Editorial Open Access
Insight into Zeta Potential Measurements in Biopolymer Film Preparation
Mohammed Sabbah, Marilena Esposito, Prospero Di Pierro, C. Valeria L. Giosafatto, Loredana Mariniello and Raffaele Porta*
Department of Chemical Sciences, University of Naples “Federico II”, Complesso Universitario di Monte Sant’Angelo, via Cintia, 80126 Napoli, Italy
- *Corresponding Author:
- Raffaele Porta
Department of Chemical Sciences
University of Naples “Federico II”
Complesso Universitario di Monte Sant’Angelo
via Cintia, 80126 Napoli, Italy
E-mail: portaraf@unina.it
Received April 26, 2016; Accepted April 27, 2016; Published May 04, 2016
Citation: Sabbah M, Esposito M, Pierro PD, Giosafatto CVL, Mariniello L, et al. (2016) Insight into Zeta Potential Measurements in Biopolymer Film Preparation. J Biotechnol Biomater 6:e126. doi:10.4172/2155-952X.1000e126
Copyright: © 2016 Sabbah M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
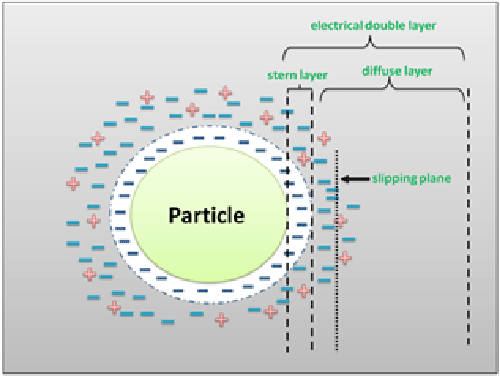
Micro- and nano-particle charge is one of the main factors determining the physical stability of both emulsions and suspensions and can be quantified by measuring their so called “zeta potential”. When all the particles have a large either negative or positive zeta potential value, they will repel each other and, as a consequence, the suspension becomes stable. By contrast, whether the zeta potential is close to 0 mV, the tendency for flocculation increases. Zeta potential is, however, a feature of the particle in its environment and not of the particle itself. In fact, its net charge in solution affects the ion distribution surrounding the particle, thus resulting in an increase in the concentration of counter-ions. The region over which this influence extends is called “electrical double layer” (EDL) and EDL splits into two regions (Figure 1). In the first, called “stern layer”, the ions are of opposite charge with respect to the particles and, being strongly bound to them, move with them. The second layer, conversely, is a “diffuse layer” where the ions are less strongly attached and, inside it, there is a boundary line between the ions moving with the particles and the not moving ones. This region, called “slipping plane”, is known as the surface of hydrodynamic shear and the potential existing in the slipping plane is called zeta potential [1].
Electrophoresis is the most widely used technique for measuring zeta potential. By directly analysing the electrophoretic mobility of a particle, the zeta potential may then be determined using the “Henry equation”:
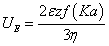
where UE is the electrophoretic mobility, ε is the dielectric constant, z is the zeta potential, η is the viscosity and f(Ka) is Henry’s function. For measuring zeta potential in aqueous solutions of moderate electrolyte concentration, a Henry’s function value of 1.5 is used (Smoluchowski approximation) whereas, if zeta potential is measured in a non-polar solvent, f(Ka) is set to 1.0 (Huckel approximation).
Zeta potential is attracting an increasing interest for the characterization of electrochemical surface properties of both microand nano-particles being a key parameter for a number of applications, including characterization of biomedical polymers, electrokinetic transport of particles or blood cells, biocompatibility tests for pharmaceuticals and medical devices, membrane separation, protein purification, mineral processing, water treatment, characterization of clothing material properties in the textile industry [2,3].
The use of biodegradable and/or edible films was proposed in the late sixties, originally to extend the shelf life of various fresh, frozen and manufactured food items and to improve their quality [4]. Many biomacromolecules including proteins and carbohydrates have been thus far used, blended or not, as edible films and coatings [4-7]. Proteinand polysaccharide-based films show good tensile features, whereas lipid-based films have proved to be good water vapour barriers [8-10]. The formation of biopolymer supra-molecular structures induced by electrostatic interactions is related to the nature of raw materials, as well as to other factors such as concentration of the components, their mix ratio, pH, temperature and ionic strength [11-13]. In addition, Porta et al. [14,15] studied the effect of transglutaminase (TGase), an enzyme able to form protein inter- and/or intra-molecular crosslinks, on the mechanical and barrier properties of protein-based films showing that the enzyme is a very useful tool to produce innovative bio-plastics from renewable biomass sources. Giosafatto et al. [16] characterized citrus pectin (PEC) edible films containing TGase-modified phaseolin, and reported that the mechanical, as well as barrier properties to CO2, O2 and water vapor of the latter were comparable to the ones of commercial plastics. More recently, Porta et al. [17] investigated the microstructure and some features of bitter vetch (Vicia ervilia, BV) seed protein films reinforced by microbial TGase. BV, an annual grain legume crop thus far widely cultivated only for forage because of its high nutritional value, shows several favourable characteristics, such as having high yields and being a cheap and abundant protein source [18,19]. Arabestani et al. [20] found that the very low gas permeability, in addition to good mechanical properties, of BV protein films prepared in the presence of the enzyme conferred to these new bio-materials potential practical applications not only as edible coatings in food packaging but also as biodegradable containers.
On the other hand, polysaccharides as starch, alginate, cellulose, chitosan, carrageenan, PEC, or their derivatives, impart hardness, crispness, compactness, thickening quality, viscosity, adhesiveness, and gel forming ability to a variety of films. These films, because of the make up of the polymer chains, exhibit excellent gas permeability properties, resulting in desirable modified atmospheres that enhance the shelf life of the products without creating anaerobic conditions [21]. Additionally, polysaccharide films and coatings can be used to extend the shelf life of muscle foods by preventing dehydration, oxidative rancidity and surface browning, even though their hydrophilic nature makes them poor barriers for water vapour [22].
Among the different polysaccharides, PEC appears to be suitable for low moisture foods [21]. PEC, mainly extracted from citrus peel and apple pomace, is a heterogeneous group of acidic macromolecules well known for their long and safe use in the food industry as a thickening and stabilizer agents. Because of its gelling characteristics, bio-adhesivity, biocompatibility and non-toxicity properties, PEC is also a promising biopolymer as a drug delivery vehicle. In fact, it has been used in mucoadhesive systems to increase the retention time of the dosage form in the gastrointestinal tract, thus enhancing drug absorption after its oral administration [23,24]. The ability of PEC to adhere to mucous membranes seems to be dependent on the different type of polysaccharide employed [25,26]. In particular, the degree of esterification or amidation of the galacturonic acid residues inside the macromolecule is often used to characterize PEC and describes the different properties of the various PEC preparations [27].
All the above literature indicates that the variations of the film physical properties are closely related to polyelectrolyte nature of the biopolymer component(s) and to their capacity to influence the microstructural network by their ionisable groups. The charge on the polymer chains is related to pH and ionic strength of the solution and could affect the polyelectrolyte aggregation with formation of nano-complexes. Therefore, a careful analysis of this relationships needs experimental details that may be provided by zeta potential measurements carried out on the film forming (FF) mixtures used to prepare the differently tailored biodegradable/edible films. Moreover, the analysis of the various factors able to influence the zeta potential value of each FF solution/suspension could be useful to render “stable” the latter. It is worthy to note that the mixing of the different components of a specific FF solution/suspension -as well as the pH, the ionic strength, the polyelectrolyte ratio, or even the method of adding of each component and the speed of mixing- may markedly influence the zeta potential and the size of the obtained micro- or nano-complexes.
To this aim, the zeta potential of FF solutions of pure polysaccharide (PEC) and protein (phaseolin), as well as of a protein mixture (BV seed protein concentrate, BVPC), was measured at different pH values by titration from pH 8.0 to pH 2.0 using the Zetasizer nano-ZSP (Malvern, Worcestershire, UK). The results reported in Table 1 showed that PEC negative zeta potential progressively decreased from -37.50 mV, recorded at pH 8.0, to -6.84 mV recorded at pH 2.0, indicating that PEC FF solution starts to move from a stable to a more unstable form at about pH 5.0. Nguyen et al. [28] found that the adsorption of PEC onto positive liposomes yielded a reproducible increase in particle size and a shift of the zeta potential from positive to negative side, whereas PEC adsorption onto negative liposomes did not render any significant changes probably due to electrostatic repulsion. Furthermore, zeta potential is commonly used also to investigate protein solution/ suspension stability [29]. Yin et al. [30] recently studied the surface charge and conformational properties of phaseolin, the major globulin occurring in red kidney beans (Phaseolus vulgaris L) [31], and reported that the zeta potential of phaseolin, measured in the absence of NaCl, increased from -44.0 mV at pH 9.4 to 27.9 mV at pH 3.0, the protein isoelectric point occurring at pH 4.2. In addition, zeta potential decrease was observed by increasing NaCl concentration from 0 to 200 mM at pH below the isoelectric point. Table 1 summarizes the results of our recent experiments concerning the changes in the zeta potential values of phaseolin when the protein was dissolved in 125 mM NaCl at 25°C as a function of pH. Also in this case the zeta potential was observed to increase (from -13.80 mV at pH 8.0 to 3.48 mV at pH 2.0). Finally, Table 1 reports also the changes in the zeta potential values measured with the mixture of proteins extracted from BV seeds. The detected zeta potential of BVPC was found to increase from -17.00 mV at pH 8.0 to 23.50 mV at pH 2.0, indicating that the electrostatic repulsion pattern may be gradually modified as a result of the gradual deprotonation of carboxyl groups and protonation of the amino groups of each BV protein composing the mixture. Further preliminary experiments (unpublished data) allowed to correlate film mechanical properties with the increase of the negative zeta potential of BV protein/PEC nano-complexes occurring in the FF solution. Our findings are in agreement with previous studies [32,33] explaining the observed increase of flexibility of both polysaccharide/essential oil and whey protein/gelatin composite films by the influence of the nanoemulsion/ solution electrical charge. In this respect, it was suggested that the repulsive forces among macromolecules of the same charge could increase the distance between the polymer chains and, consequently, determine a plasticizing effect in the case of charged polymeric film structures.
| Zeta potential (mV) | |||
|---|---|---|---|
| pH | PEC | Phaseolin | BVPC |
| 8 | -37.50± 1.06 | -13.80 ± 0.21 | -17.00 ± 0.01 |
| 7 | -38.50 ± 1.13 | -13.02 ± 0.70 | -15.80 ± 1.20 |
| 6 | -37.60 ± 1.13 | -11.20 ± 1.27 | -17.90 ± 0.56 |
| 5 | -30.90 ± 1.82 | -6.39 ± 0.64 | -9.30 ± 0.03 |
| 4 | -27.30 ± 1.48 | -6.13 ± 0.06 | 9.33 ± 0.07 |
| 3 | -10.55 ± 1.41 | 0.44 ± 0.13 | 13.50 ± 0.21 |
| 2 | -6.84 ± 1.48 | 3.48 ± 0.62 | 23.50 ± 0.21 |
Table 1: Zeta potential of PEC, phaseolin and BVPC measured at different pHs.
In conclusion, zeta potential measurement is proposed as an useful tool for assessing biopolymer interactions in each specific FF solution/ suspension, before casting, during the preparation of bio-based edible films. When zeta potential value results either less negative than -10mV or lower than 10mV, the particle solution/suspension is extremely unstable and, in turn, the physical properties of the derived edible film would be hard to tailor for specific applications.
References
- Anon (2004) Zetasizer Nano Series User Manual. Worcestershire, Malvern Instruments Ltd., United Kingdom.
- Sze A, Erickson D, Ren L, Li D (2003) Zeta-potential measurement using the Smoluchowski equation and the slope of the current-time relationship in electroosmotic flow. J Colloid Interface Sci 261: 402-410.
- Koch S, Woias P, Meixner LK, Drost S, Wolf H (1999) Protein detection with a novel ISFET-based zeta potential analyzer. BiosensBioelectron 14: 413-421.
- Kester JJ, Fennema OR (1986) Edible films and coatings: a review. Food Tech 40: 47-59.
- Stuchell YM, Krochta JM (1994) Enzymatic treatments and thermal effects on edible soy protein films. J Food Sci 59: 1332-1337.
- Gontard N, Ring S (1996) Edible wheat gluten film: influence of water content on glass transition temperature. J Agr Food Chem 44: 3474-3478.
- Krochta JM, DeMulder-Johnston C (1997) Edible and biodegradable polymer films: challenges and opportunities. Food Technol 51: 61-74.
- Anker M, Berntsen J, Hermansson AM, Stading M (2002) Improved water vapor barrier of whey protein films by addition of an acetylated monoglyceride. Innov Food SciEmergTechnol 3: 81-92.
- Diaz-Sobac A, Garcia H, Beristain CI (2002) Morphology and water vapour permeability of emulsion based on mesquite gum. J Food Process Preserv 26: 141-144.
- Morillon V, Debeaufort F, Blond G, Capelle M, Voilley A (2002) Factors affecting the moisture permeability of lipid-based edible films: a review. Crit Rev Food SciNutr 42: 67-89.
- Longares A, Monahan FJ, O’ Riordan ED, O’Sullivan M (2005) Physical properties of edible films made from mixtures of sodium caseinate and WPI. Int Dairy J 15: 1255-1260.
- Kim SJ, Ustunol Z (2001) Thermal properties, heat sealability and seal attributes of whey protein isolate/lipid emulsion edible films. J Food Sci 66: 985-990
- Wang LZ, Liu L, Holmes J, Huang J, Kerry JF, et al. (2008) Effect of pH and addition of corn oil on the properties of whey protein isolate-based films using response surface methodology. Int J Food SciTechnol 43: 787-796.
- Porta R, Di Pierro P, Sorrentino A, Mariniello L (2011) Promising perspectives for transglutaminase in “bioplastics” production. J Biotechnol Biomaterial 1: 102e.
- Porta R, Mariniello L, Di Pierro P, Sorrentino A, Giosafatto CV (2011) Transglutaminasecrosslinked pectin- and chitosan-based edible films: a review. Crit Rev Food SciNutr 51: 223-238.
- Giosafatto CV, Di Pierro P, Gunning P, Mackie A, Porta R, et al. (2014) Characterization of Citrus pectin edible films containing transglutaminase-modified phaseolin. CarbohydrPolym 106: 200-208.
- Porta R, Di Pierro P, Rossi Marquez G, Mariniello L, Khadivar M, et al. (2015) Microstructure and properties of bitter vetch (Viciaervilia) protein films reinforced by microbial transglutaminase. Food Hydrocoll 50: 102-107.
- Bellido LL (1994) Grain Legumes for Animal Feeds. Neglected Crops: 1492 from a Different Perspective, Plant Production and Protection Series No. 26. Hernando Bermejo JE, Leon J (Eds.). FAO, Rome.
- Sadeghi GH, Mohammadi L, Ibrahim SA, Gruber KJ (2009) Use of bitter vetch (Viciaervilia) as a feed ingredient for poultry. World PoultSci J 65: 51-64.
- Arabestani A, Kadivar M, Amoresano A, Illiano A, Di Pierro P, et al. (2016) Bitter vetch (Viciaervilia) seed protein concentrate as possible source for production of bilayered films and biodegradable containers. Food Hydrocoll 60: 232-242.
- Baldwin EA, Nisperos-Carriedo MO, Baker RA (1995) Use of edible coatings to preserve quality of lightly (and slightly) processed products. Crit Rev Food SciNutr 35: 509-524.
- Nisperos-Carriedo MO (1994) Edible coatings and films based on polysaccharides. In: Krochta JM, Baldwin EA, Nisperos-Carriedo MO (Eds.), Edible coating and film to improve food quality. Technomic Pub Co., Lancaster, PA.
- Liu L, Fishman ML, Hicks KB, Kende M (2005) Interaction of various pectin formulations with porcine colonic tissues. Biomaterials 26: 5907-5916.
- Liu LS, Fishman ML, Hicks KB (2007) Pectin in controlled drug delivery: a review. Cellulose 14: 15-24.
- Hagesaether E, Sande SA (2007) In vitro measurements of mucoadhesive properties of six types of pectin. Drug DevInd Pharm 33: 417-425.
- Thirawong N, Nunthanid J, Puttipipatkhachorn S, Sriamornsak P (2007) Mucoadhesive properties of various pectins on gastrointestinal mucosa: an in vitro evaluation using texture analyzer. Eur J Pharm Biopharm 67: 132-40.
- Rolin C (1993) Pectin. In: Whistler RL, Bemiller JN (Eds), Industrial Gums: Polysaccharides and Their Derivatives. Academic Press, New York.
- Nguyen S, Alund SJ, Hiorth M, Kjøniksen AL, Smistad G (2011) Studies on pectin coating of liposomes for drug delivery. Colloids Surf B Biointerfaces 88: 664-673.
- Jachimska B, Wasilewska M, Adamczyk Z (2008) Characterization of globular protein solutions by dynamic light scattering, electrophoretic mobility, and viscosity measurements. Langmuir 24: 6866-6872.
- Yin SW, Li huan K, Tang CH, Yang XQ, Wen QB, et al. (2011) Surface charge and conformational properties of phaseolin, the major globulin in red kidney bean (Phaseolus vulgaris L): effect of pH.Int J Food Sci Tech 46: 1628-1635.
- Giosafatto CV, Mariniello L, Ring S (2007) Extraction and characterization of Foeniculumvulgarepectins and their use for preparing biopolymer films in the presence of phaseolin protein. J Agric Food Chem 55: 1237-1240.
- Jiang Y, Li Y, Chai Z, Leng X (2010) Study of the physical properties of whey protein isolate and gelatin composite films. J Agric Food Chem 58: 5100-5108.
- Acevedo-Fani A, Salvia-Trujillo L, Rojas-Graü MA, Martín-Belloso O (2015) Edible films from essential-oil-loaded nanoemulsions: physicochemical characterization and antimicrobial properties. Food Hydrocoll 47: 168-177.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 13414
- [From(publication date):
June-2016 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 12349
- PDF downloads : 1065