo University, Japan | ||
| 2FANCL Institute, FANCL Corporation, 12-13 Kamishinano, Japan | ||
| Corresponding Author : | Takayoshi Mamiya Department of Chemical Pharmacology Graduate School of Pharmacy, Meijo University, Japan Tel: +81-52-839-2737 Fax: +81-52-834-8090 E-mail: mamiya@meijo-u.ac.jp |
|
| Received October 28, 2013; Accepted November 29, 2013; Published December 02, 2013 | ||
| Citation: Mamiya T, Ukai M, Morikawa K, Kise M (2013) Intake of food pellets containing pre-germinated brown rice alleviates cognitive deficits caused by β-amyloid peptide25-35 in mice: Implication of lipid peroxidation. J Rice Res 1:116. doi: 10.4172/jrr.1000116 | ||
| Copyright: © 2013 Mamiya T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Rice Research: Open Access
Abstract
In this study, we investigated whether the food pellets containing pre-germinated brown rice (PGBR; hatsuga genmai in Japanese) were effective on the impairments of cognitive function induced by ß-amyloid peptide25-35 (Aß25-35) in mice. To evaluate the effects of PGBR, mice were received AIN-93G (as control pellets) or PGBR-added food pellets (PGBR pellets) during this study. Aß25-35 (3 nmol/3 ßmol i.c.v.) was injected to mice on the day 22. On the days 30 and 31, we assessed the tasks related to visible cognition using novel object recognition tests. By the injection of Aß25-35 in the control pellets-fed mice impairments were observed, but the mice fed PGBR-added food pellets did not show the deficits. After the behavioral tests, we found Aß25-35 increased lipid peroxidation in the hippocampus of control pellets-fed mice but not PGBR pellets-fed mice. Taken together, these results suggest that continuous feeding of food pellets containing PGBR (i) attenuates the Aß25-35-induced impairments of cognitive function, and (ii) inhibited increases in lipid peroxidation in the hippocampus.
| Keywords | |
| Pre-germinated brown rice; Novel object recognition; Lipid peroxidation; Hippocampus | |
| Introduction | |
| Rice is an important energy source for people worldwide. The rice grain consists of an endosperm, a bran layer and a germ. However, polished rice lacks the bran and germ, which are abundant in dietary fiber, vitamins and minerals. Polished rice is one of the main staples in Asian countries. On the other hand, pre-germinated brown rice (PGBR) is rapidly becoming popular as a health food in Japan. PGBR is brown rice soaked in water to induce slight germination and is known to contain an abundance of nutrients, including 13 times the amount of ß-oryzanol, a potent antioxidant, present in polished rice. In a recent study, we revealed that ferulic acid (4-hydroxy-3-methoxycinnamic acid; FA), a main and active metabolite of ß-oryzanol attenuated oxidative stress-induced memory impairment in mice [1]. | |
| Alzheimer’s disease (AD) is the most common cause of a progressive decline in cognitive function in the elderly, and is characterized by the presence of numerous senile plaques and neurofibrillary tangles associated with the loss of neurons in the brain [2]. Senile plaques that essentially consist of Aß, a peptide that is thought to be the leading cause of neurotoxicity. There is accumulating evidence that oxidative stress contributes to Aβ-induced neurotoxicity [3]. Elevated levels of Aß a major component of senile plaques induce oxidative stress, which mediates the damage seen in AD patients [4]. The involvement of oxidative stress, including protein oxidation and lipid peroxidation, has been widely reported in AD brain samples and experimental models of AD [4,5]. Thus, one promising mechanism for the prevention of Aß25- 35-induced memory deficits may be the suppression of oxidative stress, for example, via radical scavenging. Antioxidants such as ß-tocopherol and other reagents with anti-oxidative activity protect neurons against Aß-induced cytotoxicity and behavioral deficits [6,7]. In addition, it has been reported that imbalance in oxidative homeostasis and lipid peroxidation is involved in AD [8]. Therefore, the scavenging effects of natural, safe ingredients from foods may be a rational alternative to preventive and therapeutic interventions. | |
| However, it remains unclear whether the effects of PGBR in this AD animal model are related to lipid peroxidation. Here, in order to investigate whether intake of PGBR pellets is effective against Aß25- 35-induced memory impairments, we performed the novel object recognition test and then measured lipid peroxidation levels in the hippocampus. | |
| Materials and Methods | |
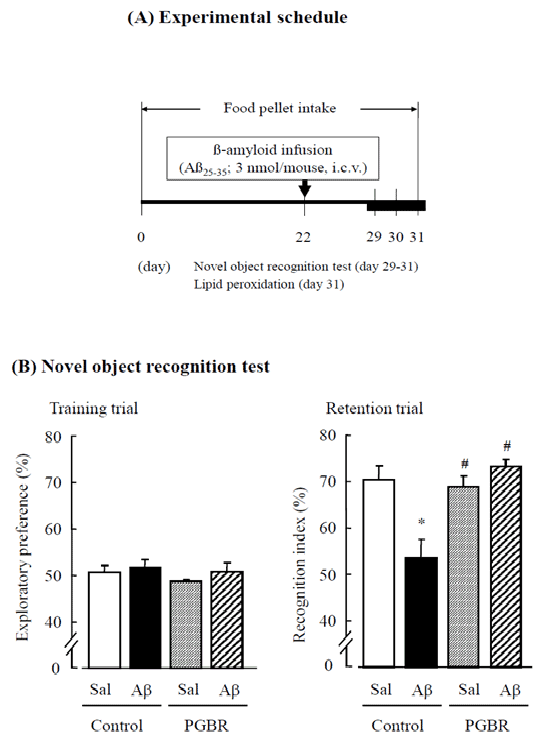
| Animals and food: Five-week-old male ICR mice (Nihon SLC Co., Shizuoka, Japan) were purchased. We performed experiments as shown in Figure 1A. We received mice from 11h00 and 13h00, then put mice into the home cage and gave adequate food pellets (day 0). Animals were housed in a controlled environment (23 ± 1°C, 50 ± 5% humidity) and given access to water ad libitum. Room lights were on between 7:30 and 19:30. We used control pellets (AIN-93G; Oriental Yeast, Tokyo, Japan), the ingredients of which are as follows: 39.7% cornstarch, 20% casein, 0.3% L-cystine, 13.2% ß-cornstarch, 10% sucrose, 7% bean oil, 5% cellulose powder, 3.5% minerals, 1% vitamins, 0.25% choline bicitrates, and 0.0014% butylhydroquinone. | |
| The rice we used is grown in Hokkaido area in Japan (Oryza sativa subsp. japonica (Hoshino-yume)). PGBR was prepared at 25–30% water content to induce germination and dried to 15% according to a patented procedure (Patent No. 3738025, JP, November 4, 2005). PGBR were manufactured as powdered feed by Oriental Yeast. The nutrients in the PGBR pellets were the same as those in AIN-93G, except that cornstarch was replaced with PGBR, as reported previously [9,10]. All experiments were approved with the Guidelines for Animal Experiments of Meijo University (Approved number: Yaku-Jitsu-12) and the Guiding Principles for the Care and Use of Laboratory Animals approved by the Japanese Pharmacological Society (2007). | |
| Aß25-35 treatment: Aß25-35 (Bachem AG, Budendorf, Switzerland) was dissolved in sterile distilled water at a concentration of 1 mg/ ml and allowed to aggregate by incubation at 37°C for 4 days, as reported previously [9]. Peptide and vehicle (3 μL) were administered intracerebroventricularly (i.c.v.) for approximately 20 s under light ether anesthesia on day 22. The site of administration was checked by injecting India ink in preliminary experiments. Neither insertion of the needle nor injection of the vehicle had a significant influence on survival, behavioral response or cognitive function [9]. | |
| In the novel object recognition test, we used 13 mice in each group. Each mouse was individually habituated in a black plastic cage (30×30 cm floor×50 cm height) for 15 min on days 29 and 30. On day 31, in the training trial, two same-sample objects (white plastic film cases, Ø3×6 cm) were placed in the cage, and the mouse was allowed to explore them freely for 5 min. The time spent exploring each object was recorded manually using a stopwatch. In the retention trial that was conducted immediately after the training trial, the mouse was removed once and then placed back in the same cage, and one of the objects used in the training trial was replaced with a novel object (black dry battery cell, Ø3×6 cm) for the mouse to explore freely for 5 min. Exploratory preference (%), which is the ratio of time spent exploring any one of the two same-sample objects in the training trial or the novel object in the retention trial against the total time spent exploring both objects, was used to express recognition memory [1]. | |
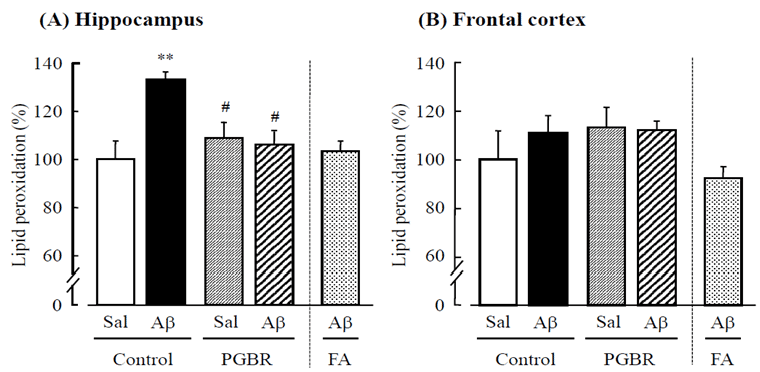
| Lipid peroxidation (FOX) assay was performed on day 31. This assay is a direct measure of lipid hydroperoxide; it is based on the formation of an Fe(III) xylenol orange complex that is measurable at 560-580 nm. After the novel object recognition test, we randomly selected 5 mice from each group. We also prepared 5 mice treated with FA (15 mg/kg/day orally) based on a previous report [11]. Mouse brains were rapidly removed and the hippocampus was dissected out on an ice-cold glass plate. Brain tissue (about 100 mg) was added to 0.9 mL of homogenization buffer (100 mM KH2PO4/KOH buffer, pH. 7.4) and homogenized in a sonicator. The homogenized tissue was centrifuged at 2,000 rpm, and the supernatant (100 ßL) was placed in an Eppendorf tube. Fox reagent was made by adding the following sequentially into a flask: 2.5 mM FeSO4, 250 mM H2SO4, 1 mM xylenol orange and distilled water to make a final volume of 100 mL. Fox reagent (0.9 mL) was added to the 100-ßL sample and incubation was conducted at room temperature for 30 min in the dark, after which absorbance was read at 580 nm. Blanks were prepared by replacing tissue extract with phosphate buffer. One millimolar cumene hydroperoxide (5 ßL) was added to the tissue samples, which were left to incubate at room temperature for another 30 min, and the absorbance at 580 nm was read again. Levels of lipid peroxidation (%) are expressed as cumene hydroperoxide equivalents [12]. | |
| All results were expressed as means ± SEM for each group. Data were analyzed by one-way ANOVA, followed by Tukey’s test. The level of significance was set at P<0.05. | |
| Results | |
| Novel recognition object test: As shown in Figure 1B, there were no differences in exploratory preference among the groups in the training trial (F3,51=0.07, P=0.98). In the retention trial of control pellets group, the Sal-treated group exhibited greater preference for the novel object than the familiar object. The duration of exploration (recognition) of the novel object was decreased by Aß25-35 peptide injection without affecting ambulation. PGBR pellets attenuated the decrease in exploratory preference induced by Aß25-35 peptide to a value comparable to that obtained in Sal-treated group (F3,51=5.11, P<0.01, Tukey’s test; P<0.05). | |
| Lipid peroxidation (FOX) assay: To determine the effects of PGBR pellets on the brain oxidative load, we measured the index of lipid peroxidation (levels of cumene hydroperoxide equivalent) in the hippocampus (Figure 2). On day 31, we confirmed that Aß25-35 increased lipid peroxidation to 130.1% vs. the control group (F3,19=12.07, P<0.001, Tukey’s test; P<0.01). On the other hand, PGBR pellets significantly inhibited this increase (F3,19=12.07, P<0.001, Tukey’s test; P<0.05) to levels comparable to those in mice treated with FA (Figure 2A). In the frontal cortex (Figure 2B), there were no differences among the groups (F3,19=0.92, P=0.11). | |
| Discussion | |
| In this study, we clarified that intake of PGBR inhibited the cognitive impairments and lipid peroxidation in the hippocampus of the Aß25-35-treated mice. Previously, we have already reported that the Aß25-35-induced impairments of spontaneous alternation behavior in the Y-maze are reversed by intake of PGBR for 4 weeks [9]. However, as the behavior can also reflect drug-related changes in sensory/ attentional, motivational and performance processes, spontaneous alternation behavior should not be unquestionably accepted as a measure of memory alone [13]. Therefore, we used the novel object recognition task to evaluate the effects of PGBR here. In the training trial, we could not find any differences on the exploratory preference among four groups. Additionally, our previous report has been shown that there are no significant changes on the counts of ambulation, rearing, grooming under non-stimulating condition in the open field test [14]. No serious healthy accidents have been reported to happen in spite of daily intake of PGBR in Japan for the past 30 years, so that our data support that PGBR is healthy and safe food. | |
| In the retention trial of control pellets group, the Sal-treated group exhibited greater preference for the novel object than the familiar object. The duration of recognition of the novel object was decreased by Aß25-35 peptide. PGBR pellets attenuated the decrease in recognition induced by Aß25-35 peptide to a value comparable to that obtained in Sal-treated group. In addition, we have reported that 1-day intake of PGBR has no effect on behavioral impairments [9]. These facts suggest that repeated intake of PGBR may prevent the induction of cognitive dysfunction by Aß25-35 peptide. | |
| In AD patients, elevated levels of Aß induce oxidative stress, which mediates the neuronal and functional damage [4]. The basic studies have revealed that oxidative stress contributes to the Aβ1–42-induced toxicity in vivo, as shown by induction of cytosolic Cu, Zn-superoxide dismutase (SOD) and mitochondrial Mn-SOD in the hippocampus and cortex. Production of malondialdehyde (lipid peroxidation) and protein carbonyl (protein oxidation) remains elevated 10 days after Aβ1–42 injection [14]. Similarly, Aβ25–35 induces significant oxidative stress, measured within 1 week after injection, as an increase in lipid peroxidation and superoxide generation [6,15]. | |
| PGBR includes much antioxidants, not only ferulic acid (FA) but also γ-oryzanol, which is mainly metabolized to FA in liver. In the very recent, Umnajkitikorn’s group have been shown that antioxidant properties of germinated rice (Oryza sativa L.) which is same type we used, are enhanced [16]. FA has also been shown to possess free radical scavenging activity and to reduce peroxidative damage in vitro [7,17]. PGBR pellets contain 9.1 mg/100 g total FA, of which about 50-60% is oryzanol. Because the average PGBR pellets intake was 4.5 ± 0.10 g/day/mouse during the study [9], the amount of FA consumed was 0.41 mg/mouse/day. In addition, body weight was 27.6 ± 0.2 g at the beginning of administration and 41.3 ± 0.5 g at sacrifice, which gives a dose of approximately 10-15 mg/kg/day during the experiment. Yan et al. showed that pretreatment with FA in drinking water (at 14-19 mg/ kg/day) for 28 days prevented Aß1-42-induced impairment of learning and memory in mice [11]. Taken together, these results suggest that FA contributes to improving cognitive dysfunction in this AD model. | |
| We found that only in the hippocampus, Aß25-35 significantly increased lipid peroxidation in control pellet-fed mice, whereas in PGBR-fed mice, we did not observe such increases. Thus, it is likely that there are specific brain regions that are responsive to oxidative stress, and we found that the hippocampus is more sensitive to peroxidation by Aß25-35 than the frontal cortex. Functionally, the hippocampus receives inputs from the perirhinal cortex, which is itself the site of several information entrances as visual, olfactory, and somatosensory stimulus, all of them involved in object recognition [18,19]. Therefore, it is consistent that systemic intake of PGBR may alleviate cognitive dysfunction accompanied with hippocampal lipid peroxidation by Aß25-35. | |
| Whereas we have already clarified that PGBR increases serotonin contents in the frontal cortex [10], the relationship between increased serotonin in the frontal cortex and PGBR on cognitive impairment is unclear, so that we aim to clarify the correlations between PGBR and neurotransmitters in the brain in a future study. In conclusion, we surmise that the reversal effects of PGBR on Aß25-35-induced cognitive dysfunction are at least partially due to the radical scavenging activity of FA in the PGBR. Therefore, it is possible that the scavenging effects of PGBR may prevent the neurotoxicity induced by Aß25-35. | |
| Acknowledgements | |
| This work was supported by JSPS KAKENHI (Grant Numbers 24590304 and 22790233); the Research Project Supported by the Takeda Science Foundation; the Meijo Asian Research Center; the Smoking Research Foundation Grant for Biomedical Research; a Sasakawa Scientific Research Grant from the Japan Science Society; and Basic Research Grants from the Japan Health and Research Institute and the Aichi Health Promotion Foundation. We are thankful to Messrs. Keita Onogi, Yuya Hasegawa, Yoshiaki Kawai and Takamasa Asanuma for help in the experiment. | |
References |
|
|
|
Figures at a glance
 |
 |
|
| Figure 1 | Figure 2 |
Relevant Topics
- Basmati Rice
- Drought Tolerence
- Golden Rice
- Leaf Diseases
- Long Grain Rice
- Par Boiled Rice
- Raw Rice
- Rice
- Rice and Aquaculture
- Rice and Nutrition
- Rice Blast
- Rice Bran
- Rice Diseases
- Rice Economics
- Rice Genome
- Rice husk
- Rice production
- Rice research
- Rice Yield
- Sticky Rice
- Stress Resistant Rice
- Unpolished Rice
- White Rice
Recommended Journals
Article Tools
Article Usage
- Total views: 14797
- [From(publication date):
December-2013 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 10165
- PDF downloads : 4632
