Case Report Open Access
Injury of the Arcuate Fasciculus in a Patient with Progressive Bulbar Palsy: A Diffusion Tensor Tractography Study
| Sung Ho Jang and Min Cheol Chang* | |
| Department of Physical Medicine and Rehabilitation, College of Medicine, Yeungnam University, Republic of Korea | |
| Corresponding Author : | Min Cheol Chang Department of Physical Medicine and Rehabilitation College of Medicine, Yeungnam University 317-1 Daemyungdong, Namku, Taegu, 705-717, Republic of Korea Tel: +82-54-420-7216 Fax: +82-54-437-6005 E-mail: wheel633@hanmail.net |
| Received: June 12, 2015 Accepted: July 21, 2015 Published: July 24, 2015 | |
| Citation: Jang SH, Chang MC (2015) Injury of the Arcuate Fasciculus in a Patient with Progressive Bulbar Palsy: A Diffusion Tensor Tractography Study. OMICS J Radiol 4:197. doi:10.4172/2167-7964.1000197 | |
| Copyright: © 2015 Jang SH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Radiology
Abstract
The researchersWe report on a patient with progressive bulbar palsy (PBP) who presented with Broca’s aphasia due to injury of the left arcuate fasciculus (AF), as demonstrated on diffusion tensor tractography (DTT). A 74-yearold right handed patient with PBP and six age-matched normal subjects were recruited for this study. The patient presented with Broca’s aphasia along with typical symptoms of PBP, including dysarthria, dysphasia, and mild quadriparesis. DTT was performed at five years after symptom onset. After originating from Wernicke’s area, the left AF of the patient was discontinued at the junction between the ascending and horizontal portions. The fractional anisotropy value and tract number of the left AF were more than two standard deviations lower than those of normal subjects. In contrast, the apparent diffusion coefficient value was more than two standard deviations higher than those of normal subjects. Using the configuration and parameters of DTT, the researcherswe demonstrated injury of the left AF in a patient with PBP. The injury of the left AF in this patient appeared to coincide with Broca’s aphasia. The researchersWe recommend evaluation of the AF using DTT in patients with motor neuron disease who present with aphasia.
|
Abstract
The researchersWe report on a patient with progressive bulbar palsy (PBP) who presented with Broca’s aphasia due to injury of the left arcuate fasciculus (AF), as demonstrated on diffusion tensor tractography (DTT). A 74-year-old right handed patient with PBP and six age-matched normal subjects were recruited for this study. The patient presented with Broca’s aphasia along with typical symptoms of PBP, including dysarthria, dysphasia, and mild quadriparesis. DTT was performed at five years after symptom onset. After originating from Wernicke’s area, the left AF of the patient was discontinued at the junction between the ascending and horizontal portions. The fractional anisotropy value and tract number of the left AF were more than two standard deviations lower than those of normal subjects. In contrast, the apparent diffusion coefficient value was more than two standard deviations higher than those of normal subjects. Using the configuration and parameters of DTT, the researcherswe demonstrated injury of the left AF in a patient with PBP. The injury of the left AF in this patient appeared to coincide with Broca’s aphasia. The researchersWe recommend evaluation of the AF using DTT in patients with motor neuron disease who present with aphasia.
Keywords
Progressive bulbar palsy; Motor neuron disease; Broca’s aphasia; Diffusion tensor tractography; Arcuate fasciculus
Introduction
Motor neuron diseases (MNDs) are a group of neurological disorders characterized by progressive degeneration of upper motor neurons ([UMN], originating in the motor and premotor cortex) and lower motor neurons ([LMN], originating in the spinal cord and brain stem) [1]. MND includes primary lateral sclerosis, which selectively affects UMN, progressive muscular atrophy, which exclusively causes damage to LMN, amyotrophic lateral sclerosis (ALS), which involves both UMN and LMN, and progressive bulbar palsy (PBP), which primarily involves motor neurons in the brain stem [1]. As a result of the damage to motor neurons, Because of damage to motor neurons, patients with MND present with muscle weakness, atrophy, and fasciculation throughout the body [1].
MND is known to predominantly affect motor function. However, recent studies have reported accompanying extra-motor manifestations, including aphasia, in patients with MND [2-5], although the prevalence of the co-occurrence of motor dysfunction and extra-motor symptoms has not been clarified. The pathogenic mechanisms of aphasia in patients with MND have been reported to be the result of atrophy or hypo-perfusion in the fronto-temporal lobe, which has been demonstrated using conventional brain CT/MRI, radionuclide imaging, and post-mortem brain autopsy [2,3-5]. However, these methods are limited in that they cannot detect lesions of neural tracts. By contrast, the recently developed diffusion tensor tractography (DTT), which is derived from diffusion tensor imaging (DTI), has enabled three-dimensional visualization and detailed estimation of neural tracts [6,7]. Several studies have demonstrated the degeneration of neural tracts, including the corticospinal tract (CST), corpus callosal fibers, and uncinated fasciculus in patients with MND [8-16]. However, little is known about injury of the arcuate fasciculus (AF). In this study, the researcherswe report on a patient with PBP who presented with Broca’s aphasia due to injury of the AF in the dominant hemisphere, as demonstrated on DTT. Case Report
One patient and six right-handed age-matched normal subjects (three men; mean age, 72.6 years; range, 70-76 years) with no previous history of neurologic, psychiatric, or language problems were recruited for this study. All subjects provided informed consent for participation in the study. The study was approved by the Institutional Review Board of a university hospital (YUH-12-0421-O60).
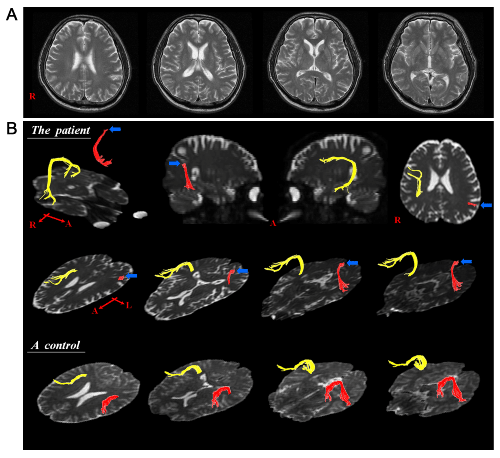
A 74-year-old right-handed man visited the neurology department of a university hospital for evaluation of language disturbance and dysphasia. He had no family history of neurological disease. Five years ago, the patient had begun to notice a slurring in his speech, which deteriorated slowly with the passage of time. One year ago, he had begun to show dysphagia (coughing during eating or drinking, difficulty in chewing and controlling food in the mouth, and drooling of saliva). On neurological examination, he was alert and oriented. He exhibited a decreased gag reflex and soft palatal movements, and the jaw jerk reflex and tongue fasciculation were observed. In addition, he had mild quadriparesis (4/5 in the right upper and lower extremities and 4+/5 in the left upper and lower extremities on the Medical Research Council Scale [17]). The deep tendon reflex was significantly increased, particularly in the left upper and lower extremities. He showed a full score of 30 points on the Mini-Mental Status Examination for evaluation of cognitive function [18]. Conventional brain MRI and electromyography/nerve conduction studies revealed no abnormal findings (Figure 1A). The patient showed symptoms of dysarthria, such as speaking in short phrases, emitting air through the nose during phonation, and an inability to sustain a normal speech volume. During the test for the assessment of dysarthria, he presented with a short maximal phonation time for the sustained vowel /a/ (2.3 seconds) and articulation errors at the sentence level (30.1%) due to an imprecise production of consonants. In addition, he had difficulty in naming and generating syntactic structures correctly and rapidly, consistent with symptoms of aphasia. On the Korean-Western Aphasia Battery (K-WAB) [19], he showed Broca’s aphasia (aphasia quotient score: 55 [56.6%ile], spontaneous speech score: 10 [30.5%ile], comprehension score: 168 [76.9%ile], repetition score: 76 [55.6%ile], naming score: 75 [75%ile], reading score: 82 [89.5%ile], writing score: 60 [67.6%ile]). As a result, based on the patient’s history and neurological examination, he was diagnosed as PBP. Diffusion tensor tractography
DTI data were acquired at five years after symptom onset using a 6-channel head coil on a 1.5 T Philips Gyroscan Intera unit (Philips, Ltd, Best, The Netherlands) by single-shot echo-planar imaging with a navigator echo. Sixty contiguous slices (acquisition matrix = 96 × 96; reconstruction matrix = 192 × 192; field of view = 240 × 240 mm2; TR = 10,726 ms; TE = 76 ms, b = 1,000 mm2s-1, NEX=1, thickness=2.5 mm) were acquired for each of the 32 noncollinear diffusion-sensitizing gradients. Fiber tracking was performed using the fiber assignment continuous tracking (FACT) algorithm implemented within the DTI task card software (Philips Extended MR WorkSpace 2.6.3). Removal of head motion effects and eddy current-induced image distortions using affine multi-scale two-dimensional registration was performed at the Oxford Centre for Functional Magnetic Resonance Imaging of Brain (FMRIB) Software Library (FSL; www.fmrib.ox.ac.uk/fsl). DTI-Studio software (CMRM, Johns Hopkins Medical Institute, Baltimore, MD, USA) was used for evaluation of the AF. Based on the method of Nucifora et al. [20], the researcherswe placed the region of interest (ROI) in both the dominant and non-dominant cerebral hemispheres for tracking of bilateral AFs (the seed ROI in the posterior parietal area of the superior longitudinal fascicle and the target ROI in the posterior temporal lobe). The seed ROI was shown in green and the target ROI was shown in blue on DTI-based color-coded maps. Termination criteria used for fiber tracking were fractional anisotropy (FA) < 0.2 and angle < 60°. The AFs were constructed with deterministic tractography. The researchersWe also measured the FA, apparent diffusion coefficient (ADC), and the tract number of the AF.
Results
On DTTs, the AFs in the right hemisphere of the patient and normal control subjects originated from Wernicke’s area, passed through the known AF pathway, and then entered into Broca’s area (Figure 1B). By contrast, in the patient, after originating from Wernike’s area, the left AF showed discontinuation at the junction between the ascending and horizontal portions (Figure 1B). The FA value (0.375) and tract number (196) of the left AF in the patient were more than two standard deviations lower than those of normal subjects (FA: 0.434 ± 0.022 [mean ± standard deviation], tract number: 432 ± 56). In contrast, the ADC value (0.891) was more than two standard deviations higher than that of normal subjects (ADC: 0.785 ± 0.041).
Discussion
In the current study, using the configuration and parameters of DTT, the researcherswe attempted to demonstrate damage of the AF in a patient with PBP who presented with Broca’s aphasia. The results of DTT showed discontinuation of the left AF (in the dominant hemisphere) of our patient between Wernicke’s and Broca’s areas, although no remarkable lesions were observed on conventional MRI. In addition, the FA value and tract number for the left AF were significantly decreased, whereas the ADC value was significantly increased in this patient, compared to those of the normal subjects. The FA value represents the white matter organization: in detail, the degree of directionality and integrity of white matter microstructures, such as axon, myelin, and microtubule, and ADC value indicates the magnitude of water diffusion [21,22]. The tract number reflects the total number of fibers in a neural tract [23]. Therefore, the decrements of FA value and fiber number, and increment of ADC value of the left AF appear to indicate injury of the left AF.
The AF is the neural tract connecting the two major speech centers (Broca’s and Wernicke’s areas), and plays a critical role in language function [24]. Injury of this tract can lead to several types of language impairment, including conduction aphasia, Broca’s aphasia, anomic aphasia, and apraxia of speech [25-28]. On DTT finding of this patient, Broca’s portion of the left AF had disappeared, whereas Wernicke’s portion was spared. The researchersWe believe that this DTT finding is compatible with Broca’s aphasia, which this patient presented with. On the other hand, dysarthria, which this patient also presented with, is thought to be caused by injury of the brain stem nuclei innervating the oropharyngeal muscles. The classic MND is characterized by progressive loss of limb, bulbar, and respiratory muscle function caused by selective degeneration of UMN and LMN, sparing the rest of the nervous system [29,30]. Accordingly, most previous DTI studies on patients with MND have focused on the CST, demonstrating the correlation of injury of the CST and deterioration of motor function. [9,10,13-16]. Regarding aphasia in patients with MND, since the study reported by Caselli in 1993, several studies have reported non-fluent aphasia in patients with MND [2-5]. In those previous studies, atrophy and hypo-perfusion in the left fronto-temporal lobe were consistently observed on conventional CT/MRI and radionuclide imaging, respectively [2,3-5]. In addition, the most common findings obtained from postmortem examinations were atrophy and neuronal loss in the left fronto-temporal lobe [2,3-5]. However, so far, no study evaluating the AF in MND patients with aphasia has been reported. Therefore, this is the first study using DTT to demonstrate injury of the left AF in a patient with MND. However, this study is limited in that it was based on a single case. Therefore, further studies involving large numbers of patients with PBP should be conducted. In addition, studies of patients with different MND subtypes, such as ALS, primary lateral sclerosis, and progressive muscular atrophy would also provide valuable insight. In conclusion, the researcherswe demonstrated injury of the left AF in a patient with PBP, using the configuration and parameters of DTT. The injury of the left AF in this patient appeared to coincide with Broca’s aphasia. The researchersWe recommend evaluation of the AF using DTT in patients with motor neuron disease who present with aphasia. Acknowledgement
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A1A4A01001873).
References
|
Figures at a glance
 |
| Figure 1 |
Relevant Topics
- Abdominal Radiology
- AI in Radiology
- Breast Imaging
- Cardiovascular Radiology
- Chest Radiology
- Clinical Radiology
- CT Imaging
- Diagnostic Radiology
- Emergency Radiology
- Fluoroscopy Radiology
- General Radiology
- Genitourinary Radiology
- Interventional Radiology Techniques
- Mammography
- Minimal Invasive surgery
- Musculoskeletal Radiology
- Neuroradiology
- Neuroradiology Advances
- Oral and Maxillofacial Radiology
- Radiography
- Radiology Imaging
- Surgical Radiology
- Tele Radiology
- Therapeutic Radiology
Recommended Journals
Article Tools
Article Usage
- Total views: 14029
- [From(publication date):
August-2015 - Jul 12, 2025] - Breakdown by view type
- HTML page views : 9470
- PDF downloads : 4559
