Innovative Approach to Diagnosis, Risk Factor, and Management of Gestational Diabetes Mellitus (Gdm)âMother and Offspring
Received: 03-Jan-2023 / Manuscript No. cpb-23-84633 / Editor assigned: 06-Jan-2023 / PreQC No. cpb-23-84633 / Reviewed: 20-Jan-2023 / QC No. cpb-23-84633 / Revised: 23-Jan-2023 / Manuscript No. cpb-23-84633 / Published Date: 30-Jan-2023 DOI: 10.4172/2167-065X.1000306
Abstract
Background: One of the main barriers for obtaining improved maternal and child health is Gestational Diabetes Mellitus. it affects around 5% of pregnancies that increases the risk of caesarean and surgical vaginal birth, macrosomia, shoulder dystocia, neonatal hypoglycaemia, and hyperbilirubinemia for both the mother and the unborn child. In this review article we focused on the various parameters that affects Gestational diabetes mellitus like, pathophysiology, epidemiology, risk factors and treatment.
Body: An extensive literature review was done from the standard databases such as Scopus, Elsevier, and PubMed using standard keywords “Gestational Diabetes”, “Diabetes”, “Pregnancy disorder”. Here, we explore the effects of Gestational Diabetes Mellitus on long- term maternal and newborn outcomes as well as health concerns that will probably last into the next generation. We discuss current clinical survey data and model of Gestational Diabetes Mellitus to better understand the underlying pathophysiology of the disease and the timely need to expand our scientific toolbox in order to identify strategies to prevent and treat Gestational Diabetes Mellitus.
Conclusion: While discussing about the gestational diabetes mellitus with the advance clinical care, in addition to the challenges currently faced in the Epidemiology, and techniques of diagnosis, Pathophysiology, risk factor, management of Gestational Diabetes Mellitus.
Keywords
Gestational diabetes; Pregnancy; Epidemiology; Pathophysiology; Diagnosis; Prevention; Management
Background
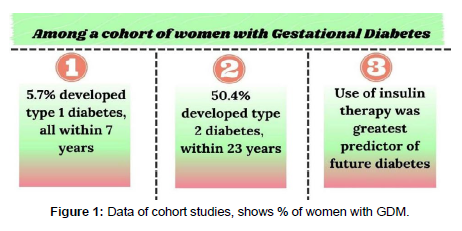
Approximately 5% of pregnancies result in gestational diabetes mellitus (GDM), though statistics can vary greatly depending on the criteria utilised and the demographics of the population. As long as the obesity pandemic persists, the prevalence is anticipated to rise. GDM-affected pregnancies increase the risk of caesarean and surgical vaginal birth, macrosomia, shoulder dystocia, neonatal hypoglycaemia, and hyperbilirubinemia for both the mother and the unborn child. Both obesity among women of childbearing age and hyperglycaemia in pregnancy (HIP) are rising to epidemic levels globally [1, 2]. We are adhering to the diagnostic guidelines for HIP provided by the International Federation of Gynaecology and Obstetrics (FIGO) [3] for our current study, which includes any level of glucose increase in pregnancy as a component of the general description of HIP. According to the Hyperglycaemia and Adverse Pregnancy Outcome (HAPO) study [4, 5], maternal BMI and hyperglycaemia had similar relationships with pregnancy problems. Both had a higher incidence of excessive foetal growth, primary caesarean delivery, clinical neonatal hypoglycaemia and foetal obesity, neonatal hyperinsulinemia, and hypertensive disorders of pregnancy. The relationship between high blood sugar and unfavourable outcomes is often linear; however the relationship between BMI and outcomes has a quadratic pattern with decreasing increments at the highest BMI categories [6]. Additionally, it may be possible to identify a distinct group of pregnant women with glucose levels that are within the normal range in the early stages of pregnancy but who have a high risk of developing "standard GDM," which is typically diagnosed at around 24 to 28 weeks' gestation, using clinical characteristics and biochemical tests. Practically speaking, it makes sense to focus early intervention efforts on women with prepregnancy hyperglycaemia, early-stage GDM, and high- risk GDM. ECVs contain a significant amount of micro RNAs, which are crucial for the metabolism of glucose. According to Yoffe et al exploratory case-control study, micro-RNA-223 and micro-RNA 23a in firsttrimester blood samples were highly predictive of later GDM (AUROC 0.91); [7]. This result for micro-RNA-233 has been corroborated by a recent cohort research [8]. The overall connections between noncoding RNAs and GDM have lately been studied in depth, and these results are encouraging [9]. These encouraging results from modest studies need to be verified in separate cohorts, as is the case with other biomarkers. In order for the essential assays to be used in normal diagnostic laboratories at low cost and high throughput, they will also need to be updated (Figure 1).
Epidemiology
Finding the true prevalence of GDM is difficult. Depending on how diverse and moral the population, the frequency varies around the globe and even within a nation's population. Therefore, compared to Caucasian women, the prevalence is higher among African (American), Hispanic (American), Pacific Islander, Native American, and Asian women (South or East) in the United States [10]. Additionally, different screening methods (universal or selective), diagnostic standards, and the incidence of T2DM in a given nation all affect GDM prevalence differently. While statistics from developed nations are rarely reported, those from western nations are frequently.
Jiwani et al. [11] and Macaulay et al. [12] recently attempted to ascertain the prevalence of GDM globally, including developing nations. The prevalence was discovered to vary between 5% in nations like Pakistan, Belgium, Denmark, Estonia, Ireland, South Korea, South Africa, and the United Kingdom, 10% in nations like Italy, Turkey, Brazil, United States, Morocco, and Australia, and 20% in nations like Bermuda and Nepal. According to a recent data According to the International Diabetes Federation, pregnancy-related hyperglycemia complicated 16% of live deliveries globally in 2013 [13], and it is most likely that the prevalence of GDM will rise as a result of the rise in risk factors like obesity and inactivity. The interaction of these environmental and genetic risk factors raises the possibility of intricate molecular mechanisms underlying GDM. According to 20 cohort studies from North America, Australia and Europe, were combined for a meta-analysis, women who are overweight, obese, or severely obese had a two to eight times higher risk of developing type 2 diabetes (GDM) than women with a normal body mass index (BMI) [14].
Gdm Diagnosis
Glycosylated haemoglobin (HbA1c): An obvious substitute for this test is glycosylated hemoglobin (HbA1c), which is frequently used to diagnose diabetes outside of pregnancy. It appears to be of minimal usefulness, with the exception of early pregnancy detection of undiagnosed hyperglycaemia, and performs poorly in both the prediction of OGTT diagnosed GDM and the prediction of pregnancy outcomes [15, 16].
Oral Glucose Tolerance Test (OGTT): The World Health Organization (WHO) [17, 18], the International Association of Diabetes in Pregnancy Study Groups (IADPSG) [19], and FIGO [20] all support "one step" OGTT testing, using thresholds of 5.1 mmol/L fasting; 10.0 mmol/L at 1 h; and 8.5 mmol/L at 2 h after a 75 gramme glucose load for diagnosis of GDM. Clearly, a cheaper, more accurate, non-fasting test would be preferred as the glucose tolerance test is cumbersome, resource-intensive and fairly poorly reproducible [21, 22]. Self-administered home OGTTs tend to function as well as laboratory testing and offer additional convenience.
Oral Glucose Challenge Test (OGCT): The diagnostic technique varies significantly between the USA [23] and Canada [24] which often choose two-step testing
Employing a non-fasting, one-hour "glucose challenge" test (GCT), followed by an OGTT (100 gram or 75 gram) if the GCT result exceeds specified thresholds. The need for early testing as well as testing during the conventional 24 to 28 week window has also been endorsed by the IADPSG, WHO, and FIGO.
Pathophysiology
Maternal tissues grow gradually less responsive to insulin during a typical pregnancy. This is thought to be brought on in part by hormones produced by the placenta and in part by other, as yet unidentified, mechanisms connected to pregnancy and obesity. The two primary sites for whole-body glucose disposal are skeletal muscle and adipose tissue. Normal pregnancy causes a 50% reduction in insulin-mediated whole- body glucose clearance, necessitating a 200%–250% increase in insulin secretion from the mother in order to maintain a euglycemic condition. [17] A progressive IR begins to form about the halfway point of a typical pregnancy and continues to worsen throughout the third trimester [25]. Possible causes of IR in pregnancy include hormones and adipokines released from the placenta, such as tumour necrosis factor (TNF)-, human placental lactogen, and human placental growth hormone. The glucose insulin balance is also upset during pregnancy due to higher levels of oestrogen, progesterone, and cortisol [26]. A woman's pancreas secretes more insulin during pregnancy to make up for the peripheral IR. When a woman's pancreas is unable to produce enough insulin to cope with the metabolic load of IR, GDM begins to develop. This condition of relative glucose intolerance is also accompanied by increased maternal adipose accumulation, decreased activity, and increased caloric consumption (Table 1).
When a pregnant woman is unable to produce enough insulin to counteract this natural insulin resistance, gestational diabetes mellitus (GDM) develops. Both lean and obese women experience GDM. However, it is thought that these populations have different pathophysiology’s for the condition. The pathogenesis of pregnancyinduced insulin resistance in obese women is essentially defined by the pre-existing elevated level of insulin resistance amplifying the pregnancy-induced insulin resistance. One known contributing cause to the metabolic syndrome is the elevated level of insulin resistance. The same components appear to be at play in slim women, although a failure in the first-phase insulin response is more significant [27]. These flaws combine to undermine insulin's ability to maintain glucose levels, which causes maternal hyperglycaemia. The placenta transmits glucose to the developing foetus. As a result, maternal hyperglycaemia prompts foetal hyperinsulinemia to balance out the excessive placental glucose transport. Foetal macrosomia (birth weight over 4000 g) is caused by the elevated insulin level in the foetus [28].
Risk Factors for Gdm
There are several risk factors connected to the emergence of GDM. Obesity, advanced maternal age, prior GDM, significant family history of diabetes, belonging to an ethnic group with a higher incidence of T2DM, polycystic ovarian syndrome, and chronic glucosuria are the most frequent risk factors. Other risk factors for GDM include a history of having large babies (birth weight >4000 g), recurrent abortion, unexplainable stillbirths, high blood pressure in the past, or pregnancyrelated high blood pressure [26].
Pregnancy-related hypertensive diseases like gestational hypertension, pre-eclampsia, and eclampsia are more common in women with GDM [29]. A higher risk of Polyhydramnios could result in a higher risk of preterm labour. In GDM, excessive foetal growth is still a significant perinatal issue. Birth trauma, maternal morbidity from caesarean births, shoulder dystocia, and neonatal hypoglycaemia are all effects of excessive foetal development [29]. Neonatal morbidities such as hyperbilirubinemia, hypocalcaemia, erythema, and respiratory distress syndrome may also be more common in new-borns of GDMaffected mothers [29]. Diabetes and cardiovascular disease in mothers are two long-term effects of GDM, while obesity and diabetes are long-term effects in children [30] Given that gestational diabetes typically develops in the latter half of the second trimester, when development is complete, congenital abnormalities do not progress more frequently in people with GDM.
Management of Gdm
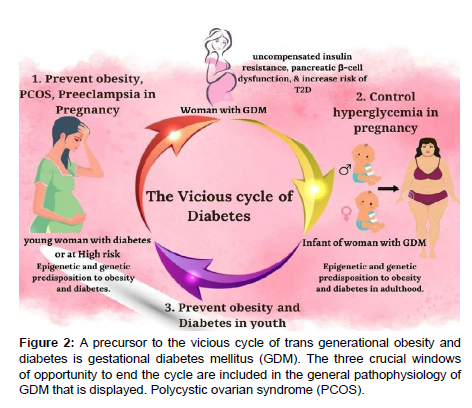
Glycaemic control is the core of GDM treatment. Lifestyle measures, such as regular exercise and medical nutrition therapy, are the first line of treatment for GDM. To ensure that the glycaemic goals are met, patients must periodically check their blood sugar levels at home. With these measures, medical treatment should be started if the glycaemic targets are not met. In comparison to standard care, the key composite result of child mortality, bone fracture, shoulder dystocia, and nerves palsy was linked with a 67% reduction with intervention that included food advising, blood glucose monitoring, and insulin administration as needed. Reduced rates of congenital malformations and average birthweight were also observed. The Maternal-Fetal Medicine Units Network randomised trial, which had 958 women with "mild" GDM, also showed similar advantages (i.e., normal fasting glucose levels on OGTT) (Figure 2). In comparison to conventional care, a similar intervention package was linked to lower clinical outcomes, such as macrosomia, Caesarean birth, shoulder dystocia, and preeclampsia [31, 32]. (Table 1) [32].
| Treatment group | Control group | Relative risk | P value | References | |
|---|---|---|---|---|---|
| Neonatal Outcomesa | n=485 | n=473 | ref. [32] | ||
| Composite perinatal end pointb | 149/460 (32.4) | 163/440 (37.0) | 0.87 | ||
| Birth Weight (g) | 3302 ± 502.4 | 3408 ± 589.4 | <0.001 | ||
| Birth Weight > 4000g | 28/477 (5.9) | 65/454 (14.3) | 0.41 (0.26-0.66) | <0.001 | |
| Large for gestational age | 34/477 (7.1) | 66/454 (14.5) | 0.49 (0.32-0.76) | <0.001 | |
| Fat mass (g) | 427.0 ± 197.9 | 464.3 ± 222.3 | 0.003 | ||
| Maternal outcomes | n=476 | n=455 | |||
| Caesarean delivery | 128 (26.9) | 154 (33.8) | 0.79 (0.64-0.99 | 0.02 | |
| Shoulder dystocia | 7 (1.5) | 18 (4.0) | 0.37 (0.14-0.97 | 0.02 | |
| Preeclampsia | 12 (2.5) | 25 (5.5) | 0.46 (0.22-0.97) | 0.02 | |
| Preeclampsia or gestational hypertension | 41 (8.6) | 62 (13.6) | 0.63 (0.42-0.96) | 0.01 | |
| Weight gain (Kg) | 2.8 ± 4.5 | 5.0 ± 3.3 | <0.001 |
Table 1: Selected Neonatal and Maternal Results from the Unite Network for Maternal-Fetal Medicine Trailc
aAt least partial delivery information was missing for 10 women in the treatment group and 19 women in the control group.
bStill birth, infant death, hypoglycaemia, hyperbilirubinemia, an elevated cord c-peptide level, and birth trauma were all part of the composite perinatal outcome.
cData are displayed as n/N (%), or n (%) or mean ± SD.
Exercise: Glycaemic control in GDM has been demonstrated to improve with exercise. If there are no medical or obstetrical contraindications, a woman with GDM is advised to engage in daily workout for at least 30 minutes. It is beneficial to GDM patients' achievement of their glycaemic goals to advise them to walk briskly or perform arm exercises while sitting in a chair for at least ten minutes following each meal [33,34].
Dietary Intervention: An crucial part of managing GDM is lifestyle intervention, which includes dietary change, physical exercise, and weight management. According to estimates, this approach may be sufficient to help 70 to 85% of women who were diagnosed based on ADA criteria reach their blood glucose goals [35]. To improve mother and foetal outcomes, gestational weight gain goals should be established on an individual basis. Although it does not specifically address GDM, an Institute of Medicine recommendation suggests weight increase targets based on pre-pregnancy BMI [35].
A recent meta-analysis of randomised controlled trials found that dietary intervention was associated with better mean maternal fasting blood glucose (13 studies; 4.07 mg/dl, 95% CI7.58 to 0.57, P = 0.02) and post - prandial glucose (9 studies; 7.78 mg/dl, 95% CI12.27 to 3.29, P = 0.0007) compared to control group as well as a lesser need for therapeutic intervention (RR 0.49, 95% CI 0. Additionally, there were decreased rates of macrosomia (RR 0.49, 95% CI 0.27-0.88, P = 0.02) and mean birthweight (170.62 g, 95% CI 333.64 to 7.60, P = 0.04) [36].
Metformin: Early in pregnancy, patients with polycystic ovarian syndrome frequently take metformin. If lifestyle changes fail to reach desired glycaemic results, it is also utilised as a medication treatment for GDM in the second and third trimesters. Numerous elements of metformin use during pregnancy have been compiled in a recent review [37].
The only oral medications used to treat GDM are metformin and glibenclamide (marketed as glyburide in the US and Canada). The rate of perinatal problems (32.0% versus 32.2%) and adverse events in the major randomised Metformin in Gestational Diabetes (MiG) trial did not differ between the metformin and insulin therapy groups [38].
However, the available data do not support the use of metformin as a GDM prevention strategyInthe (EMPOWaR) research, 449 obese women with normal baseline glucose tolerance were randomised to receive up to 2,500 g of metformin per day vs. a placebo between 12 and 16 weeks of pregnancy, and the study lasted until the baby was delivered [39].
From 12 to 18 weeks of pregnancy until delivery, Syngelaki et al. randomly assigned women with a BMI greater than 35 kg/m2 to receive 3 g of metformin or a placebo [40]. The trial was completed by 202 metformin-treated women and 198 placebo- treated women. Fetal growth did not alter in any way. Metformin caused a 1.7 kg (P 0.001) decrease in maternal GWG. GDM rates and other pregnancy outcomes were comparable between groups.
Fish oil: Additionally, it has been claimed that dietary fatty acids could be used as a treatment to lower GDM and increase the likelihood of premature birth. Before 21 weeks of pregnancy, 2399 women were randomly assigned to receive either [1] DHA- enriched fish oil 800 mg daily or [41] vegetable oil capsules without DHA until delivery for the DHA to optimise mother-infant outcome (DOMInO) RCT [42]. There was no reduction in GDM or preeclampsia, and there were no variations in the size or adiposity of the neonates. A later study of the kids at age 7 revealed no anthropometric differences [43].
Vitamin D: Low serum levels of 25-hydroxyvitamin D are undoubtedly a risk factor for the onset of GDM [44], but treatment trial outcomes have been inconsistent. According to the most recent Cochrane review [45] which mostly included studies from the Middle East, vitamin D supplementation alone "probably" lowers the population frequency of gestational diabetes mellitus (GDM) and preeclampsia (RR 0.48 (95% CI 0.30-0.79) cases. However, neither vitamin D + calcium nor vitamin d + calcium + other minerals were found to be beneficial. Therefore, supplementing with vitamin D seems like a sensible choice among groups with low baseline levels. Future research should help to clarify its actual therapeutic function (Figure 3).
Summary
In conclusion, GDM poses a significant short and long-term challenge. The early detection and treatment of GDM are unquestionably beneficial for enhancing outcomes. Mothers and newborns long-term health is also clearly correlated with these factors, although the best course of therapy has yet to be proven. This is a worldwide issue! The NCD burden of GDM as manifested in affected women and their progeny must quickly be reduced through prevention and intervention, both during and after pregnancy. Despite growing understanding in this domain, there are still few practical applications for tried-andtrue methods. Widespread adoption of very simple treatments has the potential to significantly reduce the burden of NCDs and stop the "slow motion calamity" of obesity and diabetes, as described by Dr. Margaret Chan, Director of the World Health Organization, in 2017 [46].
References
- Cho NH, Shaw JE, Karuranga S, Huang Y, Fernandes RD, et al. (2018) IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 138: 271-281.
- Vickers NJ (2017) Animal communication: when i’m calling you, will you answer too?. Current biology.
- Hod M, Kapur A, Sacks DA, Hadar E, Agarwal M, et al. (2015) The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: A pragmatic guide for diagnosis, management, and care. Int J Gynaecol Obstet
- Catalano PM, McIntyre HD, Cruickshank JK, McCance DR, Dyer AR, et al. (2012) The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes care 35: 780-786.
- Frcog AE (2011) Unravelling the Connection Between Gestational Diabetes Mellitus and Butyrylcholinesterase.
- Group HS (2010) Hyperglycaemia and Adverse Pregnancy Outcome (HAPO) Study: associations with maternal body mass index. BJOG 117: 575-584.
- Yoffe L, Polsky A, Gilam A, Raff C, Mecacci F, et al. (2019) Early diagnosis of gestational diabetes mellitus using circulating microRNAs. European journal of endocrinology. Eur J Endocrinol 181: 565-577.
- Abdeltawab A, Zaki ME, Abdeldayem Y, Mohamed AA, Zaied SM (2021) Circulating micro RNA-223 and angiopoietin-like protein 8 as biomarkers of gestational diabetes mellitus. Br J Biomed Sci 78: 12-17.
- Filardi T, Catanzaro G, Mardente S, Zicari A, Santangelo C, et al. (2020) Non-coding RNA: role in gestational diabetes pathophysiology and complications. Int J Mol Sci
- Ferrara A. (2007) Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes care.
- Jiwani A, Marseille E, Lohse N, Damm P, Hod M, et al. (2012) Gestational diabetes mellitus: results from a survey of country prevalence and practices. J Matern Fetal Neonatal Med 25: 600-610.
- Macaulay S, Dunger DB, Norris SA (2014) Gestational diabetes mellitus in Africa: a systematic review. PloS one.
- Sicree R, Shaw J, Zimmet P. (2006) Diabetes atlas international diabetes federation. Belgium: International Diabetes Federation.
- Chu SY, Callaghan WM, Kim SY, Schmid CH, Lau J, et al. (2007) Maternal obesity and risk of gestational diabetes mellitus. Diabetes care 30: 2070-2076.
- Lowe WL, Lowe LP, Kuang A, Catalano PM, Nodzenski M, et al. (2019) Maternal glucose levels during pregnancy and childhood adiposity in the Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study. Diabetologia. Apr 62: 598-610.
- Hughes RC, Williman J, Gullam JE (2016) Universal HbA1c measurement in early pregnancy to detect type 2 diabetes reduces ethnic disparities in antenatal diabetes screening: a population-based observational study. PloS one.
- Vickers NJ (2017) Animal communication: when i’m calling you, will you answer too? Current biology.
- WHO (2013) Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy. World Health Organization.
- Weinert LS (2010) International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy: comment to the International Association of Diabetes and Pregnancy Study Groups Consensus Panel. Diabetes care.
- Hod M, Kapur A, Sacks DA, Hadar E, Agarwal M, et al. (2015) The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: A pragmatic guide for diagnosis, management, and care. Int J Gynaecol Obstet.
- Bonongwe P, Lindow SW, Coetzee EJ (2015) Reproducibility of a 75G oral glucose tolerance test in pregnant women. J Perinat Med 43: 333-338.
- Persson M, Winkvist A, Mogren I (2009) Surprisingly low compliance to local guidelines for risk factor based screening for gestational diabetes mellitus-A population-based study. BMC pregnancy and childbirth 9: 1-10.
- Whiteside JL (2008) Robotic gynecologic surgery: a brave new world?. Obstet Gynecol 112: 1198-1200.
- Canadian Diabetes A (2008) clinical practice guidelines for the prevention and management of diabetes in Canada.
- Catalano PM, Tyzbir ED, Roman NM, Amini SB, Sims EA (1991) Longitudinal changes in insulin release and insulin resistance in nonobese pregnant women. Am J Obstet Gynecol 165: 1667-1672.
- Barbour LA, McCurdy CE, Hernandez TL, Kirwan JP, Catalano PM, et al. (2007) Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes care.
- Buchanan TA, Xiang AH (2005) Gestational diabetes mellitus. J Clin Invest 115: 485-491.
- Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, et al. (2005) Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. New England journal of medicine 352: 2477-352486.
- Catalano PM, McIntyre HD, Cruickshank JK, McCance DR, Dyer AR, et al. (2012) The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes care 35: 780-6.
- Dabelea D, Pettitt DJ (2001) Intrauterine diabetic environment confers risks for type 2 diabetes mellitus and obesity in the offspring, in addition to genetic susceptibility. J Pediatr Endocrinol Metab14: 1085-1092.
- Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, et al. (2005) Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. New England journal of medicine. Jun 352: 2477-2486.
- Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, et al. (2009) A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med 361: 1339-1348.
- Blumer I, Hadar E, Hadden D R, Jovanovič L, Mestman J H et al. (2013) Diabetes and pregnancy: an endocrine society clinical practice guideline. The journal of clinical endocrinology & Metabolism. 98: 4227-4249.
- Metzger BE, Buchanan TA, Coustan DR, De Leiva A, Dunger DB, et al. (2007) Summary and recommendations of the fifth international workshop-conference on gestational diabetes mellitus. Diabetes care.
- During Pregnancy WG (2009) Re-examining the guidelines. Institute of Medicine.
- Brown J, Alwan NA, West J, Brown S, McKinlay CJ (2017) Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database Syst Rev
- Lowe WL, Lowe LP, Kuang A, Catalano PM, Nodzenski M, et al. (2019) Maternal glucose levels during pregnancy and childhood adiposity in the Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study. Diabetologia 62: 598-610.
- Catalano HM, Zhang C, Desoye G, Mathiesen ER, Damm P (2019) Gestational diabetes mellitus. Nat Rev Dis Primers.
- Chiswick C, Reynolds RM, Denison F, Drake AJ, Forbes S, et al. (2015) Effect of metformin on maternal and fetal outcomes in obese pregnant women (EMPOWaR): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 3: 778-786.
- Syngelaki A, Nicolaides KH, Balani J, Hyer S, Akolekar R, et al. (2016) Metformin versus placebo in obese pregnant women without diabetes mellitus. N Engl J Med Feb 374: 434-443.
- Vickers NJ (2017) Animal communication: when i’m calling you, will you answer too?. Current biology.
- Luo X, Ma X, Hu H, Li C, Cao S, et al. (2013) Kinetic study of pentosan solubility during heating and reacting processes of steam treatment of green bamboo. Bioresource technology 130: 769-776.
- Wood K, Mantzioris E, Lingwood B, Couper J, Makrides M, et al. (2018) The effect of maternal DHA supplementation on body fat mass in children at 7 years: follow-up of the DOMInO randomized controlled trial. Prostaglandins, Leukotrienes and Essential Fatty Acids 139:49-54.
- Sadeghian M, Asadi M, Rahmani S, Akhavan Zanjani M, Sadeghi O, et al. (2020) Circulating vitamin D and the risk of gestational diabetes: a systematic review and dose-response meta-analysis. Endocrine 70: 36-47.
- Palacios C, Kostiuk LK, Peña‐Rosas JP (2019) Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev
- Chan M (2017) Obesity and diabetes: The slow‐motion disaster. Milbank Q
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Ojha KB, Anurag, Mukherjee S, Rajput R, Vinod YR (2023) Innovative Approach to Diagnosis, Risk Factor, and Management of Gestational Diabetes Mellitus (Gdm)—Mother and Offspring. Clin Pharmacol Biopharm, 12: 306. DOI: 10.4172/2167-065X.1000306
Copyright: © 2023 Ojha KB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3553
- [From(publication date): 0-2023 - Sep 23, 2025]
- Breakdown by view type
- HTML page views: 3068
- PDF downloads: 485