Editorial Open Access
Inorganic Polyphosphates: Jack of All Trades
| Tatiana Kulakovskaya* | |
| Skryabin Institute of Biochemistry and Physiology of Microorganisms, Russian Academy of Sciences, Pushchino, 142290, Russia | |
| *Corresponding Author : | Tatiana V. Kulakovskaya Skryabin Institute of Biochemistry and Physiology of Microorganisms Russian Academy of Sciences Prospect Nauki, 5, Pushchino, 142290, Russia E-mail: alla@ibpm.pushchino.ru |
| Received July 02, 2012; Accepted July 02, 2012; Published July 05, 2012 | |
| Citation: Kulakovskaya T (2012) Inorganic Polyphosphates: Jack of All Trades. Biochem Physiol 1:e107. doi:10.4172/2168-9652.1000e107 | |
| Copyright: © 2012 Kulakovskaya T This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
Prostate-Specific Antigen (PSA), a serine protease, is believed to regulate the actions of extracellular matrix proteins such as fibronectin and Insulin-like Growth Factor-Binding Proteins (IGFBPs), possible factors involved in tumor progression and metastasis. Two phosphonate ester derivatives Cbz-(4-AmPhGly)p(OPh)2 (Inh I) and Cbz- (4-AmPhe)p(OPh)2 (Inh II), first mechanism-based inhibitors for PSA were synthesized using Oleksyszyn oxidation reaction, and their inhibitory activities on cleaving fibronectin (both human and bovine) and IGFBPs were investigated. To elucidate the role of PSA in the development and progression of prostate cancer, the regulation of the PSA activity on these two physiological substrates was clearly illustrated by modulating the concentrations of inhibitors, i.e. cleavages of both fibronectin and IGFBPs by PSA are inhibited by structure specific designed diphenyl phosphonate esters, consistent with the putative degradation. The inhibition data of two synthetic phosphonate ester derivatives, as lead compounds, will facilitate the development of more specific inhibitors of PSA activity, likely to modulate the physiological control of the IGF in human prostatic cell growth.
| Introduction |
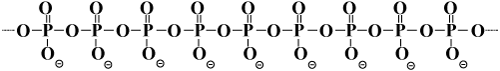
| Inorganic polyphosphates (PolyP) are linear polymers containing a few to several hundred orthophosphate residues linked by energyrich phosphoanhydride bonds (Figure 1). Until quite recently, they have been ignored in biochemistry textbooks. They were considered molecular fossils, ATP precursors in the evolution of living cells. The main function of polyphosphates was considered phosphorus storage in the cells of microorganisms. However, now it has been established that the role of polyP is not confined to phosphorus and energy storage. |
| PolyP generally have no their own rigid secondary structure in aqueous solutions and carry a negative charge. The complexing ability of PolyP is one of the major properties of this negatively charged biopolymer, determining to a considerable extent its regulatory function in living cells. In our opinion, such metabolically active molecules as PolyP do not exist in cells in the free form in large amounts. They are strongly compartmentated in cells and often form complexes with Ca2+, Mg2+, K+, polyhydroxybutyrate, nucleic acids, and proteins [1]. The interaction of PolyP with other anionic biopolymers, such as poly-beta hydroxybutyrate and probably RNA is mediated by divalent metal cations [1]. The ability of PolyP to form complexes with different components of living cells allows them to perform many specific functions in cells. |
| PolyPs are directly related to switching the genetic program characteristic of the logarithmic growth stage of bacteria over to the program of cell survival under stationary conditions. PolyP and polyphosphate kinase participate in many regulatory mechanisms in bacteria: |
| - Induction of the synthesis of RpoS, a RNA-polymerase subunit in bacteria, which is responsible for expression of the genes involved in the stationary phase and stress adaptation [2,3]; |
| - Involvement in bacterial cell motility, biofilm formation, and virulence [2,3]; |
| - Regulation of the level of the stringent response factor, guanosine 5΄-diphosphate 3΄-diphosphate (ppGpp), the second messenger in bacteria [2]; |
| - Formation of polyP/poly-β-hydroxybutyrate/Ca2+-channels involved in membrane transport [4]; |
| - Regulation of enzyme activities, especially Lon-protease [5]. |
| Quite a lot of data that have appeared in recent decades compel us to look afresh at the role of these polymers in animal and human cells. The cells of mammals possess PolyP but in lower amounts compared to the cells of microorganisms. These compounds have been found nearly in all tissues and organs and practically in all subcellular fractions: nuclei, mitochondria, plasma membranes, and microsomes [6]. However, the role of polyP in mammals is being revealed only now. These studies are a complicated task. One of the reasons is a very small amount of PolyP in animal cells. Another reason is substantial difference in metabolic enzymes in prokaryotes, where this metabolism has been studied rather thoroughly, and in eukaryotes, where no genes similar in sequence to the bacterial genes of polyP metabolism have been found. |
| PolyP Metabolizing Enzymes in Mammals |
| The BLAST search of proteins similar to the known PolyP-metabolizing enzymes of E. сoli and S. cerevisiae in the human genome yields the following results. As expected, no proteins similar in sequence to polyphosphate kinase have been found. This fact is well known from A. Kornberg’s works and gives grounds for application of polyphosphate kinase inhibitors as novel antibiotics [2,6]. The sequences similar to the gppA exopolyphosphatase of Е. colihave not been found either, and the data on 39% similarity between the unknown human protein FLJ38723, isoform CRA_c and the ppx of the bacterium are hard to interpret due to the lack of information about the activities of this protein. |
| The situation is quite different when the sequences similar to the sequences of yeast exopolyphosphatases PPN1 and PPХ1 are searched for. There is a 41% similarity between the PPN1 gene product and the acid sphingomyelinase-like phosphodiesterase (ЕC 3.1.4.12) performing the reaction: |
| Sphingomyelin + H(2)O <=> N-acylsphingosine + choline phosphate. |
| The protein is membrane bound and belongs to the nucleotide pyrophosphatase/ phosphodiesterase family. In the colon, the enzyme may play antiproliferative and antiinflammatory roles through generating ceramide, reducing the formation of lysophosphatidic acid, and inactivating the platelet-activating factor and mutations in its gene have been found in cancer cells of the intestines [7]. Hence, the study of potential exopolyphosphatase activity of this enzyme seems to be of interest. |
| The 91% similarity between the sequences of yeast exopolyphosphatase PPX1 and human protein h-prune proved to be most impressive. The human protein h-prune, a binding partner of the metastasis suppressor nm23-H1, is frequently overexpressed in metastatic cancers [8]. The protein h-prune efficiently hydrolyzes short-chain PolyP including tripoly- and tetrapolyphosphates and nucleoside 5΄-tetraphosphates [9]. Long-chain PolyP (> or 25 phosphate residues) are converted slower, whereas pyrophosphate and nucleoside triphosphates are not hydrolyzed. The reaction requires a divalent metal cofactor such as Mg2+, Co2+, or Mn2+, which activates both the enzyme and the substrate. Notably, the exopolyphosphatase activity of h-prune is suppressed by nm23-H1, long-chain polyphosphates and pyrophosphate, which may be potential physiological regulators [9]. |
| Alkaline phosphatase participates in PolyP hydrolysis in mammals [10], especially in the processes of formation of bone tissue apatite as will be noted below. |
| The PolyP synthetases in mammal cells are still unknown. |
| PolyP in Brain |
| The role of PolyP in separate organs and tissues draws special attention. The data on essential changes in PolyP content in rat brain during ontogenetic development and ageing [11] demonstrates that it is necessary to study in detail the role of these polymers in neurobiochemical processes. The PolyP level in rat brain increased 6-fold after birth. Mainly long-chain PolyP caused this increase. The maximum level of PolyP in brain was found in 12-month-old animals. In old rat brain, the total PolyP content decreased to about 50% [11]. It is not improbable that PolyP being an anionic polymer is involved in regulation of the activity of positively charged amine neurotransmitters. |
| PolyP and Cancer |
| PolyP was shown to display characteristic changes in its chain length during the apoptosis in human leukemic HL60 cells [12]. These cells contained a long chain PolyP of ~150 residues and a short chain PolyP of 25-45 residues, which could be well distinguished by electophoresis. In apoptotic cells, the long chain PolyP disappeared simultaneously with DNA fragmentation. This finding indicates that PolyP may be involved in the processes of apoptosis by affecting the stability of DNA-protein complexes or by regulation of nuclease activity [12]. PolyP enhanced the proliferation of human fibroblasts [13] and stimulated mammalian TOR, a kinase involved in the proliferation of mammary cancer cells [14]. The human protein h-prune, a binding partner of the metastasis suppressor nm23-H1, possesses an exopolyphosphatase activity and is supposed to be the missing exopolyphosphatase in animals, and the metastatic effects of h-prune are probably modulated by PolyP [9]. Unusually high accumulation of polyphosphates in the nucleoli of myeloma cells has been revealed [15]. The PNA polymerase I activity in the enriched nucleolar fraction responsible for the transcription of nucleolus is inhibited by 50% with 100 mkM of synthetic polyP of ~65 phosphate residues [15]. |
| PolyP, PolyP/Poly-β-hydroxybutyrate/Ca Complexes, and Mitochondria |
| The discovery by R. Reusch [4] of polyP/poly-β-hydroxybutyrate/ Ca2+ complexes, which proved the involvement of PolyP in the formation of channels across the cell membranes, extended our notion of the function of these compounds. The above channels formed by PolyP and poly-β-hydroxybutyrate with Ca2+ are involved in the transport processes in organisms from different evolution stages. There are ion-selective, voltage-activated channels that facilitate ion selection, solvation, and transport. |
| PolyP and PolyP/poly-β-hydroxybutyrate/Ca complexes in mitochondria participate in Сa2+ uptake [16]. PolyP level in mitochondria was dependent on the metabolic state of mitochondria: was increased in the presence of respiration substrates and reduced in the presence of the mitochondrial inhibitor (rotenone) or uncoupler (carbonyl cyanide p-trifluoromethoxyphenylhydrazone) [16]. Mitochondrial dysfunction caused by mitochondrial Permeability Transition Pore opening (mPTP) and excessive Сa2+ accumulation is the major contributor to cell and tissue damage during myocardial infarction and ischemia-reperfusion injury. The depletion of PolyP in the mitochondria of cardiac cells, although did not affect their ability to accumulate Сa2+, significantly inhibited mPTP opening. This fact suggests that PolyP is a previously unrecognized major activator of mPTP under the conditions of ischemiareperfusion injury [17]. |
| PolyP in Bone |
| The vertebrate skeleton is composed of a calcium phosphate mineral known as apatite. The skeleton is continually rebuilt, repaired and resorbed during the growth, normal remodeling, and rehabilitation after traumas and diseases. PolyP was found in bone tissue nearly ten years ago [18]. The cellular content of PolyP in osteoblast-like cells significantly decreased after the combined treatment of cells with the stimulators of osteoblast proliferation and differentiation [18]. The authors assumed that PolyP could be involved in the modulation of mineralization process in bone tissue. |
| Later, considerable advances were made in the study of PolyP contribution to bone tissue development [19-23]. |
| The modern concepts of the role of PolyP in bone tissue development come to the following [23]. The skeletal mineralization is associated with non-crystalline, calcium and phosphate containing electron-dense granules. These granules originate from mitochondria that have accumulated much calcium and lost their energy functions. The mitochondria take up Pi and condense them into PolyP and also sequester Ca2+. The formed PolyP- and Ca2+-containing granules may be transported out of the osteoclasts. They are probably transported into osteoblasts that build a new bone. The osteoblasts may embed the granules in the new unmineralized bone. Alkaline phosphatase releases Pi from PolyP, increasing local Pi and Ca2+ concentrations. As a result, apatite mineral is formed. This is the way how bone growth or posttraumatic repair and bone tissue renewal occur. |
| The enzyme responsible for PolyP synthesis in bone tissue cells has not been revealed and the signal molecules determining separate stages of this process are unknown. However, in spite of the above, the experiments with animals are already carried out and show that artificial introduction of PolyP into the bone growth or regeneration zone accelerates these processes [24-26]. |
| The finding that PolyP induces osteoblastic differentiation and bone mineralization and acts as a resource for mineralization is a basis for development of novel polyP-containing drugs for bone disease treatment and of the new PolyP-containing materials for bone substitution. |
| PolyP in Blood |
| PolyP was found in human blood more than ten years ago [12]. However, its role had remained unclear until it was shown that platelets contained PolyP-rich granules [27]. PolyP of approximately 70-75 phosphate residues was found by 31PNMR and by the ureaureapolyacrylamide gel electrophoresis of platelet extracts. Dense granules from platelets contained large amounts of calcium, potassium, and PolyP and possesses V-ATPase, a pyrophosphatase similar to the acidocalcisomes of unicellular eukaryotes [27]. The role of platelet PolyP in the blood coagulation system has been extensively studied in recent years; and it is supposed to be as follows [28-36]. Platelets release PolyP of 60-100 phosphate residues that directly bind to and activate factor XII. Platelet PolyP potently initiates fibrin formation via the factor XII-driven intrinsic pathway. PolyP is the endogenous factor XII activator in vivo linking platelet activation (primary hemostasis) and fibrin production (secondary hemostasis). PolyP enhances fibrin clot structure in a calcium-dependent manner. Fibrin clots formed in the presence of PolyP had up to 3-fold higher turbidity, had higher mass-length ratios, and exhibited thicker fibers in scanning electron micrographs. PolyP significantly shortened the clotting time in plasmas from patients with hemophilia A and B and that in the normal plasmas containing a variety of anticoagulant drugs, including unfractionated heparin, enoxaparin (a low molecular weight heparin), argatroban (a direct thrombin inhibitor) and rivaroxaban (a direct FXa inhibitor), and antagonized the anticoagulant effect of these drugs via accelerating FV activation. PolyP also shortened the clotting times of plasmas from warfarin patients. These results suggest that PolyP may have utility in reversing anticoagulation and in treating bleeding episodes in patients with hemophilia [31-32]. It was supposed that PolyP released from platelets initially promotes clot formation and stability; subsequent degradation of PolyP by blood phosphatases fosters inhibition of clotting and activation of fibrinolysis during wound healing [29]. |
| PolyP modulates the blood coagulation cascade in 3 stages: triggering the contact pathway; accelerating factor V activation; and enhancing fibrin polymerization. PolyP exerts differential effects on blood clotting, depending on polymer length [31]. Very long polymers (≥ 500 phosphate residues) are required for optimal activation of the contact pathway, while shorter polymers (100 residues) are sufficient to accelerate factor V activation and abrogate the anticoagulant function of the tissue factor pathway inhibitor. The optimal enhancement of fibrin clot turbidity by polyphosphate requires ≥ 250 residues. PolyP interacts with thrombin’s exosite II at a site that partially overlaps with, but is not identical to, the heparin-binding site. PolyP interactions with thrombin may be physiologically relevant. In addition to its role in coagulation, polyP-induced Factor XII activation mediates the release of the inflammatory mediator bradykinin by activating the kallikreinkinin system. It results in vascular leakage and edema formation in vivo. The polyP released from platelets plays a physiological role in the thrombus-forming process, as well as in inflammatory reactions [32]. The interactions of PolyP with fibrin (ogen) and its effect on fibrin structure and fibrinolysis are under study as well [31]. Electrophoretic mobility and binding assays indicate that PolyP interacts with fibrinogen and soluble fibrin. Clots formed in the presence of polyP exhibited the lower turbidity and permeability indicative of a tighter fibrin network, but these changes were not related to cross-linking or fibrinopeptide release. Microscopy showed a change in fibrin distribution in the clots formed with polyP, with formation of tight aggregates of fibrin fibers interspaced with large pores, in contrast to homogenous fiber distribution in the control clots. The lysis by tissue plasminogen activator (tPA) and plasminogen or plasmin was delayed in the clots formed with PolyP and depended on both the activator and PolyP concentration. PolyP directly influences fibrin architecture and attenuates fibrinolysis through reduced binding of fibrinolytic proteins [33]. PolyP may be useful in the therapy for some blood diseases. The addition of PolyP restored defective plasma clotting of Hermansky- Pudlak Syndrome patients, who lacked platelet PolyP [36]. |
| Conclusion |
| PolyPs are widely used as reagents in water treatment, fertilizers, flame retardants and food additives due to their unique properties, inexpensiveness, nontoxicity and biodegradability. |
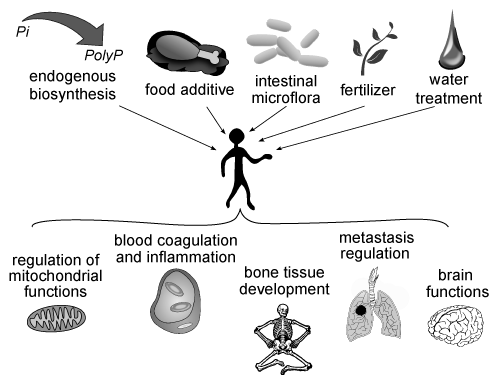
| The Figure 2 shows the main possible pathways of polyP penetration into human organism and the physiological processes they may be involved in. |
| The knowledge of PolyP metabolism is of great importance for medicine due to the following reasons: |
| - PolyPs are involved in various processes of human organism: regulation of mitochondrial functions; blood coagulation and inflammation; bone tissue development; metastatic regulation; brain function. |
| - PolyPs are widely used as food additives and, therefore, it is necessary to know how these components are utilized in the digestive system. |
| - In view of the great role of PolyP in cell metabolism, bone tissue development and blood coagulation, it is necessary to control its amounts in food. |
| - The importance of polyphosphate kinases ppk1 and ppk2 in bacterial metabolism and virulence of pathogens is of interest for the development of new inhibitors that may be prospective antibacterial compounds. |
| As a whole, polyP in human organism fairly deserves the definition of Jack of all trades specified in the title. Investigation of their numerous functions is of indisputable interest for human health protection. The new PolyP-metabolizing enzymes, especially those catalyzing PolyP biosynthesis, are still to be discovered in human organism. The modern extensive application of these compounds in industry, agriculture and food production is not neutral for human health and must be controlled and be a subject of attention from the part of biological sciences. |
References
|
Figures at a glance
 |
 |
| Figure 1 | Figure 2 |
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 6106
- [From(publication date):
October-2012 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 1671
- PDF downloads : 4435
