Inhibition of Glutamine Transporter SLC1A5 Reverses Resistance to Erlotinib in Human Non-small Cell Lung Cancer
Received: 30-Jan-2023 / Manuscript No. cmb-23-88159 / Editor assigned: 01-Feb-2023 / PreQC No. cmb-23-88159(PQ) / Reviewed: 15-Feb-2023 / QC No. cmb-23-88159 / Revised: 20-Feb-2023 / Manuscript No. cmb-23-88159(R) / Accepted Date: 22-Feb-2023 / Published Date: 27-Feb-2023 DOI: 10.4172/1165-158X.1000257
Abstract
Purpose: The acquired resistance to erlotinib, an EGFR tyrosine kinase inhibitor (EGFR-TKI), is a great challenge in the targeted therapy of non-small cell lung cancer (NSCLC). Targeting glutamine import could re-sensitize the resistant cancer cells to erlotinib. Our results indicated that the glutamine transporter solute carrier 1 family member 5 (SLC1A5) could be a potential target for the treatment of NSCLC, and the application of GPNA, an SCL1A5 inhibitor, may reverse the acquired resistance to erlotinib.
Materials and Methods: The cell proliferation was measured by crystal violet staining assay. The expression levels of proteins were checked by western blot. The flow cytometry was used to analyze the cell cycle. CO-IP was used to detect the ubiquitination of indicated proteins.
Results: The expression of SLC1A5 in erlotinib-resistant cell lines (PC9ER, HCC827ER) was significantly higher than those in the erlotinib-sensitive cell lines (PC9, HCC827). The proliferation of erlotinib-resistant cells was more dependent on glutamine. Inhibition of SLC1A5 significantly suppressed the growth of NSCLC cells, especially for the erlotinib-resistant cells. Moreover, inhibition of SLC1A5 aggravated the inhibitory efficacy of erlotinib on cell proliferation in erlotinib-resistant cells and induced cell cycle arrest in G0/G1 phase. Mechanistically, SLC1A5 inhibition facilitated PDK1 degradation through the ubiquitin-proteasome pathway, and decreased the expression of p-AKT, Cyclin B1 and CDC42.
Discussion: Glutamine and its transporters play important roles in the development of various numbers of cancers. In this study, we showed that the proliferation of erlotinib resistance NSCLC cells exhibited greater dependence on glutamine. Our study highlighted the role of SLC1A5 in promoting the proliferation of NSCLC cells, especially in erlotinib-resistant NSCLC cells. The effect of SLC1A5 on cell growth in NSCLC was related with its metabolism regulation function and its impact on PDK1-AKT signaling pathway. Our results indicated that SLC1A5 would be a potential target for the treatment of EGFR-TKIs resistant NSCLC.
Keywords
SLC1A5; Erlotinib resistance; Glutamine; Cell cycle; Non-small cell lung cancer
Introduction
Lung cancer is the highest mortality and morbidity of cancer in the world. Non-small cell lung cancer (NSCLC) is the major type of lung cancer, accounting for more than 85% of all lung cancers [1, 2]. Clinically, the great majority of patients with NSCLC have been in advanced stage when diagnosed and have a dismal 5-year survival rate of approximately 15% [3, 4]. Significant advances have been achieved in the management of NSCLC over the last few years. Newer chemotherapy agents, targeted therapy and, more recently, immunotherapy, prolong survival and improve quality of life in patients with good performance status [5-7]. Among them, targeted therapy is pushing the boundary to significantly improve patient outcomes and quality of life [8, 9].
Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs), including erlotinib and gefitinib, can be used as a classical treatment for advanced NSCLCs harbouring certain mutations in the EGFR gene. EGFR-TKIs have a significant effect in improving the overall survival and progression-free survival of patients with advanced NSCLC [10-12]. However, after a period of clinical treatment, many patients with NSCLC would ultimately become resistance to EGFRTKIs, which leads to poor prognosis. Some molecular mechanisms of acquired resistance have been elucidated such as T790M secondary mutation, MET amplification, and so on [13-15]. However, many of the remaining mechanisms of resistance are still unknown. Therefore, more work needs to be done to reveal additional mechanisms and provide new therapeutic targets to reverse drug resistance.
Solute-linked carrier family 1A, member 5 (SLC1A5), also known as ASCT2, encodes a Na+-dependent neutral amino acid transporter [16]. SLC1A5 is a glutamine transporter that plays an important role in glutamine uptake, which is involved in energy supply, macromolecular synthesis, redox homeostasis, and nucleotide biosynthesis [17-19].
More recently, SLC1A5 got more attention since its expression was elevated in various malignancies, including lung cancer, triple-negative basal-like breast cancer, and renal cell carcinoma [20-21]. In NSCLC, SLC1A5 was demonstrated as a major transporter of glutamine and its expression is closely associated with tumor aggressiveness and prognosis [20, 23-25]. Targeting SLC1A5 may affect glutamine uptake, cell growth, ATP generations, autophagy, and cell death in lung cancer cells, which are likely mediated in part by mTOR-AKT signaling [20, 24]. L-γ-Glutamyl-p-Nitroanilide (GPNA) was proposed as an SCL1A5 inhibitor [26] and has been subsequently widely used to this purpose [24, 27, 28], although it is known that it also inhibits other sodiumdependent amino acid transporters [29]. Currently, the role of SLC1A5 in the EGFR-TKIs resistant-NSCLC remains unclear.
PDK1 (3-phosphoionositide-dependent protein kinase 1) is one of downstream effectors of PI3K (phosphoinositide 3-kinase), which belongs to the family of AGC kinases [30]. Which phosphorylates AKT-Thr308 to activate the AKT kinase [31, 32] PDK1 participates in PI3K/AKT pathway in many cellular processes, containing cell cycle, cell survival, oncogenesis, and Epithelial-Mesenchymal Transition (EMT) [33]. Because of the auto-phosphorylation and activation property, there are few studies investigating the direct regulation of PDK1. It has been reported that PDK1 could be modified by monoubiquitination, although the function of PDK1 mono-ubiquitination is still unknown [34].
In several cancers, the expression of PDK1 is regulated via ubiquitination and degradation, then suppresses AKT kinase activity and oncogenic functions [35, 36]. Exploring the regulation of PDK1 protein will provide insights for understanding PI3K- AKT roles in biological process.
In this study, we will investigate the regulatory role of SLC1A5 in erlotinib-resistant NSCLC cells and try to clarify the exact molecular mechanism underlying that effect.
Materials and Methods
Cell culture
The non-small cell lung cancer (NSCLC) cells from ATCC were cultured in RPMI- 1640 (Invitrogen, C11875500BT) supplemented with 10% FBS (Excel, FCS) at 37°C in the presence of 5% CO2. The erlotinib-resistant cell lines (PC9ER and HCC827ER) were obtained and cultured as previously described [37].
Reagents
Erlotinib hydrochloride was obtained from Bio vision Inc. L-Glutamic acid γ-(p-nitroanilide) (GPNA, SLC1A5 inhibitor) and SLC1A5 siRNA were obtained from Santa Cruz Biotechnology. MG132 was purchased from Bio vision (1791–5). Cycloheximide was bought from Sigma (CHX; C7698). Primary antibodies against β-actin (66009– 1-lg), SLC1A5 (20350-1-AP), PDK1 (17086-1-AP), CDK1 (19532-1- AP), CDC42 (10155-1-AP), Cyclin B1 (55004–1-AP) and UB (201–2- AP) were purchased from Proteintech. Primary antibody against Phos- AKT1 (Ser473) (9271S) was purchased from Cell signaling technology. Crystal violet and other analytical grade chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA).
siRNA transfection experiment
For knockdown experiments, the cells were seeded the day before SLC1A5 siRNA transfection to reach 50% confluence at the time of transfection. Trilencer-27 universal scrambled negative control siRNA duplex (SR30004; Origene Technologies) was used as a negative control. The knockdown efficiency was determined by western blot using appropriate antibodies.
Cell growth assay
To carry out cell growth assays under glutamine (Gln) free condition, cells were seeded in 12-well plates at a density of 105 cells per well in 2 ml of complete culture medium. The medium was changed to Gln free medium supplemented with 10% FBS on the following day. Medium was changed every 2 days. After 6 days, the cell number was counted. For cell growth assays with erlotinib or GPNA treatment, cells were seeded in 12-well plates at a density of 105cells per well in 2 ml medium with 10% FBS. On the following day, the medium was changed to RPMI 1640 + 10% FBS + DMSO or RPMI 1640 + 10% FBS + erlotinib, or RPMI 1640 + 10% FBS + GPNA. Medium containing erlotinib or GPNA was changed every 2 days. After 6 days, the cell number was counted. For cell growth assays following knockdown of SLC1A5, cells were transiently transfected with SLC1A5 siRNAs and seeded in 24-well plates at 105 cells per well in 0.5 ml medium containing 10% FBS. The medium was changed every 2 days. Cells were fixed in 4% formaldehyde at the indicated times and stained with 0.1% crystal violet. Dye was extracted with 10% acetic acid and the relative proliferation rate was assessed from the increase in absorbance at 595 nm.
Western blot analysis
PC9, PC9ER, HCC827 and HCC827ER cells were cultured in 100- mm culture dishes at a density of 3 × 105 cells/dish. After treatment, cells were lysed in RIPA buffer (Beyotime, P0013), containing protease inhibitor cocktail (Sigma, P2714) and phenylmethylsulfonyl fluoride (DINGGUO, WB0180). Then, the cell lysates were centrifuged at 10,000 g for 20 min at 4°C. The supernatants were precleared with protein G agarose beads (Roche, 11243233001) for 1 h at 4°C. Then, the supernatants were combined with the indicated antibodies supplemented with protein G agarose beads to incubate overnight at 4°C. On the second day, the immunocomplexes were washed with lysis buffer. The supernatants were suspended with loading buffer and boiled for 10 min.
For western blotting, the proteins were subjected to 10% or 12% SDS-PAGE and transferred to PVDF membranes (Millipore, IPVH00010). The membranes were blocked with 5% skim milk (BD, 232,100) for 1 h at room temperature and incubated with the indicated antibodies overnight at 4°C. The membranes were washed 3 times at room temperature with 1× TBST (20 mM Tris-HCl, 150 mM NaCl, 0.05% Tween-20) for 10 min every time, followed by incubation for 1 h at room temperature with horseradish peroxidase-conjugated antimouse (Thermo Fisher Scientific, 31,430) or 166 anti-rabbit (Thermo Fisher Scientific, 31,460) secondary antibodies. Protein bands were visualized after incubation using the Pro-Light chemiluminescence detection kit (TIANGEN, PA112-01) and a digital gel image analysis system (TANON 5500), and the band intensities were quantified with ImageJ software.
Cell cycle analysis
The cells were seeded in 6-well plates with each treatment. Then, the cells were harvested and washed with 1× PBS, and 70% alcohol in PBS was added to the cells on ice for 2 h; the cells were washed with 1× PBS and re-suspended with 400 μl guava cell cycle reagent (Millipore, 4700- 0160) (PI:585/29). After incubation at 37°C for 15 min, the cells were analyzed using a BD FACS JazzTM Cell Sorter (Becton Dickinson).
Statistical analysis Each value corresponded to the mean ± Standard Error of Mean (S.E.M) of three independent experiments in triplicates. The statistical analyses were performed using GraphPad Prism 5.0 software. Statistical significance was tested by one-way ANOVA, with P-value of less than 0.05 considered statistically significant.
Results
The expression of SLC1A5 was elevated in erlotinib-resistant NSCLC cells
In this study, to examine the involvement of SLC1A5 in erlotinib resistance, we performed western blot to detect its expressions in erlotinib-resistant NSCLC cells (PC9ER and HCC827ER) and the parental erlotinib-sensitive NSCLC cells (PC9 and HCC827). Results showed that SLC1A5 expression was significantly higher in erlotinibresistant cells than in the parental cells (Figure 1A and 1B). When cells were incubated with glutamine free medium or treated with GPNA, an inhibitor of SLC1A5, the cell growth was inhibited from Day 6 in PC9 and HCC827 cells (Figure 1C and 1E), while the growth of PC9ER and HCC827ER cells was inhibited since Day 4 (Figure 1D and 1F). Thus, the growth of erlotinib-resistant cells appears more dependent on SLC1A5 and glutamine metabolism than that of erlotinib-sensitive cells.
Figure 1: The expression of SLC1A5 was elevated in erlotinib resistant NSCLC cells. (A, B) The protein level of SLC1A5 was detected by western blot in PC9, PC9ER, HCC827 and HCC827ER cells. (C-E) Cell growth was determined in PC9, PC9ER, HCC827 and HCC827ER cells treated with glutamine free medium or SLC1A5 inhibitor GPNA (0.5 and 1mM). Values represent mean ± S.E.M. *P < 0.05, **P < 0.01 and ***P<0.001 vs. negative control group.
SLC1A5 inhibition reversed the resistance of HCC827ER to erlotinib
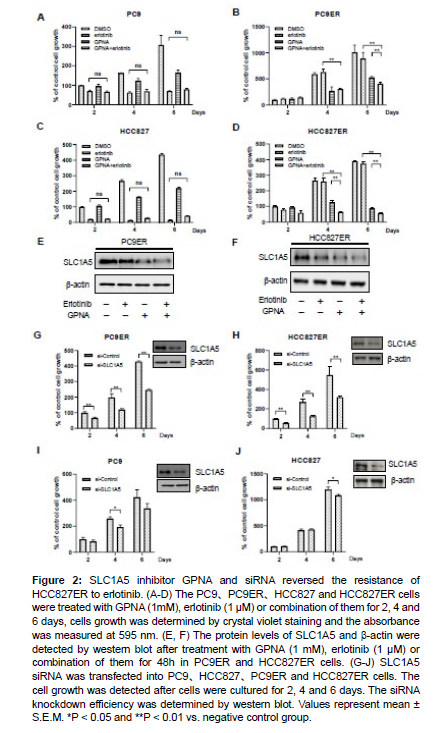
To evaluate the role of SLC1A5 in erlotinib resistance, cells were treated with GPNA or erlotinib. The combination treatment of GPNA and erlotinib showed greater efficiency in cell growth inhibition than the treatment with GPNA or erlotinib alone in PC9ER cells (Figure 2B), and there were no significant differences between the treatment with erlotinib alone and the combination treatment with GPNA and erlotinib in PC9 cells (Figure 2A). Similar results were also observed in HCC827 and HCC827ER cells (Figure 2C and 2D). Moreover, the protein levels of SLC1A5 were examined and the results showed that both GPNA and erlotinib decreased the expression of SLC1A5 (Figure 2E and 2F).
Figure 2: SLC1A5 inhibitor GPNA and siRNA reversed the resistance of HCC827ER to erlotinib. (A-D) The PC9、PC9ER、HCC827 and HCC827ER cells were treated with GPNA (1mM), erlotinib (1 μM) or combination of them for 2, 4 and 6 days, cells growth was determined by crystal violet staining and the absorbance was measured at 595 nm. (E, F) The protein levels of SLC1A5 and β-actin were detected by western blot after treatment with GPNA (1 mM), erlotinib (1 μM) or combination of them for 48h in PC9ER and HCC827ER cells. (G-J) SLC1A5 siRNA was transfected into PC9、HCC827、PC9ER and HCC827ER cells. The cell growth was detected after cells were cultured for 2, 4 and 6 days. The siRNA knockdown efficiency was determined by western blot. Values represent mean ± S.E.M. *P < 0.05 and **P < 0.01 vs. negative control group.
To further demonstrate the role of SLC1A5 in erlotinib resistance in NSCLC cells, the expression of SLC1A5 was knocked down using SLC1A5 siRNA, and knockdown efficiency of SLC1A5 siRNA was determined by western blot. Consistent with the results of cells treated with GPNA, knockdown of SLC1A5 by siRNA significantly inhibited the growth of PC9ER and HCC827ER cells from Day 2 to Day 6 (Figure 2G and 2H), while the growth of PC9 and HCC827 was slightly inhibited by SLC1A5 siRNA (Figure 2I and 2J). These results indicate that SLC1A5 inhibition is a potential way to reverse the acquired resistance to erlotinib.
SLC1A5 inhibition resulted in cell cycle arrest in erlotinibresistant cells
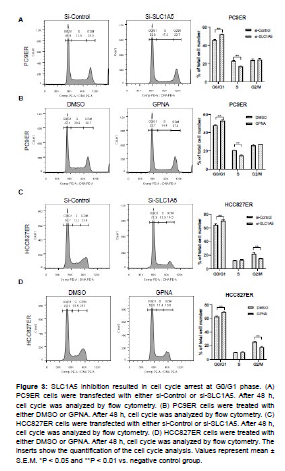
To further examine the role of SLC1A5 in cell proliferation, we detected the effects of SLC1A5 inhibition on cell cycle progression. Knockdown of SLC1A5 by siRNA in PC9ER cells induced an accumulation of cells at G0/G1 phase and a reduction of cells at S phases (Figure 3A). GPNA treatment also arrested the cell cycle at G0/ G1 phase (Figure 3B). Same results were also observed in HCC829ER cells (Figure 3C and 3D). In this context, it is demonstrated that the inhibition of cell proliferation is associated with SLC1A5 mediated cell cycle arrest.
Figure 3: SLC1A5 inhibition resulted in cell cycle arrest at G0/G1 phase. (A) PC9ER cells were transfected with either si-Control or si-SLC1A5. After 48 h, cell cycle was analyzed by flow cytometry. (B) PC9ER cells were treated with either DMSO or GPNA. After 48 h, cell cycle was analyzed by flow cytometry. (C) HCC827ER cells were transfected with either si-Control or si-SLC1A5. After 48 h, cell cycle was analyzed by flow cytometry. (D) HCC827ER cells were treated with either DMSO or GPNA. After 48 h, cell cycle was analyzed by flow cytometry. The inserts show the quantification of the cell cycle analysis. Values represent mean ± S.E.M. *P < 0.05 and **P < 0.01 vs. negative control group.
SLC1A5 promoted erlotinib resistance through PDK1-AKT signaling pathway in NSCLC cells
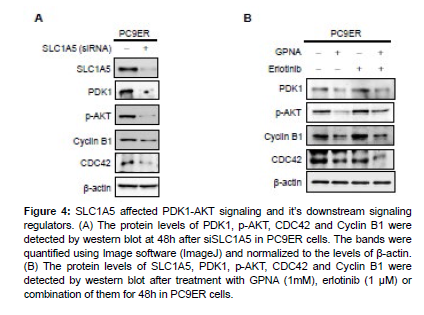
To further investigate the downstream signaling pathways of SLC1A5, the expression of regulators in PDK1-AKT signaling pathway were examined. Intriguingly, SLC1A5 knockdown by siRNA significantly decreased the protein levels of PDK1 and p-AKT. Furthermore, CDC42 and Cyclin B1 were also down-regulated following SLC1A5 knockdown (Figure 4A). As expected, treatment with GPNA alone or the combination of GPNA and erlotinib markedly suppressed the expression of PDK1, p- AKT, CDC42 and Cyclin B1 in PC9ER cells (Figure 4B). These results suggest that SLC1A5 inhibition may decrease the activation of PDK1-AKT signaling pathway, resulting in the down regulation of CDC42 and Cyclin B and cell cycle arrest. Furthermore, for the first time, our study bridges cell cycle proteins with SLC1A5 and erlotinib resistance, and this work might shed new light on cancer treatment by targeting cancer metabolism.
Figure 4: SLC1A5 affected PDK1-AKT signaling and it’s downstream signaling regulators. (A) The protein levels of PDK1, p-AKT, CDC42 and Cyclin B1 were detected by western blot at 48h after siSLC1A5 in PC9ER cells. The bands were quantified using Image software (ImageJ) and normalized to the levels of β-actin. (B) The protein levels of SLC1A5, PDK1, p-AKT, CDC42 and Cyclin B1 were detected by western blot after treatment with GPNA (1mM), erlotinib (1 μM) or combination of them for 48h in PC9ER cells.
SLC1A5 inhibition facilitates PDK1 degradation via the ubiquitin-proteasome pathway
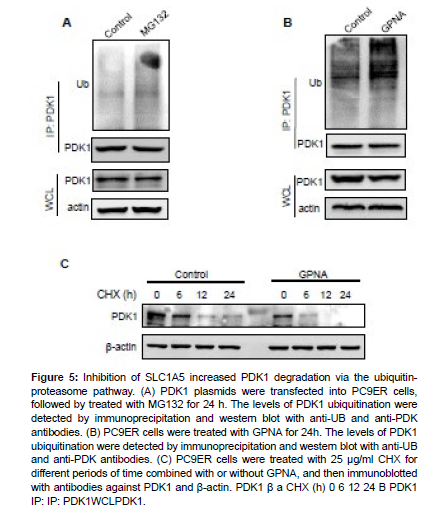
GPNA treatment has been shown to increase protein ubiquitination and proteasomal degradation [38]. Thus, we speculate that SLC1A5 and glutamine metabolism might be involved in PDK1 degradation.
Our results showed that the ubiquitination of PDK1 was promoted by MG132 treatment (Figure 5A), suggesting that PDK1 protein was degraded in a proteasomal degradation pathway. Furthermore, GPNA treatment also promoted the ubiquitination of PDK1 (Figure 5B). To determine whether SLC1A5 regulates the stability of PDK1, we examined the protein levels of PDK1 in the presence of CHX under GPNA-treated condition. As shown in Figure 5C, treatment of GPNA accelerated the degradation rate of PDK1. These results suggest that inhibition of SLC1A5 promote the degradation of PDK1 through the ubiquitin-proteasome pathway, which might contribute to the impact of SLC1A5 on erlotinib response.
Figure 5: Inhibition of SLC1A5 increased PDK1 degradation via the ubiquitinproteasome pathway. (A) PDK1 plasmids were transfected into PC9ER cells, followed by treated with MG132 for 24 h. The levels of PDK1 ubiquitination were detected by immunoprecipitation and western blot with anti-UB and anti-PDK antibodies. (B) PC9ER cells were treated with GPNA for 24h. The levels of PDK1 ubiquitination were detected by immunoprecipitation and western blot with anti-UB and anti-PDK antibodies. (C) PC9ER cells were treated with 25 μg/ml CHX for different periods of time combined with or without GPNA, and then immunoblotted with antibodies against PDK1 and β-actin. PDK1 β a CHX (h) 0 6 12 24 B PDK1 IP: IP: PDK1WCLPDK1.
Discussion
Glutamine and its transporters play important roles in the development of various numbers of cancers [29, 30]. In this study, we showed that the proliferation of erlotinib resistance NSCLC cells exhibited greater dependence on glutamine. Thus, concentrating on factors that potentially affect the glutamine metabolism could be a promising therapeutic strategy for NSCLC. GPNA, a major inhibitor of SLC1A5 along with other members of SNAT family, has been widely used to block glutamine intake [24, 27, 28]. In different erlotinib resistance NSCLC cell lines, we showed that inhibition of SLC1A5 by GPNA significantly enhanced the inhibitory efficacy of erlotinib. Furthermore, GPNA or SLC1A5 siRNA induced cell cycle arrest at G0/ G1 phase. Taken together, our results suggest that SLC1A5 may be a potential target to ameliorate the erlotinib resistance in NSCLC.
The involvement of SLC1A5 in erlotinib response led us to study the mechanism by which SLC1A5 affects the erlotinib efficacy. A previous study reported that SLC1A5 inhibition induced apoptosis [39]. In esophageal cancer cells, SLC1A5 down regulation induced cell cycle arrest in G0/G1 phase, though the regulated mechanism still unknown [40]. In this study, the results of flow cytometry showed that inhibition of SLC1A5 by siRNA or GPNA in erlotinib resistant NSCLC cells arrested the cell cycle at G0/G1 phase. Cyclin B1 expression accumulates at the G2-M cell cycle transition, accompanied with its translocation to the nucleus. We also detected the expression of cellcycle proteins and found that the protein levels of Cyclin B1 and CDC42 were reduced following SLC1A5 inhibition. PI3K/AKT signaling pathway plays multiple roles in the regulation of cell cycle [41]. Accordingly, we consider that the inhibition of SLC1A5 might attribute to the inhibition of PI3K/AKT signaling pathway. Most strikingly, our work revealed that SLC1A5 induced the degradation of PDK1 and down-regulated the expression of p-AKT, which might contribute to the decreased expression of cell-cycle proteins and cell cycle arrest. These findings enriched our understandings of SLC1A5 and provided more experimental evidence for the idea of developing SLC1A5 as anti-tumor target. Conclusions Our study provides insights into understanding the role of SLC1A5 in promoting the proliferation of NSCLC cells, especially in erlotinibresistant NSCLC cells. The effect of SLC1A5 on cell growth in NSCLC was related with its metabolism regulation function and its impact on PDK1-AKT signaling pathway. Our results indicated that SLC1A5 would be a potential target for the treatment of EGFR-TKIs resistant NSCLC. Declarations Conflicts of Interest The authors declare that there are no conflicts of interests. Funding This study was partially supported by the Natural Science Foundation of Jiangxi Province (20212BAB206069), the National Natural Science Foundation of China (82002761, 82260563), Science and Technology Plan Projects of Health Commission of Jiangxi Province (202310001), Science and Technology Plan Projects of Administration of Traditional Chinese Medicine of Jiangxi Province (2022B063) and Science and technology research project of education department of Jiangxi province (GJJ2203555).
Data availability statement
The datasets used and/or analyzed during this study are available from the corresponding author on reasonable request.
Author Contributions
Zhuo Lu, Tao Wang: Project administration, Writing-original draft, Methodology, Validation, Software, Data curation. Zhen Chen: Project administration, Methodology. Shi-Yin Chen: Statistical consultation, Formal analysis. You-Kun Jie, Zhu-Yun Fu: Visualization, Conceptualization, Resources, Writing-review & editing, Supervision.
Acknowledgements
The authors thank Professor Xiao-Lei Li (The first affiliated Hospital of Nanchang University, China) for providing PC9 and PC9ER cells and Professor Shi-Yong Sun (Department of Hematology and Medical Oncology; Emory University School of Medicine and Winship Cancer Institute, USA) for providing HCC827 and HCC827ER cells.
References
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, et al. (2015) Global cancer statistics, 2012. CA Cancer J Clin 65: 87-108.
- McIntyre A, Ganti AK (2017) Lung cancer-A global perspective. J Surg Oncol 115: 550-554.
- Reck M, Heigener DF, Mok T, Soria JC, Rabe KF (2013) Management of non-small-cell lung cancer: recent developments. Lancet 382: 709-719.
- Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA (2008) Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 83: 584-594.
- Maione P, Rossi A, Sacco PC, Bareschino MA, Schettino C, et al. (2010) Advances in chemotherapy in advanced non-small-cell lung cancer. Expert Opin Pharmacother 11: 2997-3007.
- Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, et al. (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13: 239-246.
- Santarpia M, Giovannetti E, Rolfo C, Karachaliou N, González-Cao M, et al. (2016) Recent developments in the use of immunotherapy in non-small cell lung cancer. Expert Rev Respir Med 10: 781-798.
- Chan BA, Hughes BG (2015) Targeted therapy for non-small cell lung cancer: current standards and the promise of the future. Transl Lung Cancer Res 4: 36-54.
- Boolell V, Alamgeer M, Watkins DN, Ganju V (2015) The Evolution of Therapies in Non-Small Cell Lung Cancer. Cancers (Basel) 7: 1815-1846.
- Shea M, Costa DB, Rangachari D (2016) Management of advanced non-small cell lung cancers with known mutations or rearrangements: latest evidence and treatment approaches. Ther Adv Respir Dis 10: 113-129.
- Medical Advisory Secretariat (2010) Epidermal Growth Factor Receptor Mutation (EGFR) Testing for Prediction of Response to EGFR-Targeting Tyrosine Kinase Inhibitor (TKI) Drugs in Patients with Advanced Non-Small-Cell Lung Cancer: An Evidence-Based Analysis. Ont Health Technol Assess Ser 10: 1-48.
- Greenhalgh J, Dwan K, Boland A, Bates V, Vecchio F, et al. (2016) First-line treatment of advanced epidermal growth factor receptor (EGFR) mutation positive non-squamous non-small cell lung cancer. Cochrane Database Syst Rev.
- Nguyen KS, Kobayashi S, Costa DB (2009) Acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancers dependent on the epidermal growth factor receptor pathway. Clin Lung Cancer 10: 281-289.
- Stewart EL, Tan SZ, Liu G, Tsao MS (2015) Known and putative mechanisms of resistance to EGFR targeted therapies in NSCLC patients with EGFR mutations-a review. Transl Lung Cancer Res 4: 67-81.
- Zhang K, Yuan Q (2016) Current mechanism of acquired resistance to epidermal growth factor receptor-tyrosine kinase inhibitors and updated therapy strategies in human non-small cell lung cancer. J Cancer Res Ther 12: C131-C137.
- Utsunomiya-Tate N, Endou H, Kanai Y (1996) Cloning and functional characterization of a system ASC-like Na+-dependent neutral amino acid transporter. J Biol Chem 271: 14883-14890.
- Kanai Y, Hediger MA (2004) The glutamate/neutral amino acid transporter family SLC1: molecular, physiological and pharmacological aspects. Pflugers Arch 447: 469-479.
- Fuchs BC, Bode BP (2005) Amino acid transporters ASCT2 and LAT1 in cancer: partners in crime? Semin Cancer Biol 15: 254-266.
- Pochini L, Scalise M, Galluccio M, Indiveri C (2014) Membrane transporters for the special amino acid glutamine: structure/function relationships and relevance to human health. Front Chem 2: 61.
- Hassanein M, Hoeksema MD, Shiota M (2013) SLC1A5 mediates glutamine transport required for lung cancer cell growth and survival. Clin Cancer Res 19: 560-570.
- van Geldermalsen M, Wang Q, Nagarajah R, Qian J, Harris BK, et al. (2016) ASCT2/SLC1A5 controls glutamine uptake and tumour growth in triple-negative basal-like breast cancer. Oncogene 35: 3201-3208.
- Liu YD, Yang L, An HM, Chang Y, Zhang W, et al. (2015) High expression of Solute Carrier Family 1, member 5 (SLC1A5) is associated with poor prognosis in clear-cell renal cell carcinoma. Sci Rep 5.
- Shimizu K, Kaira K, Tomizawa Y, Sunaga N, Kawashima O, et al. (2014) ASC amino-acid transporter 2 (ASCT2) as a novel prognostic marker in non-small cell lung cancer. Br J Cancer 110: 2030-2039.
- Hassanein M, Qian J, Hoeksema MD, Wang J, Jacobovitz M, et al. (2015) Targeting SLC1a5-mediated glutamine dependence in non-small cell lung cancer. Int J Cancer 137: 1587-1597.
- Hassanein M, Hight MR, Buck JR, Tantawy MN, Nickels ML, et al. (2016) Preclinical Evaluation of 4-[F-392 18]Fluoroglutamine PET to Assess ASCT2 Expression in Lung Cancer. Mol Imaging Biol 18: 18-23.
- Esslinger CS, Cybulski KA, Rhoderick JF (2005) N-gamma-Aryl glutamine analogues as probes of the ASCT2 neutral amino acid transporter binding site. Bioorg Med Chem 13: 1111-1118.
- Bolzoni M, Chiu M, Accardi F, Vescovini R, Airoldi I, et al. (2016) Dependence on glutamine uptake and glutamine addiction characterizes myeloma cells: a new attractive target. Blood 128: 667-679.
- van Geldermalsen M, Wang Q, Nagarajah R, Marshall AD, Thoeng A, et al. (2016) ASCT2/SLC1A5 controls glutamine uptake and tumour growth in triple-negative basal-like breast cancer. Oncogene 35: 3201-3208.
- Broer A, Rahimi F, Broer S (2016) Deletion of Amino Acid Transporter ASCT2 (SLC1A5) Reveals an Essential Role for Transporters SNAT1 (SLC38A1) and SNAT2 (SLC38A2) to Sustain Glutaminolysis in Cancer Cells. J Biol Chem 291: 13194-13205.
- Pearce LR, Komander D, Alessi DR (2010) The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol 11: 9-22.
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, et al. (1997) Characterization of a 3-phosphoinositide- dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol 7: 261-269.
- Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, et al. (2004) PI3K/Akt signalling pathway and cancer. Cancer Treat Rev 30: 193-204.
- Chen J, Yuan CB, Yang B, Zhou X (2021) Baicalin Inhibits EMT through PDK1/AKT Signaling in Human Nonsmall Cell Lung Cancer. J Oncol 2021: 4391581.
- Uras IZ, List T, Nijman SMB (2012) Ubiquitin-Specific Protease 4 Inhibits Mono-Ubiquitination of the Master Growth Factor Signaling Kinase PDK1. PLoS One 7: e31003.
- Jiang Q, Zheng N, Bu L, Zhang X, Zhang X, et al. (2021) SPOP-mediated ubiquitination and degradation of PDK1 suppresses AKT kinase activity and oncogenic functions. Mol Cancer 20: 100.
- Wang R, Huang KL, Xing LX (2022) TRIM35 functions as a novel tumor suppressor in 423 breast cancer by inducing cell apoptosis through ubiquitination of PDK1. Neoplasma 69: 370-382.
- Xie CF, Jin JB, Bao XJ, Zhan W-H, Han T-H, et al. (2016) Inhibition of mitochondrial glutaminase activity reverses acquired erlotinib resistance in non-small cell lung cancer. Oncotarget 7: 610-621.
- Ma H, Wu Z, Peng J, Li Y, Huang H, et al. (2018) Inhibition of SLC1A5 sensitizes colorectal cancer to cetuximab. Int J Cancer 142: 2578-2588.
- Corti A, Dominici S, Piaggi S, Belcastro E, Chiu M, et al. (2019) gamma-Glutamyltransferase enzyme activity of cancer cells modulates L-gamma-glutamyl-p-nitroanilide (GPNA) cytotoxicity. Sci 432 Rep 9: 891.
- Lin J, Yang T, Peng Z, Xiao H, Jiang N, et al. (2018) SLC1A5 Silencing Inhibits Esophageal Cancer Growth via Cell Cycle Arrest and Apoptosis. Cell Physiol Biochem 48: 397.
- Chang F, Lee JT, Navolanic PM, Steelman LS, Shelton JG, et al. (2003) Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia 17: 590-603.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Citation: Lu Z, Wang T, Chen Z, Chen SY, Fu ZY, et al. (2023) Inhibition ofGlutamine Transporter SLC1A5 Reverses Resistance to Erlotinib in Human NonsmallCell Lung Cancer. Cell Mol Biol, 69: 257 DOI: 10.4172/1165-158X.1000257
Copyright: © 2023 Lu Z, et al. This is an open-access article distributed under theterms of the Creative Commons Attribution License, which permits unrestricteduse, distribution, and reproduction in any medium, provided the original author andsource are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2958
- [From(publication date): 0-2023 - Nov 23, 2025]
- Breakdown by view type
- HTML page views: 2561
- PDF downloads: 397