Research Article Open Access
Inhibitory Potential of Yucca Gloriosa L. Extract and Isolated Gloriosaol Isomeric Mixture on Ovalbumin Induced Airway Hyperresponsiveness in Balb/C Mice
| Saurabh Gupta1*, Basavan Duraiswamy2, Satish Kumar Muthureddy Nataraj2, Rama Satyanarayana Raju K2, Babu UV3, Sharath Kumar LM3, Omji Porwal4, and Renu Gupta5 | |
| 1 Department of Pharmacology, Indore institute of Pharmacy, M.P, India | |
| 2 Department of Pharmacology and Pharmacognosy, J.S.S. College of Pharmacy, TN, India | |
| 3 The Himalaya Drug Company, Makali, Bangalore, India | |
| 4 National Institute of Pharmaceutical Education and Research (NIPER-A), Ahmedabad, India | |
| 5 Dr. Batra’s, Nirala Bazaar, Aurangabad, India | |
| Corresponding Author : | Saurabh Gupta Associate Professor Department of Pharmacology Indore institute of Pharmacy Pithampur road Opp. IIM, Rau, Indore (M.P.) 453331, India Tel: +919407178028 E-mail: saurabhgupta80@gmail.com |
| Received January 20, 2014; Accepted February 21, 2014; Published February 24, 2014 | |
| Citation: Gupta S, Duraiswamy B, Muthureddy Nataraj SK, Rama Satyanarayana Raju K, Babu UV, et al. (2014) Inhibitory Potential of Yucca Gloriosa L. Extract and Isolated Gloriosaol Isomeric Mixture on Ovalbumin Induced Airway Hyperresponsiveness in Balb/C Mice. Clin Pharmacol Biopharm S2:002. doi:10.4172/2167-065X.S2-002 | |
| Copyright: © 2014 Gupta S, et al. This is an open-access article distributed underthe terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
Yucca gloriosa (Agavaceae) is used in Indian traditional medicine as an antiallergic drug. The ethanol extract was prepared with aerial parts of Yucca gloriosa (YGE). Gloriosaol isomeric mixtures (GLM) are isolated from the extract, structurally confirmed FIA-MS/MS. Both YGE and GLM are evaluated against ovalbumin-induced airway hyperresponsiveness (AHR) in Balb/C mice. The test drugs (GLM or YGE or Dexamethasone) are administered p.o. prior to challenge with aerosolized 2.5% w/v ovalbumin. Total and differential leucocytes count, Nitrite (NO2), Nitrate (NO3), Tumor necrosis factor- α (TNF-α), Interlukin-6 (IL-6), and Interlukin-13 (IL-13) are estimated in bronchoalveolar lavage fluid (BALF). Similarly, Myeloperoxidase (MPO) and Malonaldehyde (MDA) are estimated in lungs. The results reveal a significant increase in total and differential leucocytes counts, NO2, NO3, TNF-α, IL-6, and IL-13 in ovalbumin induced AHR. However, these parameters are significantly decreased in YGE and GLM treated mice at test doses (YGE 100 & 200 mg/kg and GLM 30 mg/kg). Similar observations are recorded for Myeloperoxidase (MPO) and Malonaldehyde (MDA) in lungs. Pro inflammatory mediators are (TNF-α, IL-6, and IL-13) known that responsible for AHR. Histopathology reveled justify the effectiveness. The present investigations suggest both YGE and GLM are interesting molecules for the further research the treatment of asthma, with an approach through pro-inflammatory inhibitory pathway.
| Keywords |
| Gloriosaol isomeric mixtures (GLM); Yucca gloriosa (YGE); Tumor necrosis factor- α (TNF- α); Interlukin-6 (IL-6) and Interlukin-13 (IL-13) |
| Introduction |
| Asthma is one of the world’s most common long-term respiratory disorders characterized by a specific pattern of inflammation [1]. Global burden of asthma reported (GBAR), over 50 million people in Central and Southern Asia are suffers from asthma. The disease is estimated to affect as many as 300 million people worldwide, a number that could increase by further 100 million by 2025 [2-3]. WHO also estimates that presently 235 million peoples suffer from asthma worldwide. Asthma statistics estimated that in India 57,000 deaths were reported in 2004 and was considered as one of the leading cause of morbidity and mortality [4].Asthma is characterized by episodic, reversible bronchoconstriction resulting from increased airway hyper responsiveness (AHR) of tracheobronchial tree to various stimuli [5]. There is some evidence for the pathophysiological causes of asthma show increased infiltration of inflammatory cells like eosinophils, [6] neutrophils, [7] lymphocytes, monocytes [8] and mast cells [9]. These are intensely found in airway wall biopsies and in broncho alveolar lavage fluid (BALF) from asthmatic patients. In allergic asthma mast cells play a pivotal role in inflammatory and immediate type I hypersensitive reactions, such as acute bronchoconstrictor responses and hyperventilation [10]. Allergic asthma pathophysiology inflammatory mediators derived from these cells such as histamine, [11] cytokines, [12] leukotrienes [13], prostaglandins [14], platelets [15], free radicals like reactive oxidative species (ROS) and reactive nitrogen species (RNS) [16] etc. Previous studies have demonstrated common approach to quantify histamine in plasma while total and differential cell counts in BALF. While, it was experimentally documented proved that elevated levels of histamine, increases in number and percentage of eosinophils in short-term high-level challenge murine animal’s model in BALF, lung tissue and plasma [17,18]. |
| We have recently described a model of chronic asthma in balb/c mice but experimental systems which employ antigens that bear little relationship to triggers of human asthma pathophysiologically. This method involved systemic sensitization of balb/c mice with ovalbumin and subsequent OVA inhalational challenge. In contrast to reported studies which suggest that the long-term challenge is associated with down-regulation of the inflammatory and immunological response. This approach consistently elicited lesions typical of chronic human asthma. The models based upon sensitization by systemic administration of protein antigens such as ovalbumin which produces Th2-dominated response by recruiting and activating inflammatory cells like eosinophils, tumor necrosis factor-α (TNF-α), interleukin-4 (IL-4), IL-5, IL-6 and IL-13 etc. [19,20]. Th2 cytokines increased production promoted by T helper cells, which was activated by eosinophils and mast cells. While, significant changes observe in specimens of lung tissues biopsy like lamina propria with accumulation of lymphocytes, plasmacytoid lymphocytes and macrophages; together with epithelial hypertrophy, mucous cell hyperplasia and subepithelial fibrosis etc. [21]. The current therapeutic approaches for the treatment have limitation in their ability to target all the features of the disease. Thus, there is an unmet need for developing new drugs to target these features along with improved efficacy and without side effects. Due to these reasons, there is a need of new-targeted therapeutic agent required to treat asthma. So in this direction we are focus toward complimentary alternatives medicine for asthmatic disorder. |
| Yucca gloriosa L. (family- Agavaceae) commonly known as Spanish Dagger, is a stem less plant. The leaves of this plant are 2-3 feet long, 2 inches wide, long pointed, often toothed margin, mostly in rosettes at the ground level or at the ends of the trunk. Flowers have many cups or saucer shaped, hanging, greenish white to reddish, fragrant, born mostly in erect panicles that usually overtop the leaves [22]. Y. gloriosa widely grow in California, Mexico and India. In India it is widely available in Tamilnadu, Andhra Pradesh and Himalayas. An ethnobotanical survey claim that in ancient times tribal people used this plant leaf for bronchitis, asthma, dysentery, phthisis, menstrual disorder, hemorrhagic, septicemia [22,23], anti-inflammatory agents, arthritis, potent free-radical scavenging effects [24], cancer [25], cerebral ischemia, hepatoprotective, leprosy, ulcer, anti-helminthic, anti-microbial [26] etc. The fruit is used as a blood purifier, cholagogue and as a purgative. The rhizomes are used in the manufacture of detergent [23]. |
| The previous literature reported that the phytochemical constituents of Y. gloriosa leaves contain sapogenins- titogenin derivatives, smilagenin, 12- β- hydroxysmilagenin and β- sitosterol, tigogenin 3-O-β-D-xylopyranosyl- β-lycotetraoside, gitogenin 3-O-β-Dxylopyranosyl- β-lycotetraoside, gitogenin 3-O-α-L-rhannopyranosyl- β-lycotetraosid, pro-type of gitogenin 3-O-β-D-xylopyranosyl-β- lycotetraoside [22,23,27], sitosterol, stigmasterol, campesterol and cholesterol [28]. LC-MS/MS of Methanolic extract of roots and bark of Y. gloriosa are rich source of phenolic derivatives and the constituents are structurally related to resveratrol. These phenolic compounds are yuccaols (A-E) and gloriosaols (A-E) having good source of antioxidant property [29-31]. Flowers of Y. gloriosa contain yuccaloesides A, B, C, E, degalactotigonin and gitogenin 3-O-α-L-rhamnopyranosyl-β- lycotetraosidesteroidal glycosides [32]. The existence of 24 steroidal glycosides, including 13 furostanols from methanolic extract of Y. gloriosa leaves. These compounds are derivatives of tigogenin [33]. The phytochemistry of Y. gloriosa has been well developed and demonstrated in various literatures. Y. gloriosa rich structural diversity and complexity has prompted us to further intensify investigation. We hypothesized that Y. gloriosa extract and isolated compound would have an antiasthmatic effect on AHR in murine mice model of allergic asthma which is not reported so far. |
| Materials and Methods |
| Collection of plant |
| Yucca gloriosa L. aerial (leaf) part were collected in the month of August, 2010, from Tirupati district, Andhra Pradesh, India. Dr. K. Madhava Chetty, Botanist, Department of Botany, Sri Venkateswara University, Tirupati authenticated the collected plant. Voucher specimen has been preserved in our laboratory (SVU/SC/08/24/10-11) for future reference. |
| Drugs and chemicals |
| Ova Albumin, O-dianisdine, Flavin adenine dinucleotide (FAD), Bovine serum albumin (BSA), May-grunwald, Griess reagent (NED: 0.2% (w/v), sulfanilamide: 2% (w/v), solubilized in double distilled water: 95% and phosphoric acid: 5% (v/v)), 1,1,3,3 Tetraethoxypropane, Thio barbituric acid, N-[2-hydroxyethyl] piperazine-N’-[2-ethanesulphonic acid] (HEPES), Reduced nicotinamide adenine dinucleotide phosphate (NADPH), Hexa decyl trimethyl ammonium bromide (HTAB), Nitrate reductase, Lowry reagent (A mixture 100 ml sodium carbonate (2% w/v), 1ml of sodium potassium tartrate (2% (w/v), 1 ml of 1 % (w/v) of cupric sulfate), Hematoxylin, Periodic acid schiff stain were purchased from sigma Pvt. Ltd. etc. Thiopentone sodium was procured from Neon Laboratories, India. ELISA Kits IL-6 & TNF-α were procured from Koma Biotech, South Korea where as IL-13 from Ray Biotech, Inc., USA for scientific estimation of cytokine assay. All chemicals used were of analytical grade. Dexamethasone was obtained as a gift sample from Ranbaxy Pvt. Ltd. |
| Preparation of extraction |
| The dried (aerial) leaf of Y. gloriosa (1 kg) powdered was ground using a milling and extracted with cold maceration process using absolute ethanol (99.5%) by intermittent shaking for 10 days. The solvent were evaporated by rotary flash evaporator (Rota vapor, R-210/215, Buchi, Switzerland) under vacuum and reduced pressure at a temperature of maximally 550C. The concentrate the extract reached upto semi solid material and then kept in a desiccator for drying to give sticky dark brown ethanolic 3.16 % on the dry weight henceforth called YGE, respectively. The extract was subjected for phytochemical tests [34]. |
| ESI-MS/MS analysis: A qualitative estimation: ESI–MS/MS Flow injection analysis (FIA) fingerprints of the YGE extract by API 2000 (Applied biosystem/MDS SCIEX, Canada) mass spectrometer attached with ESI (Electro spray ionization; Turbo spray) source and a chromatographic system. Batch acquisition and data processing was controlled by Analyst 1.5 version software. The optimized parameters like declustering potential (DP) 40 V, focusing potential (FP) 400 V, entrance potential (EP) 10 V, curtain gas (N2) (CUR) 20 psi, Ion spray voltage (IS) 5500 V, temperature (TEM) 0°C, Ion source gas (O2) (GS1) 30 psi and (GS2) 40 psi were optimized with respect to ionization intensity response. Acquisition was performed by setting the mass of the analysts with suitable scan range. The extracts were diluted in a solution containing 100% (v/v) HPLC grade methanol (Merck, India). The extracts were analyzed by direct infusion directly by means of a syringe pump (Harvard Apparatus) at a flow rate 20 μl/min constantly in mass spectrometer. Intensity of ionization response of YGE was analyzed by direct insertion into negative and positive ionization in both modes of ESI-MS/MS fingerprinting. This method provides a sensitive and selective method for the identification of polar organic molecule with acidic sites, such as phenolic compounds found in Y. gloriosa. Selected reported compounds mass ion and their ESI-MS/MS compared to those found in references, for the identification of these compounds. |
| Isolation of compound: The 20 g of YGE was charged over silica gel (60-120 mesh) column (100 cm × 5 cm) eluted gradually with solvents and the solvent mixtures of increasing polarities. Fractions were collected in 50 ml portions and monitored on TLC and the fractions showing similar spots were combined. The fraction 5, eluted in chloroform mobile phase, showed one major spot when subjected for TLC using the mobile phase showed one major spot in chloroform: methanol: 1drop formic acid (4:1:1drop) solvent system and obtained the Rf value of 0.51 with minor impurities (yield: 350 mg). Recrystallization of fraction 5 in chloroform yielded a brown crystalline powder designated as compound 1 (GLM) (yield: 310 mg). The structure of GLM was elucidated by ESI-MS/MS. |
| Animal husbandry: Specific pathogen-free female Swiss albino mice and male balb/c mice (20-28 g) were obtained from the animal house, J.S.S. College of Pharmacy, Ootacamund, India, and were housed under standard environmental conditions (12-h dark-light cycle, 22-28°C, 60-70% relative humidity). The animals were housed in stainless steel cages and had free access of standard laboratory feed (M/S Hindustan Lever Ltd., Bangalore, India) and water ad libitum. The experimental protocol was approved by the institutional animal ethics committee (IAEC) constituted in accordance with the rules. The experiments were conducted as per the guidelines of the committee for the purpose of control and supervision on experimental animals (CPCSEA), Chennai, India (Approval no. JSSCP/IAEC/Ph.D/P. Cog/02/2011-12). |
| Acute toxicity |
| The procedure was followed according to the OECD guidelines 423 (Acute toxic class method). Adult female Swiss albino of weight (22-28 g) was used for acute toxicity study. The dose level was selected from one from the defined doses (5, 50, 300, 2000 mg/kg body weight) orally (p.o.) and the results allow a substance to be ranked and classified according to the globally harmonized system (GHS). The mice were observed for mortality, body weight changes, urine color analysis and clinical signs daily for a period of one month and at the end of the study period, all the animals were scarified and subjected to gross necropsy [35]. |
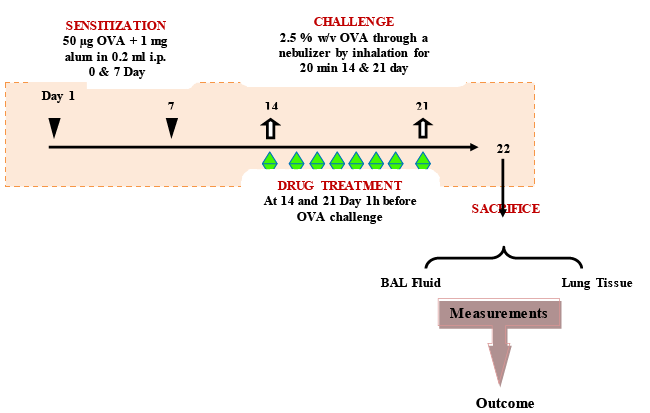
| Sensitization and ovalbumin challenge: Male balb/c mice (20- 28 g) were injected i.p. with a combination containing ovalbumin (OVA) (50 μg) and alum (1 mg) in 0.2 ml of normal saline apart from vehicle control group on day 0 and 7. At day 14 and 21, the mice were challenged with 2.5 % (w/v) OVA aerosol through a nebulizer (Omron, UK, Model No. CX3) delivering particles of 0.5-5 μ size at a pressure range of 30-36 psi for 20 min. The vehicle control mice were exposed to saline aerosol for 20 min on day 14 and 21 [36]. |
| Treatment schedule: Each group consists of eight male balb/c mice. 1 h before each OVA sensitization and challenge on day 14 and 21st day after the initial sensitization. YGE (50, 100 and 200 mg/kg), GLM (15 and 30 mg/kg each was administered orally (p.o.) once daily on days 14-21. OVA control (negative control) and positive control mice were treated orally with phosphate buffered saline (PBS) and dexamethasone (DEXA; 30 mg/kg; p.o.) [37], respectively, once daily 14-21 days. Animals were sacrificed after 48 h of the last challenge at day 22 to characterize the inhibitory effects of tested drug. Schematic experimental protocol of the treatment schedule describe above (Figure 1). |
| BALF collection: The mice were sacrificed by administering excess of thiopentone (80 mg/kg, i.p.) after 24 h of the last challenge. A tracheal cannula was inserted and lavaged twice with the 1 ml of 4°C cold PBS, pH=7.4. After collecting the BALF, the lungs of the mice were removed and it was stored at -20°C for the estimation of MDA, MPO and total protein [38]. |
| Measurement total and differential count in BALF: The BALF was centrifuged at 170 g for 10 min at 4°C and the supernatant was removed and stored at -80°C for the estimation of further analysis. The pellets obtained after the centrifugation was resuspended in the 0.5 ml of the PBS and total leukocyte count (TLC) was performed using neubauers chamber and WBC diluting fluid. A smear was prepared using the BALF. The air dried smeared slide was stained for 10 min with giemsa and washed with millipore water for 8 min. Counter staining was latter carried out with may-grunwald stain for 10 min. The differential cell count (DLC) was carried out using a digital light microscope (Motic, Japan, Cat. No. B1 Series) at 100x magnification by oil immersion technique and calculate least 100 cells were differentiated on each slide to find the percentage of macrophage and mononuclear inflammatory cells, such as monocytes, neutrophils and eosinophils [38]. |
| Measurement of nitric oxide (NO) in BALF: BALF supernatant (100 μl) was incubated for 30 min at 37°C with HEPES acid free buffer (50 mM; pH=7.4), FAD (5 μM), NADPH (0.1 mM), millipore water 290 μl and nitrate reductase (0.2 U/ml). Any unreacted NADPH in the solution was oxidized by incubating the solution with potassium ferricyanide (1 mM) at 25°C for 10 min for the conversion of nitrate to nitrite. Later on the sample solution was incubated with 1 ml of the griess reagent for 10 min and the absorbance was measured at 543 nm using a UV spectrophotometer (Perkin Emler, Model No. Lamda 25). For the evaluation of nitrite in identical set of tubes, nitrate reductase was omitted. A standard curve plotted with absorbance (543 nm) vs. concentration (μM) standards and was used to determine the concentrations of nitric oxide metabolites in the BALF of tested sample [39]. |
| Enzyme–linked immunosorbent assay (ELISA): Estimation of TNF-α (Cat#: K0331186), IL-6 (Cat#: K0331230) (Koma Biotech, South Korea) and IL-13 (Cat#: ELM-IL13-001) (Ray Biotech, Inc., USA) in the BALF. Standard method of ELISA kits was performed using according to the manufacturer’s instructions. |
| Measurement of lung malonyldialdehyde (MDA) activity: The (100 mg) of lung tissue was homogenated with 1 ml normal saline using teflon coated glass high speed homogenizer (Remi, India ; Cat. No. 4148). From that 1 ml of the tissue homogenate was mixed with the 2 ml mixture of thiobarbituric acid 0.375% (w/v), trichloroacetic acid 5% (v/v) and HCl (0.25 N). The mixture in the test tube was incubated in the boiling water (water bath) for 15 min. Later it was cooled and centrifuged at 1500 rpm for 10 min. The pink colored solution was obtained and measured at 535 nm using a UV spectrophotometer. 1,1,3,3 tetrae thoxypropane served as an external standard. The standard plot was used for the estimation of MDA. MDA levels in the lungs were expressed as nM/mg of tissue protein [40]. |
| Measurement of lung myeloperoxidase (MPO) activity: The frozen harvested lung were weighted and homogenized with 1 ml of the 50 mM phosphate buffer (pH=6) using teflon coated glass high speed homogenizer. The homogenate was centrifuged at 40,000 g at 4°C for 15 min. The pellets were resuspended in 1 ml 50 mM potassium phosphate buffer (PPB) (pH=6) containing 0.5% (w/v) HTAB to neutralize the pseudoperoxidase activity of hemoglobin and solublize membrane bound MPO. The suspension was frozen and thawed 3 times and sonicated (Bandlin Sonorex, Germany; Model No. RK100H) on ice for 10 sec. 0.1 ml of supernatant sample was added with 2.9 ml of PPB (50 mM) containing O-dianisdine (0.19 mg/ml). The solution was transferred to the cuvette and hydrogen peroxide (H2O2) (0.0005% (v/v) was added. Immediately the absorbance was measured at 460 nm and then after 3 min using UV spectrophotometer. The MPO activity was calculated by formula: MPO activity (μg/lung) = ΔA × 4.05/ lung weight (g), where (ΔA= rate of change in absorbance at 460 nm between 1 and 3 min). The MPO levels in lung were expressed as mU/ mg tissues protein [41,42]. |
| Measurement of total lung protein (TLP) activity: The (100 mg) of lung tissue was homogenated with 1 ml normal saline. One ml of the tissue homogenate was mixed with the 5 ml of the Lowry reagent and 0.5 ml of the folin-ciocateu’s reagent. The mixture was mixed thoroughly and incubated for 30 min at 25°C. The absorbance of the blue colored solution was measured at 750 nm using a UV spectrophotometer. Standard plot was obtained using BSA at concentrations of 10-100 μg/ ml [43]. |
| Histopathology of lung tissue: Lung sections were stained with heamtoxylin and eosin (H&E) for routine histology and periodic schiff acid stain (PAS) for goblet cell study. Briefly, one portion of the harvested lung as fixed with 10% buffered formalin. The paraffin embedded blocks were cut into 50 μm section using a microtome (Lecia, UK; Model No. RM2135) mounted and stained with heamtoxylin and eosin (H&E) and periodic-acid schiff (PAS) [44]. |
| Statistical analysis: Significant differences between selected groups were estimated by using One-way Analysis of Variance (ANOVA) followed by Tukey’s multiple comparison tests. Statistical significance was set at P<0.05. The analysis was carried out using Graph Pad Prism software V 6.01. |
| Results |
| Qualitative phytochemical screening |
| The phytochemical screening on Y. gloriosa revealed the presence of primary metabolites such as carbohydrates, proteins and amino acids. The secondary metabolites found included alkaloids, glycosides, saponins, sterols, flavonoids, phenolic compounds and tannins. |
| ESI -MS/MS qualitative studies of Y. gloriosa ethanolic extract |
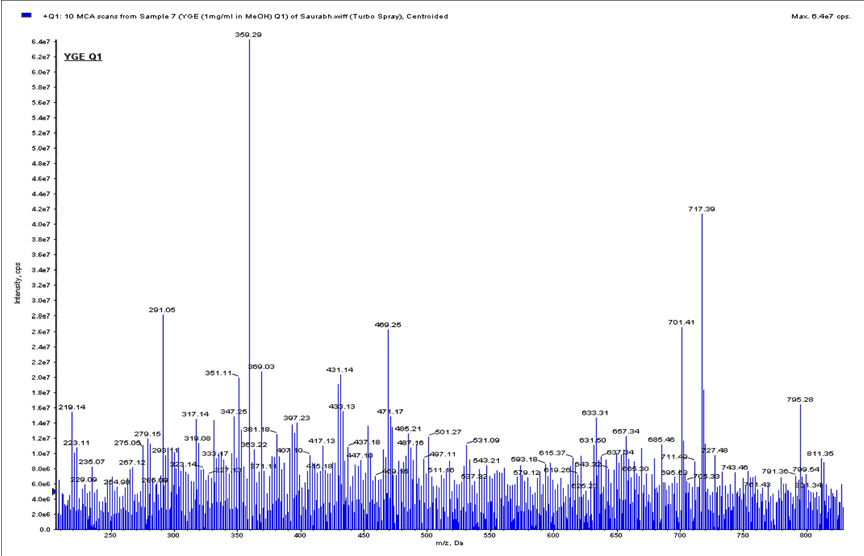
| The intense response were observed in positive ion [M-H]+, fingerprinting of ESI-MS/MS for YGE. The event was a full scan ESIMS/ MS. FIA studies to acquire data on ions in the range 100-1400 m/z (Figures 2A & 2B). The thirteen good intense response were identified with YGE were precursor ion at represented in (Table 1). |
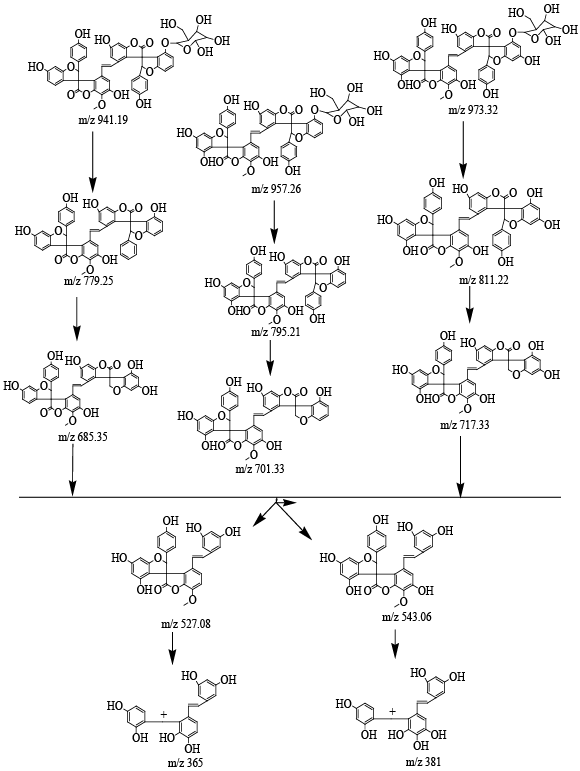
| Characterization of Isolated compound 1: Brown crystalline powder; m.p.: 300°C responds to shinoda test, thereby indicating its phenolic nature. IR (KBr), Vmax spectra shows absorption bands at C-O-C (1075 and 1259 cm-1), -CH=CH- (1656 cm-1), C=O (1738 cm-1), -CH3 (2852 cm-1) and Ar-OH (3370 cm-1) groups. The fragmentation patterns of steroidal saponins were analyzed by ESI-MS in positive ion mode and MS/MS detection of a specific multiple reactions monitoring was developed for their structure determination the phenol pseudomolecular ions. The first event was positive mode scan of ESI-MS to acquire data in the range of 100-1000 m/z and product ion scan was performed on selected mass ions 543 m/z and 275 m/z. The fragmentation patterns were more informative when spectra were recorded in positive ion mode, and this operative mode was preferred for the ESI-MS/MS method development. For these compounds beginning from the selected protonated molecular ions, [M+H]+ were taken into consideration are m/z 973.32, 957.26 and 941.19. After the elimination of the glucoside unit, the product ions yielded are at m/z 811.22, 795.21 and 779.25 respectively. This indicates the presence of the three compounds with m/z 973.32, 957.26 and 941.19. The following scheme was used in these experiments: |
| Compound 1. precursor ion at 973.32m/z and the product ion at 811.22 m/z |
| Compound 2. precursor ion at 957.26m/z and the product ion at 795.21 m/z |
| Compound 3. precursor ion at 941.19m/z and the product ion at 779.25 m/z |
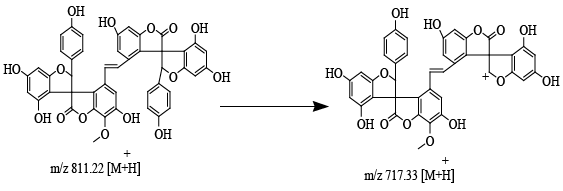
| Structure of precursor ion at m/z 811.22; product ion at m/z 717.33 (Figure 3) after eliminate the neutral phenol moiety to yield the ions at m/z 717.33, 701.33, 685.35. |
| d. Compound 1. Precursor ion at 811.22m/z and the product ion at 717.33m/z |
| e. Compound 2. Precursor ion at 795.21m/z and the product ion at 701.33m/z |
| f. Compound 3. Precursor ion at 779.25m/z and the product ion at 685.35m/z |
| g. Compound 1. Precursor ion at 717.33m/z and the product ion at m/z 543.06 and 527.08 |
| h. Compound 2. Precursor ion at 701.33m/z and the product ion at m/z 543.06 and 527.08 |
| Compound 3 Precursor ion at 685.35 m/z and the product ions at m/z 543.06 and 527.08 |
| Structure of precursor ion at m/z 543.21; product ion at m/z 451.30, 437.00 and 543. Further Precursor ion at m/z 275; product ion at m/z 229 (by the elimination of two -OH groups and a methyl group), finally selected precursor ion at m/z 229; product ion at m/z 140 (methoxylated phloroglucinole moiety) from the critical comparison of ESI-MS/MS signals indicated that since these compounds differ only for stereochemistry they will occur in the same reaction monitoring chromatogram and compound 1 proved its proposed structure are an steroidal phenolic compound isomeric gloriosaol (GLM). The fragmentation pattern of mention below (Figure 4). |
| Acute toxicity study of Y. gloriosa extract |
| According to OECD guideline 423, acute toxicity study was carried out none of the mice show o abnormal clinical signs and no mortality upon single administration of YGE (2000 mg/kg) on day one. Observations recorded twice daily for 14 days also did not reveal any drug related observable changes. Based on the LD50 value 1/10 (200 mg/ kg), 1/20 (100 mg/kg), and 1/40th (50 mg/kg) of LD50 dose was selected for pharmacological studies. |
| Total and DLC influx in recoverable BALF |
| Massive statistically significant (p<0.001) increase in total cell and DLC influx was observed after OVA aerosol exposure in the BALF collected at 22th day when compared to vehicle control. The total number of cells in recoverable BALF was significantly (p<0.001) lowered upon YGE (200 mg/kg) and GLM at tested dose (30 mg/kg) treatment when compared to OVA control. A corresponding significant decrease in circulating eosinophils (p<0.001) and neutrophils (p<0.001) were observed significantly decreased by YGE and isolated GLM at maximum tested dose treated animals (Table 2). However, the mean value indicate that DEXA 30 mg/kg and YGE 200 mg/kg proved to be more potent in reducing the DLC in BALF when compared to all the other treatment groups. |
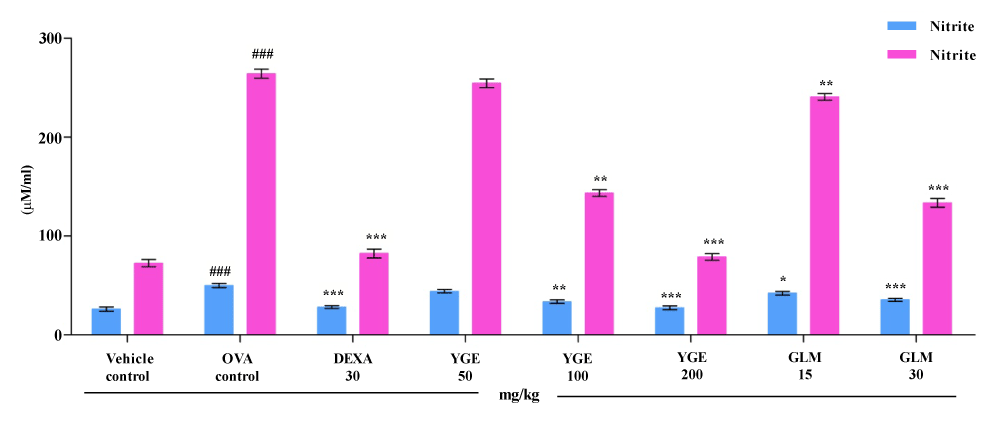
| Nitrite (NO2) and Nitrate (NO3) activity in BALF |
| An elevated level of NO2 and NO3 in lungs causes formation of NO due to that increases in oxidative stress level. This increased oxidative stress and NO form potent peroxynitrite radical which causes nitrosylation of proteins in the airway walls. The NO2 and NO3 activity in OVA control BALF (μM) was significantly elevated (p<0.001) when compared to vehicle control group (Figure 5). When compared to OVA challenged group, elevated NO2 and NO3 levels were significantly decreased (p<0.001) by YGE at 200 mg/kg and GLM at 30 mg/kg. However, the mean value indicate that DEXA 30 mg/kg and YGE 200 mg/kg found to be more potent in decreasing the NO2 and NO3 free radicals levels in BALF when compared among all the treated groups. |
| Cytokine levels in BALF |
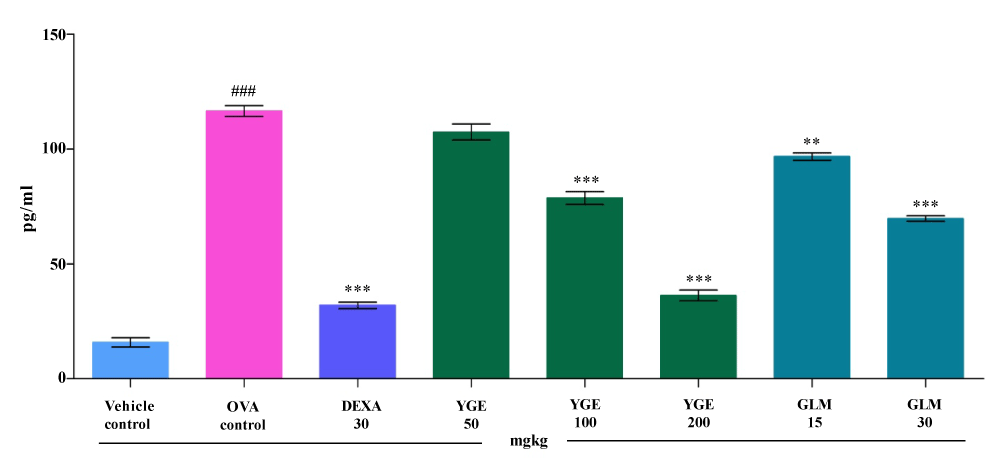
| TNF- α level in the BALF: In asthmatic condition increased amount of TNF-α mediator are produced which activate the proinflammatory transcription factors due to that so many inflammatory genes in the asthmatic airway. The TNF-α levels in OVA control BALF (pg/ ml) was significantly increased (p<0.001) when compared to vehicle control group (Figure 6). The elevated TNF-α levels were significantly decreased (p<0.001) TNF-α levels when compared with OVA challenge group by YGE 200 mg/kg and GLM at 30 mg. However, the mean value indicate that DEXA 30 mg/kg and YGE 200 mg/kg showed statistically significant (p<0.001) decrease the elevated TNF-α activity among all the treatment group. |
| IL-6 level in the BALF: IL-6 is the lead cause of smooth muscle hyperplasia and fibroblast asthmatic condition. Massive statistically significantly increase (p<0.001) in IL-6 levels in BALF (pg/ml) was observed in OVA control when compared to vehicle control. When compared to OVA control, elevated IL-6 levels were significantly decreased (p<0.001) by YGE at 200 mg/kg dose levels (Figure 7). Whereas GLM at 30 mg/kg also showed significantly (p<0.001) decreased elevated IL-6 levels. However, mean value indicate that DEXA 30 mg/kg and YGE 200 mg/kg show significant decrease (p<0.001) in the elevated IL-6 level among the entire treatment group animal. |
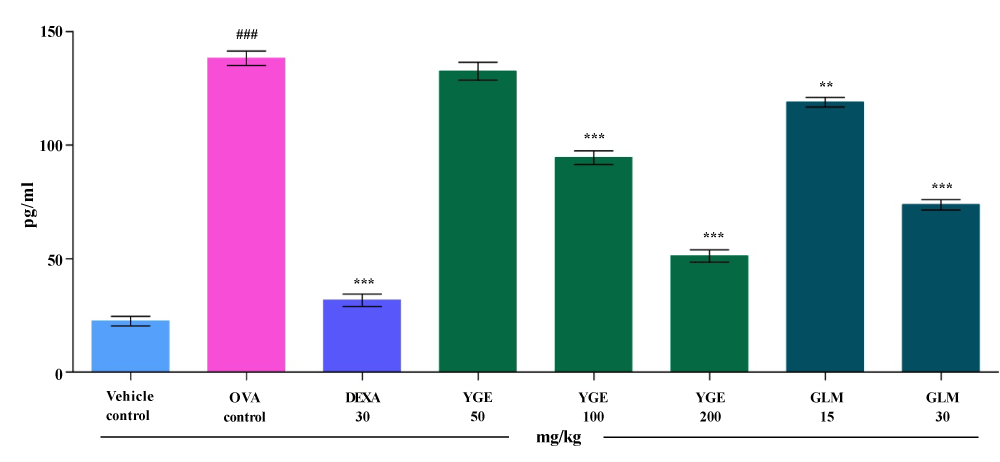
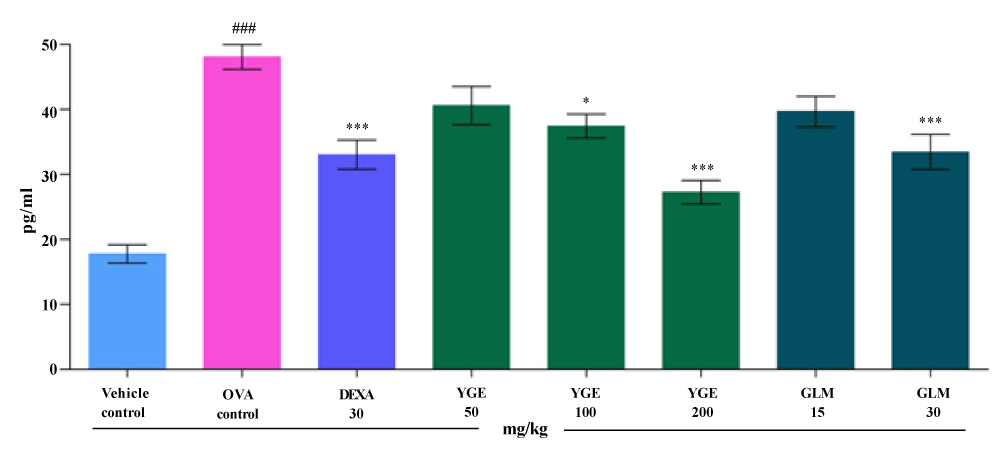
| IL-13 level in the BALF: Activation of IL-13 generates allergic inflammatory response and cause formation of IgE antibody. The IL-13 levels in BALF (pg/ml) were significantly increased (p<0.001), in the OVA control when compared with vehicle control (Figure 8). When compared to OVA group, elevated IL-13 levels were significantly decreased (p<0.001), by YGE at 200 mg/kg dose level. Whereas, GLM at 30 mg/kg showed significantly (p<0.01) decreased the activity of IL-13. However, mean value indicate that YGE 200 mg/kg (p<0.001) showed potent significant decrease in elevated IL-13 activity among the entire treatment group. |
| MDA level in lung |
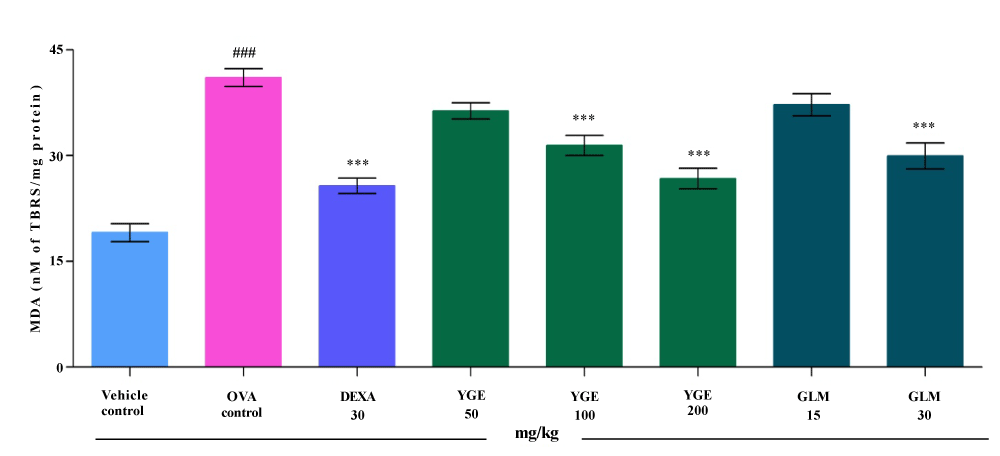
| Estimation of lipid peroxidation give the degree of denatures protein taking place in the lung tissue. OVA exposure significantly (p<0.001) elevated the MDA level in lung tissue (nM of TBAR/mg protein) when compared with vehicle control. When compared to the OVA challenged group, the elevated MDA level was significantly (p<0.001) decreased by YGE at 200 mg/kg. The compound GLM at 30 mg/kg significantly (p<0.001) decreased the MDA activity (Figure 9). However, the mean value indicated that a dose of DEXA 30 mg/kg and YGE at 200 mg/kg (p<0.001) showed a significant decrease in elevated MDA activity among all the treatment. |
| MPO level in lung |
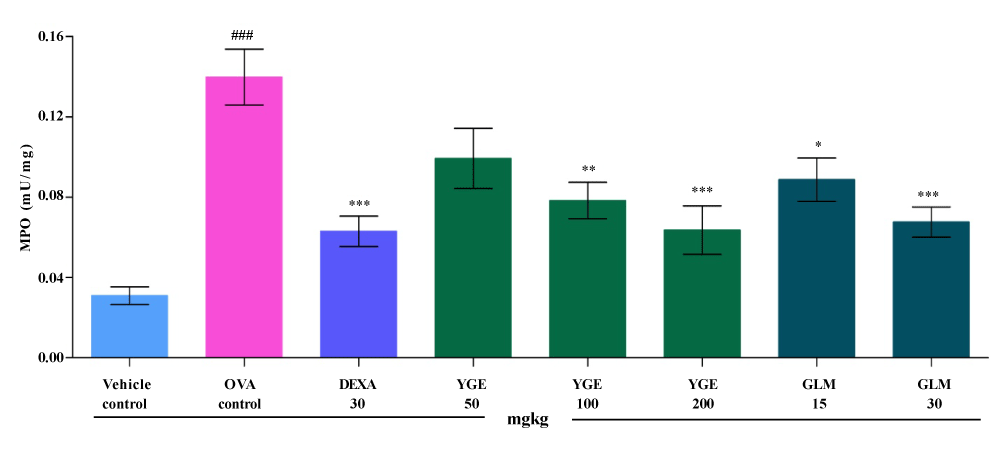
| MPO is the major constituents of neutrophils. Elevated concentration of MPO causes cell lesions in the lungs tissue. Lung tissue (mU/mg) MPO activity was significantly (p<0.001) elevated, in OVA control when compared to vehicle control group (Figure 10). The elevated MPO level was significantly (p<0.001) decreased by YGE at 200 mg/kg dose levels in comparison with OVA challenged group. Whereas, GLM at 30 mg/kg significantly (p<0.001) decreased the MPO level. However, the mean value indicated that treatment with DEXA 30 mg/kg and YGE at 200 mg/kg dose level proved significant reduction (p<0.001) in the elevated MPO level. |
| TLP level in lung |
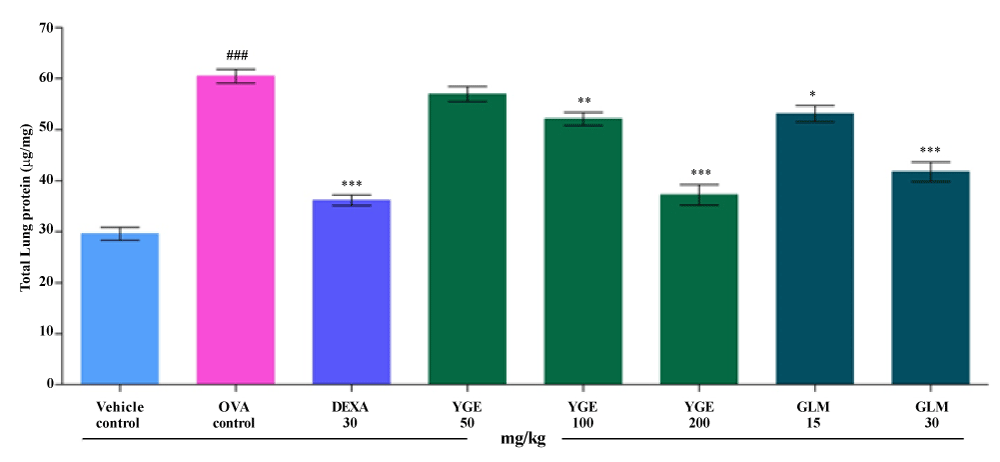
| In asthma elevated level of protein shows the protein degeneration of lung tissue cells. TLP activity in lung tissue (μg/mg) was significantly (p<0.001) elevated in the OVA control when compared with vehicle control mice. Elevated TLP level was significantly decreased (p<0.001) by YGE at 200 mg/kg dose levels when compared with OVA challenged group. Whereas, GLM at 30 mg/kg showed significantly decrease (p<0.001) TLP level in lung tissue. However, the mean value indicated that treatment with DEXA 30 mg/kg and YGE 200 mg/kg displayed a significant (p<0.001) decrease in elevated TLP level among the entire dose level (Figure 11). |
| Histopathology |
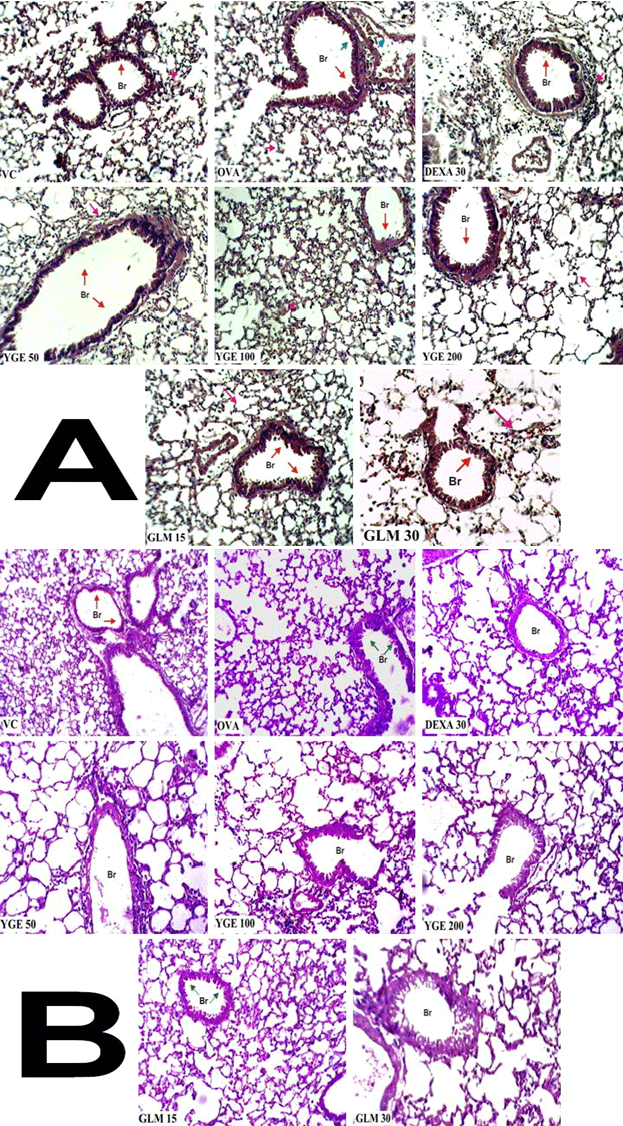
| Bronchi histology with hematoxylin and eosin (H&E): In asthma disorder histology is very important evidence, for finding the structural changes in bronchi. Histological of lung sections revealed that OVA control group, stained with H&E, showed massive increase in peribronchial inflammation, Increased vascularity, epithelial fragility, angiogenesis, increased matrix deposition in the airway wall, increased airway smooth muscle mass, abnormalities in elastin, cuffing and active metaplastic goblet cells when compared to vehicle control group. Maximum restoration of bronchi was observed in YGE (200 mg/kg) which showed presence of diffused but mild inflammation in interstitial spaces and functional goblet cells with no much secretion in bronchi when compared with OVA control. However, mice treated with DEXA 30 mg/kg showed similar observation. The histological plates are shown below (Figure 12A). |
| Bronchi globlet cell histology with periodic acid-Schiff (PAS): Bronchi globlet cell histology carried out by PAS stain to know the architecture of goblet cells condition and arrangement. Histological of lung sections revealed that OVA control group showed lost architecture and metaplastic goblet cell arrangement. Treatment with YGE 200 mg/ kg showed maximum restoration of goblet cells were observed showed no metaplasia and cells are arranged typically reaching towards normal when compare with OVA control. However, mice treated with DEXA 30 mg/kg showed similar observation. The histological plates are shown below (Figure 12B). |
| Discussion |
| Asthma is one of the most prevalent diseases of pulmonary disorders. Recently new therapeutic approaches have been developed but many severe side effects are reported. Effective treatments for asthma existing, but morbidity and mortality rate is high and increases burden of asthma significantly in developed and developing countries [45]. Asthma caused by number of factors and a unified theory of the pathogenesis of asthmatic bronchoconstriction is still not clarified because asthma is multifactorial disorder which involves both genetic and environmental factors making it expensive and more difficult for the scientists to make significant steps for developing new-targeted therapies [46]. On the scientific side, researchers are now teaming up in networks and focus on projects to understand more clearly the heterogeneous phenotypes that underlie asthma. Nowadays present research scenario for herbal antiasthmatic therapy which is available to treat asthma is very few in number. The plant-based drugs provide outstanding contribution to modern therapeutics. The natural medicine are attracting renewed attention from both practical and scientific view points, but the mode of action of traditional herbal medicines and related products from nature is more complex than of a single bioactive factor. So there is a need to find potential leads from medicinal plants that may be effective against different type of hypersensitivity reactions which involved in various cellular pathway of allergic asthma. On the basis of ethnobotanical claims Y. gloriosa plants having antiasthmatic properties. The phytochemistry of Y. gloriosa has been well developed and demonstrated in various literatures. Their rich structural diversity and complexity has prompted us to further intensify investigation. The present study was undertaken to evaluate ethanolic and aqueous extracts of Y. gloriosa and its isolated GLM to assess the in vivo antiasthmatic activity on OVA induced animal models. Efforts were also made to identify the phytoconstituents that are responsible for the antiasthmatic activity, which is not reported so far. |
| The qualitative phytochemical investigation on the extracts of Y. gloriosa has proved the presence of carbohydrates, amino acids, glycosides, saponins, sterols, flavonoids, phenolic compounds and tannins. The phytochemicals tests found to be within pharmacopoeial standards range [47]. The qualitative analysis on the crude extracts has been carried out by ESI- MS/MS by FIA methods. For observing the peak intensities are exactly matching with reported compounds, which are claimed to be present in Y. gloriosa as per the literature. The result revealed that in positive mode, thirteen good intense response peaks were identified with YGE extract and are characterized as resveratrol at m/z 229.09, trans-3,3’,5,5’–tetrahydroxyl-4-methoxystilbene at m/z 275.05, yuccaol A-B at m/z 497.11, yuccarol C-E at m/z 543.2, gloriosaol A-E at m/z 811.35 and yuccaloeside B at m/z 1211.91. Paola Montoro et al [29,32] have reported the presence of phenolic components in the leaves, bark and flower of the methanolic extract of Y. gloriosa by LC/ MS/MS. They have also elucidated the various chemical structures of phytoconstituents present in Y. gloriosa. Bassarello C et al [32] suggests that the gloriosaols A-E and the yuccaols A-E present in the Y. gloriosa plant are the derivatives of resveratrol, trans-3,3’,5,5’-tetrahydroxy- 4’-methoxystilbene, and the spirobiflavonoid larixinol along with very unusual spirostructures. Further Paola Montoro et al [29] had suggested that multifunctional activity has been due to the presence of resveratrol together with gloriosaols A-E and yuccaols A-E. A strong radical scavenging activity has also been observed in Y. gloriosa. On the bases of IR and MS/MS spectral data it has been found that the isolated compound may be a mixture of gloriosaol, which was further confirmed by ESI-MS/MS. The fragmentation pattern has justified the compound gloriosaols are spiro-structures made up of the same basic C15 and C14 structural units of yuccaols C–E but differing from yuccaols C–E in the occurrence of two C15 units instead of one. Gloriosaols A and B exhibit the two p-hydroxyphenyl rings of the C15 units at the opposite side of the stilbenic moiety, gloriosaol C shows the two p-hydroxyphenyl rings at the same side of the stilbenic moiety, and in gloriosaols D and E, a p-hydroxyphenyl ring is oriented to the same side of the stilbenic moiety in gloriosaols D and the other one is located to the opposite side in gloriosaols E this is also evidenced by ESI-MS/MS spectral data of compound also reveled that compound is steroidal nucleus compound 1 isomeric gloriosaol (GLM). ESI-MS/MS data were consistent matched with reported literature Paola Montoro et al [29]. |
| In the acute toxicity study, the extracts YGE at the dose of 2000 mg/kg have shown no abnormal clinical signs and no mortality. Based on the GHS guideline LD50 values have been selected for in vivo studies. Previous literature reported that the presence of various phytochemical constituents, such as phenolics, saponin, alkaloids and flavonoids containing plants such as Areca semen [48], Cedrus deodar (Roxb) Loud [49] Moringa oleifera [50] Glycyrrhiza glabra [51] Bacopa monniera [52] Platycodi Radix (CK) [53] etc. possessing antiasthmatic activity. Plants containing saponin like Gardenia latifolia, Ginko biloba, Zizipbus polygala and Panax ginseng have been useful in the treatment of bronchitis and asthma. These plants shown interesting results by inhibiting different type of mediator’s viz., leukotrienes, lipoxygenase, cyclooxygenase, platelet activating, phosphodiesterase and cytokine, for treatment of asthma [54]. The use of “Classical” ovalbumin challenge in murine model has significantly increased the understanding of the basic mechanisms of allergic inflammation and asthmatic pathway which is difficult to answer in patients [55]. The basic mechanism behind, ovalbumin model in early phase inhaled allergen bind with antigen resulting initiation of IgE-dependent hypersensitivity reaction driven by mast cell-derived mediators. But later these responses activate antigen-specific T cells due to that secretion of cytokines which activated by CD4+T-lymphocytes due to that damage in airway epithelial cells [56]. In acute sensitization administration of the allergen (OVA) with aluminum hydroxide (Al(OH)3) which promote the Th2 cytokines have been highlighted by numerous studies in murine animal models [57]. The eosinophils contributions in asthma are phenotype in nature, and there is some controversy using ovalbumin induced asthmatic animal models [55]. The present investigation focus to find out the inhibitory potential of Y. gloriosa on targeted cytokines like IL- 6, IL-13, TNF-α and also understand the role of eosinophils in signaling pathways and cellular mechanism. |
| It has been well documented that total and DLC play a central role in asthma. Circulation of eosinophils may also be recruited to site of allergen provocation by coordinate expression of a range of inflammatory cytokines and chemokine secreted from inflammatory cells. Activation of eosinophils and neutrophils causes initiation of IL-8 pathway which induces sputum in asthma and causes acute AHR. In our study, we found, massive significant (p<0.001) increase in number of total cell count and in DLC increased number of eosinophils and neutrophils has been observed in BALF of OVA challenged group. However, these cell numbers, especially eosinophils and neutrophils, significantly (p<0.001) decreased by YGE treatment when compared to all the other extracts and isolated GLM. Therefore, these results provide evidence that YGE treatment attenuating the proliferation and transmigration of eosinophils and neutrophils in the lung. Eosinophils play crucial role in allergic induced asthma by producing eosinophil epoxidase (EPO). This EPO generate free radicals hypobromous acid (HOBr) that damage the lung tissue which contribute the generation of other leukocyte dependant mediator cells [58,59]. So we say that eosinophils amplify the inflammatory response generated by T cells. From the results it could be suggested that Y. gloriosa decreases the inflammation by inhibiting the eosinophils infiltration and neutrophils. An elevated level of NO2 and NO3 in lungs causes formation of NO due to that increases in oxidative stress level. This increased oxidative stress and NO form potent peroxynitrite radical which is a cytotoxic species causes nitrosylation of proteins in the airway walls and damage the tissues [16]. The elevated level of NO2 and NO3 has been observed in BALF of OVA challenged group which was significantly (p<0.001) inhibited by YGE at 200 mg/kg dose level among the entire tested dose and showed most potent inhibitory activity of NO free radicals, further strengthening the antioxidant mechanism. Similar to the deleterious effects of ROS, RNS too have been strongly implicated in the inflammatory response in asthmatic individuals [16]. Since it has been well demonstrated that activation of the nuclear transcription factor (NF- κB) plays an important role in the expression of nitric oxide synthase (iNOS) enhanced NF-κB activation with a subsequent iNOS expression may contribute to the inflammatory process. As previously author reported that Y. gloriosa is the rich source of polyphenolics compound, including resveratrol and number of other stilbenes like (yuccaols (A-E) and gloriosaols (A-E)). These phenolic compounds showed anti-inflammatory activity via inhibiting the NF-κB. NF-κB stimulates synthesis of inducible iNOS, which causes formation of the inflammatory agent nitric oxide [24,25,60]. |
| Our results are strongly supported by other studies indicating the antioxidant capability of Y. gloriosa in suppressing iNOS and inhibition of NF-κB activation. Thus, Y. gloriosa considered as adjuvant in the treatment of asthma. Similarly, the level of TNF-α, IL-6 and IL-13 has performed in the recoverable BALF. The elevated level of TNF-α, IL-6 and IL-13 was observed in the BALF of OVA challenged group which was significantly (p<0.001) attenuated by YGE at 200 mg/ kg demonstrated uniform inhibition of TNF-α level, IL-6 and IL-13 levels in the collected BALF. While, GLM at 30 mg/kg also showed significant (p<0.01) inhibition of IL-13 levels in the collected BALF compare with TNF-α and IL-6 activity. As we discuss above Y. gloriosa contain poly-phenolic compounds which have capability to attenuate the inflammation, via inhibition of prostaglandin production and to decrease the activity of phosphorylation of ERK1/2, cyclooxygenase-2, and various transcription factors including such as NF-κB, STAT3, HIF-1α, and β-catenin [24]. Resveratrol and their derivative also having ability to inhibit protein kinases such as PI3K, JNK, and Akt and production of inflammatory mediators like IFN-γ, TNF-α, COX- 2, iNOS and various interleukin’s [60,25]. These findings suggest that resveratrol and its derivative can be very attractive compounds for treating asthma since these compounds having multiple therapeutic effect like anti-oxidative, anti-inflammatory, vascular protective property and antiasthmatic activity. |
| In additionally, we measured tissue MPO, MDA and TLP activity in OVA exposed balb/c mice. Neutrophil migration in lung tissue, show the presence of MPO enzyme, which is considered as marker for inflammation. MPO is an enzyme present in the azurophilic granules of neutrophils and increase in MPO activity which reflects neutrophil migration to the tissue which is the index of inflammation [42]. The elevated level of MPO has been observed in lung tissue of OVA sensitized mice which was significantly (p<0.001) decreased by YGE at 200 mg/kg showed most significantly reduced (p<0.001) elevated MPO activity. In asthmatic patients recruitment of neutrophils and macrophages at the site of lung tissue injury produces a burst of ROS and RNS which is responsible for tissue damage either directly by oxidizing various cellular components like lipids, proteins and nucleic acids or indirectly by activating various downstream pathogenic pathways involved in complication of allergic asthma [16]. We measured LPO in the form of MDA is reliable marker for ROS [40]. We have observed significant (p<0.001) increase in MDA activity in the lungs of the OVA challenged balb/c mice. Treatment with YGE at 200 mg/kg has significantly (p<0.001) reduced MDA activity, when compared to entire treatment group. Similarly YGE at 200 mg/kg dose level showed most significant reduction (p<0.001) in the elevated TLP levels. At the outset, the extract YGE at 200 mg/kg demonstrated uniform inhibition of MDA level, and a corresponding ability to decrease MPO and TLP activity in the lung tissue. Y. gloriosa extract showed inhibitory activity on NOS, LPO, TLP and MPO implicate the anti-inflammatory activity via antioxidant pathway. Histological examination of the lung sections stained with H and E and PAS further supports the inflammatory cell influx into the bronchioles and alveoli upon OVA challenged. Due to OVA challenge structural changes observed bronchi and globlet cell due to heavy infiltration of inflammatory cells. This may alter the lung function which included peribronchial inflammation, increased vascularity, epithelial fragility, enlarged sub mucosal mucus glands, accumulation of proteinaceous fluid in bronchial wall, increased smooth muscle mass, cuffing and abnormalities in elastin observed in H AND E stained [61]. However, PAS stained were analyzed the globlet cell arrangement feature including goblet cell hyperplasia, hypertrophy and goblet cell metaplastic condition of the bronchi tissue [62]. The histology of lung tissue stained with H and E and PAS reveal that YGE 200 mg/kg effectively reduced this inflammatory cell influx into the lungs. The reduced inflammatory cell influx may be responsible for improved airway clearance with least fluid accumulation and ultimately shows restoration of airway function. |
| Above result showed that, YGE extract are more effective in ovalbumin-induced AHR in balb/c mice. The isolated GLM also shows good activity at the dose of 30 mg/kg dose in the respective parameters but further more assessment is required with combination of synthetic drugs. This antiasthmatic activity exerted by Y. gloriosa due to the presence of multiple constituents like resveratrol and yuccaols (A-E) and gloriosaols (A-E) etc. these poly-phenolics compounds having potential anti-inflammatory and antioxidant activity. Whole Y. gloriosa plant powder contain resveratrol which known for strong anti-inflammatory and antioxidant activity [24,25]. Marzocco et al [63] demonstrated that yuccaols and gloriosaols inhibit inducible iNOS. During inflammatory responses of nitric oxide is a main inflammatory agent. The iNOS is controlled by NF-κB, a transcription factor that regulates gene expression. These phenolic compounds and resveratrol showed anti-inflammatory activity via inhibiting the NF-κB [63]. These YGE extract mixtures act as synergistically and in additive fashion till the concentration attains a threshold dose. After which the very same constituents which are present in the mixture could antagonize the therapeutic effect. These compounds might be responsible for the one or more mechanisms for antiasthmatic activity of YGE extract. In this study, we highlighted the possible mode of action of YGE extract to bring about anti-inflammatory activity in situ to in situ by investigating its efficacy against T-cell-derived cytokine production and subsequent cytokine-mediated cellular infiltration and capacity to block NF- κB mediator release into the local tissues like lung. Nevertheless, the present study has strongly demonstrated the antiasthmatic activity of Y. gloriosa in allergic animal models. |
| Conclusion |
| In conclusion, the results of present investigation observe OVA do play a major role in inducing bronchial dysfunction. The results also reveal that Y. gloriosa inhibits ovalbumin-induced AHR in murine model of asthma. Our results indicated that temporal association between the recruitment of eosinophils and other inflammatory cells like neutrophils, lymphocytes, and macrophages also NO2, NO3, TNF-α, IL-6 and IL-13 are estimated in the BALF and lung tissues of OVA-induced murine asthma model; that MDA, MPO, TLP levels were massively increased; and Y. gloriosa reduced significantly the severity of airway inflammation and the accompanying oxidative stress viz T-cell-derived cytokine production and subsequent cytokinemediated cellular infiltration and capacity to inhibit NF-κB mediator release in lung. GLM showed promising activity at the dose of 30 mg/kg. Our observations support and suggest that Y. gloriosa and isolated GLM molecule is promising candidate as complementary and alternative medicine for AHR patients. It seems clear that there is still great potential in Y. gloriosa for therapy to treat asthma. |
| Conflict of Interest Statement |
| The author(s) declared no potential conflicts of interests with respect to the authorship and/or publication of this article. |
| Acknowledgements |
| This work was financially supported by the JSS University, Mysore. The authors are thankful to Genxbio healthcare Pvt. Ltd., Delhi, India for providing IL- 13 as a gift to carry out the study. The authors are also thankful to Ranbaxy Pvt. Ltd. for providing Dexamethasone as a gift to carry out the study. |
| Declaration of Interest |
| The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. |
| References |
References
- Cookson WO, Moffatt MF (2000) Genetics of asthma and allergic disease. Hum Mol Genet 9: 2359-2364.
- World asthma days (2004) Global Burden of Asthma Report, Embargoed.
- South Asia Network for chronic Disease, Asthma in India.
- Ganesh Kumar S, Premarajan KC, Sarkar S, Sahu K Swaroop, Sahana, et al (2012) Prevalence and factors associated with asthma among school children in rural Puducherry, India. CurrPediatr Res 16: 159-163.
- Saria A, Lundberg MJ, Skoftsch G, Lembeck F (1983) Vascular protein leakage in various tissues is induced by substance P, capsaicin, bradykinin, serotonin, histamine, and by antigen challenge. NaunynSchmiedebergs Arch Pharmacol324:212-218.
- Wardlaw AJ, Dunnette S, Gleich GJ, Collins JV, Kay AB (1988) Eosinophils and mast cells in bronchoalveolar lavage in subjects with mild asthma. Relationship to bronchial hyperreactivity. Am Rev Respir Dis 137: 62-69.
- Sur S, Crotty TB, Kephart GM, Hyma BA, Colby TV, et al (1993) Sudden-onset fatal asthma. A distinct entity with few eosinophils and relatively more neutrophils in the airway submucosa? Am Rev Respir Dis 148: 713-719.
- Romagnani S (2000) The role of lymphocytes in allergic disease. J Allergy ClinImmunol 105: 399-408.
- Peachell P (2005) Targeting the mast cell in asthma. CurrOpinPharmacol 5: 251-256.
- Barnes PJ (2002) Are mast cells still important in asthma?. Rev FrAllergolImmunolClin42:20-27.
- Liu MC, Hubbard WC, Proud D, Stealey BA, Galli SJ, et al (1991) Immediate and late inflammatory responses to ragweed antigen challenge of the peripheral airways in allergic asthmatics. Cellular, mediator, and permeability changes. Am Rev Respir Dis 144: 51-58.
- Chung KF, Barnes PJ (1999) Cytokines in asthma. Thorax 54: 825-857.
- Medina-Tato DA, Watson ML, Ward SG (2006) Leukocyte navigation mechanisms as targets in airway diseases. Drug Discov Today 11: 866-879.
- Wenzel SE, Szefler SJ, Leung DY, Sloan SI, Rex MD, et al (1997) Bronchoscopic evaluation of severe asthma. Persistent inflammation associated with high dose glucocorticoids. Am J RespirCrit Care Med 156: 737-743.
- Moritani C, Ishioka S, Haruta Y, Kambe M, Yamakido M (1998) Activation of platelets in bronchial asthma. Chest 113: 452-458.
- Kirkham P, Rahman I (2006) Oxidative stress in asthma and COPD: antioxidants as a therapeutic strategy. PharmacolTher 111: 476-494.
- Ind PW, Barnes PJ, Brown MJ, Causon R, Dollery CT (1983) Measurement of plasma histamine in asthma. Clin Allergy 13: 61-67.
- Fulkerson PC, Rothenberg ME, Hogan SP (2005) Building a better mouse model: experimental models of chronic asthma. ClinExp Allergy 35: 1251-1253.
- Temelkovski J, Hogan SP, Shepherd DP, Foster PS, Kumar RK (1998) An improved murine model of asthma: selective airway inflammation, epithelial lesions and increased methacholine responsiveness following chronic exposure to aerosolized allergen. Thorax 53:849-856.
- Elwood W, Lötvall JO, Barnes PJ, Chung KF (1991) Characterization of allergen-induced bronchial hyperresponsiveness and airway inflammation in actively sensitized brown-Norway rats. J Allergy ClinImmunol 88: 951-960.
- Bradley BL, Azzawi M, Jacobson M, Assoufi B, Collins JV, et al (1991) Eosinophils, T-lymphocytes, mast cells, neutrophils, and macrophages in bronchial biopsy specimens from atopic subjects with asthma: comparison with biopsy specimens from atopic subjects without asthma and normal control subjects and relationship to bronchial hyperresponsiveness. J Allergy ClinImmunol 88: 661-674.
- Anonymous (2009). The Wealth of India, Raw Materials. New Delhi: National institute of science communication and Information Resourses CSIR XI: 14-16.
- Kirtikar KR, Basu BD, Khare CP(1999) Indian medicinal plant(Edn. 2) Dehradun: International books distributor, Dehradun, India, pp 2502-2625.
- Cheeke PR, Piacente S, Oleszek W (2006) Anti-inflammatory and anti-arthritic effects of Yucca schidigera: a review. J Inflamm (Lond) 3: 6.
- Fulda S (2010) Resveratrol and derivatives for the prevention and treatment of cancer. Drug Discov Today 15: 757-765.
- Thamizhvanan K (2012) Anthelmintic and antimicrobial activity of petroleum ether extract of Yucca gloriosa L. whole plant. Int J of Preclin Pharm Res 3: 20-22.
- Nakano K, Matsuda E, Tsurumi K, Yamasaki T, Murakami K, et al (1988) The steroidal glycosides of the flowers of Yucca gloriosa. Phytochem27:3235-3239.
- Gogoberidze MK, Mamaladze MN, Zambakhidze NE (1992) Steroidal substance in Yucca gloriosaL. cell and tissue culture and their formation during morphogenesis. Plant sci84:201-207.
- Montoro P, Skhirtladze A, Bassarello C, Perrone A, Kemertelidze E, et al (2008) Determination of phenolic compounds in Yucca gloriosa bark and root by LC-MS/MS. J Pharm Biomed Anal 47: 854-859.
- Bassarello C, Bifulco G, Montoro P, Skhirtladze A, Kemertelidze E, et al (2007)Gloriosaols A and B, two novel phenolics from Yucca gloriosa: structural characterization and configurational assignment by a combined NMR quantum mechanical strategy. Tetrahedron63:148-154.
- Bassarello C, Bifulco G, Montoro P, Skhirtladze A, Benidze M, et al (2007) Yucca gloriosa: a source of phenolic derivatives with strong antioxidant activity. J Agric Food Chem 55: 6636-6642.
- Montoro P, Skhirtladze A, Perrone A, Benidze M, Kemertelidze E, et al (2010) Determination of steroidal glycosides in Yucca gloriosa flowers by LC/MS/MS. J Pharm Biomed Anal 52: 791-795.
- Kemertelidze E, Benidze M, Skhirtladze A (2011) Steroidal Glycosides from the Leaves of Yucca GloriosaL.. Bull Georg NatlAcadSci5: 158-163.
- Kokate CK (1988) Practical pharmacognosy (Edn 2) Vallabhprakasham, New Delhi, India. pp 142-159.
- Organization for economic co-operation and development guidelines for testing chemicals (1992). Acute oral toxicity Paris: OECD:98-101.
- Epstein MM (2006) Are mouse models of allergic asthma useful for testing novel therapeutics? ExpToxicolPathol 57 Suppl 2: 41-44.
- Vasconcelos JF, Teixeira MM, Barbosa-Filho JM, Lúcio AS, Almeida JR, et al (2008) The triterpenoidlupeol attenuates allergic airway inflammation in a murine model. IntImmunopharmacol 8: 1216-1221.
- Thompson AB, Teschler H, Wang YM, Konietzko N, Costabel U (1996) Preparation of bronchoalveolar lavage fluid with microscope slide smears. EurRespir J 9: 603-608.
- Toward TJ, Broadley KJ (2000) Airway reactivity, inflammatory cell influx and nitric oxide in guinea-pig airways after lipopolysaccharide inhalation. Br J Pharmacol 131: 271-281.
- Aruoma OI, Halliwell B, Laughton MJ, Quinlan GJ, Gutteridge JM (1989) The mechanism of initiation of lipid peroxidation. Evidence against a requirement for an iron(II)-iron(III) complex. Biochem J 258: 617-620.
- Bradley PP, Christensen RD, Rothstein G (1982) Cellular and extracellular myeloperoxidase in pyogenic inflammation. Blood 60: 618-622.
- Zhang T, Zhang X, Shao Z, Ding R, Yang S, et al (2005) The prophylactic and therapeutic effects of cholinolytics on perfluoroisobutylene inhalation induced acute lung injury. J Occup Health 47: 277-285.
- LOWRY OH, ROSEBROUGH NJ, FARR AL, RANDALL RJ (1951) Protein measurement with the Folin phenol reagent. J BiolChem 193: 265-275.
- Aich J, Mabalirajan U, Ahmad T, Agrawal A, Ghosh B (2012) Loss-of-function of inositol polyphosphate-4-phosphatase reversibly increases the severity of allergic airway inflammation. Nat Commun 3: 877.
- Bousquet J, Bousquet PJ, Godard P, Daures JP (2005) The public health implications of asthma. Bull World Health Organ 83: 548-554.
- Ruth L (2007) A breath of hope. Researchers are combining their efforts to study the genetic and environmental factors in asthma. EMBO Rep 8: 319-321.
- Shantha TR, Vasanth Kumar KG, Gopa Kumar K (2007) Pharmacognosy of tala fruits (Borassusflabellier L.). AryaVaidyan20:199-205.
- Lee JH, Chang SH, Park YS, Her E, Lee HY, et al (2004) In-vitro and in-vivo anti-allergic actions of Arecae semen. J Pharm Pharmacol 56: 927-933.
- Shinde UA, Kulkarni KR, Phadke AS, Nair AM, Mungantiwar AA, et al (1999) Mast cell stabilizing and lipoxygenase inhibitory activity of Cedrusdeodara (Roxb.) Loud. wood oil. Indian J Exp Biol 37: 258-261.
- Mahajan SG, Banerjee A, Chauhan BF, Padh H, Nivsarkar M, et al (2009) Inhibitory effect of n-butanol fraction of Moringaoleifera Lam. seeds on ovalbumin-induced airway inflammation in a guinea pig model of asthma. Int J Toxicol 28: 519-527.
- Ram A, Mabalirajan U, Das M, Bhattacharya I, Dinda AK, et al (2006) Glycyrrhizin alleviates experimental allergic asthma in mice. IntImmunopharmacol 6: 1468-1477.
- Channa S, Dar A, Yaqoob M, Anjum S, Sultani Z, et al (2003) Broncho-vasodilatory activity of fractions and pure constituents isolated from Bacopamonniera. J Ethnopharmacol 86: 27-35.
- Choi JH, Hwang YP, Lee HS, Jeong HG (2009) Inhibitory effect of Platycodi Radix on ovalbumin-induced airway inflammation in a murine model of asthma. Food ChemToxicol 47: 1272-1279.
- Mali RG, Dhake AS (2011) A review on herbal antiasthmatics. Orient Pharm Exp Med 11: 77-90.
- Kumar RK, Herbert C, Foster PS (2008) The "classical" ovalbumin challenge model of asthma in mice. Curr Drug Targets 9: 485-494.
- Kumar RK, Temelkovski J, McNeil HP, Hunter N (2000) Airway inflammation in a murine model of chronic asthma: evidence for a local humoral immune response. Clin Exp Allergy 30: 1486-1492.
- Nials AT, Uddin S (2008) Mouse models of allergic asthma: acute and chronic allergen challenge. Dis Model Mech 1: 213-220.
- Drazen JM, Arm JP, Austen KF (1996) Sorting out the cytokines of asthma. J Exp Med 183: 1-5.
- Riffo-Vasquez Y, Spina D (2002) Role of cytokines and chemokines in bronchial hyperresponsiveness and airway inflammation. PharmacolTher 94: 185-211.
- Park HS, Kim SR, Kim JO, Lee YC (2010) The roles of phytochemicals in bronchial asthma. Molecules 15: 6810-6834.
- Bai TR, Knight DA (2005) Structural changes in the airways in asthma: observations and consequences. ClinSci (Lond) 108: 463-477.
- Olmez D, Babayigit A, Erbil G, Karaman O, Bagriyanik A, et al (2009) Histopathologic changes in two mouse models of asthma. J InvestigAllergolClinImmunol 19: 132-138.
- Marzocco S, Piacente S, Pizza C, Oleszek W, Stochmal A, et al (2004) Inhibition of inducible nitric oxide synthase expression by yuccaol C from Yucca schidigeraroezl. Life Sci 75: 1491-1501.
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2A | Figure 2B | Figure 3 | Figure 4 |
 |
 |
 |
 |
| Figure 5 | Figure 6 | Figure 7 | Figure 8 |
 |
 |
 |
 |
| Figure 9 | Figure 10 | Figure 11 | Figure 12 |
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 14839
- [From(publication date):
specialissue-2014 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 10189
- PDF downloads : 4650
Peer Reviewed Journals
Make the best use of Scientific Research and information from our 700 + peer reviewed, Open Access Journals
