Inhibition of Adipocyte Differentiation and Adipogenesis by the Extract from Sophora japonica Fruit
Received: 26-Nov-2022 / Manuscript No. JOWT-22-81185 / Editor assigned: 28-Nov-2022 / PreQC No. JOWT-22-81185 (PQ) / Reviewed: 12-Dec-2022 / QC No. JOWT-22-81185 / Revised: 16-Dec-2022 / Manuscript No. JOWT-22-81185 (R) / Published Date: 23-Dec-2022 DOI: 10.4172/2165-7904.1000531
Abstract
The world-wide rate of obesity is increasing continuously, representing a serious medical threat since it is associated with a variety of diseases including type 2 diabetes, cardiovascular disease, and numerous cancers. Sophora japonica is used as a traditional herb for medicinal purposes in eastern Asia. However, the anti-obesity effects of S. japonica fruit have not been explored. The aim of this study is to investigate the inhibition of adipocyte differentiation and adipogenesis by an ethanol extract of S. japonica fruit (EESF) in 3T3-L1 pre-adipocytes. Our results demonstrate that EESF suppressed the terminal differentiation of 3T3-L1 pre-adipocytes in a dose-dependent manner, as confirmed by a decrease in lipid droplet number and lipid content through Oil Red O staining. EESF significantly reduced the accumulation of cellular triglyceride, which was associated with a significant inhibition of the levels of pro-adipogenic transcription factors, including PPARγ, C/EBPα and C/EBPβ. In addition, EESF potentially down regulated the expression levels of adipocyte-specific proteins, including aP2 and leptin. In particular, EESF treatment effectively enhanced the activation of the AMPK signaling pathway; however, the co-treatment with compound C, an inhibitor of AMPK, significantly restored the EESF-induced inhibition of pro-adipogenic transcription factors and adipocyte-specific genes. These results indicate that EESF may exert an anti-obesity effect by controlling the AMPK signaling pathway, suggesting that the fruit extract of S. japonica may be a potential anti-obesity agent.
Keywords
Adipogenic transcription factors; Anti-adipogenesis; Lipid droplet; Sophora japonica
Abbreviations
AMPK: AMP-activated protein kinase; ACC: acetyl CoA carboxylase; C/EBPs: cytidine-cytidine-adenosine-adenosinethymidine (CCAAT)/enhancer binding proteins; SREBP1c: sterol response element binding protein 1c; PPARγ: peroxisome proliferatoractivated receptor γ; aP2: adipocyte-specific lipid binding protein; DMEM: Dulbecco's Modified Eagle’s Medium; PBS: phosphatebuffered saline; PMSF: phenymethylsulfonyl fluoride.
Introduction
Obesity is a metabolic disorder that is caused by an abnormal regulation of energy metabolism in the body when energy intake exceeds consumption for a prolonged period of time. Under these conditions the excess energy is stored as fat that accumulates in subcutaneous or abdominal regions in the body [1,2]. Obesity is a known risk factor for diabetes, cardiovascular diseases, neurodegenerative diseases such Parkinson's and Alzheimer's, as well as of various type of cancer including breast, endometrial, gastric, colorectal, and esophageal cancer. Of particular importance in these disease states are the adipokines produced and secreted by adipocytes [3-5]. The World Obesity Atlas 2022, published by the World Obesity Federation, predicts that one billion people globally, including 1 in 5 women and 1 in 7 men, will be living with obesity by 2030. The findings highlight that countries will not only miss the 2025 WHO target to halt the rise in obesity at 2010 levels, but that the number of people with obesity is on course to double across the globe. Obesity is thus receiving growing attention as a grave health problem [6,7]. Treatment of obesity generally involves drug administration and surgery in addition to improvements in lifestyle such as dietary control and exercise. The two most widely used drugs for obesity treatment, Sibutramine and Orlistat, cause not only cardiovascular and gastrointestinal disorders but also headache, severe thirst, constipation, insomnia, and palpitations. Therefore, the development of a therapeutic agent with proven safety and effectiveness is urgently needed [8,9]. One possible approach to this problem is through the identification of natural products with anti-obesity effects.
In general, obesity is known to be caused by two critical factors including adipocyte hypertrophy as a result of the triglyceride accumulation induced by adipogenesis, and adipocyte hyperplasia caused by the proliferation and differentiation of adipocytes [10]. Adipogenesis is the differentiation process by which adipocytes are generated from fibroblastic pre-adipocytes, and is accompanied by changes in cell morphology, gene expression, and hormone sensitivity. Adipogenesis is known to be sequentially influenced by adipogenic transcription factors including sterol response element binding protein 1c (SREBP1c), peroxisome proliferator-activated receptor γ (PPARγ), and cytidine-cytidine-adenosine-adenosine-thymidine (CCAAT)/enhancer binding proteins (C/EBPs) [11-14]. In addition, once adipocytes have been formed through adipogenesis, not only morphological characteristics, such as the triglyceride accumulation seen in white adipocytes, but also the expression of adipocyte-specific genes including adipocyte-specific lipid binding protein (aP2) and leptin is known to occur [15]. AMP-activated protein kinase (AMPK), in particular, is a key regulator of energy homeostasis that is known to control adipogenesis, and consequently has been studied as a drug target for obesity prevention and treatment [16]. The regulation of adipogenesis could be used as an important process to inhibit the inhibition of obesity since it is an essential mechanism that leads to adipocyte production.
Sophora japonica from the Fabaceae family is commonly used as a plant feedstock for the production of flavonoids. This plant is widespread in Asia and is also cultivated in the southern regions of Russia. Its buds, dried flowers, and fruit are medicinal herbs that are used in traditional Korean medicine particularly the dried fruit referred to as Sophorae Fructus [17]. Numerous biological activities of extracts, fractions, and single compounds derived from S. japonica fruits have been reported, such as anti-osteoporosis effects, anti-inflammatory effects, anti-tumor effects, reductions in menopausal symptoms, and hemostatic effects [18-24]. Although functional substances with various pharmacological activities have been reported, studies on their anti-obesity effects and the mechanisms underlying these effects are relatively very rare. The present study thus investigated the anti-obesity effects of an ethanol extract of Sophora japonica fruit (EESF) and how EESF influences adipogenesis, and reports significant findings.
Materials and Methods
Materials
The insulin, dexamethasone, and IBMX used for the differentiation of adipocytes, the Oil Red O used to detect triglyceride formation in differentiated adipocytes, and compound C, which was used as an AMPK inhibitor, were all purchased from Sigma-Aldrich (St. Louis, MO, USA). The primary antibodies used in the protein analysis were purchased from either Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA) or Cell Signaling Technology (Beverly, MA, USA) (Table 1), and the secondary antibodies used in the immunoblotting: peroxidaselabeled donkey anti-rabbit and peroxidase-labeled sheep anti-mouse immunoglobulin, were purchased from Amersham Life Science Corp. (Arlington Heights, IL, USA) (Table 1).
| Antibody | Origin | Company | Catalogue No. |
| PPARγ | Rabbit polyclonal | Santa Cruz Biotechnology, Inc. | SC-7196 |
| C/EBPα | Rabbit polyclonal | Cell Signaling Technology, Inc. | 2295 |
| C/EBPβ | Rabbit polyclonal | Cell Signaling Technology, Inc. | 3087 |
| aP2 | Goat polyclonal | Santa Cruz Biotechnology, Inc. | SC-18661 |
| Leptin | Rabbit polyclonal | Santa Cruz Biotechnology, Inc. | SC-842 |
| AMPK | Rabbit polyclonal | Santa Cruz Biotechnology, Inc. | SC-25792 |
| pAMPK | Rabbit polyclonal | Cell Signaling Technology, Inc. | 2535 |
| ACC | Rabbit polyclonal | Santa Cruz Biotechnology, Inc. | SC-30212 |
| pACC | Rabbit polyclonal | Cell Signaling Technology, Inc. | 3661 |
| Actin | Mouse monoclonal | Santa Cruz Biotechnology, Inc. | SC-47778 |
Preparation of the Ethanol Extract from Sophora japonica Fruit (EESF)
Sophora japonica fruit was obtained from Dongeui Oriental Hospital, Dongeui University College of Korean Medicine (Busan, Republic of Korea). S. japonica fruit (EESF) were air-dried at room temperature and ground to powder using a mechanical grinder. Approximately 100 g of the powder was then added to 2 L ethanol and stirred continuously at 100 rpm for 24 h at room temperature. The resulting extract was filtered and the 74 solvent was removed by rotary vacuum evaporation (N-1000S-W; EYELA, Tokyo, Japan) at Core-Facility Center for Tissue Regeneration, Dong-eui University. The extract was then dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich Chemical Co., St. Louis, MO, USA) to obtain a 100 mg/mL stock solution and stored at 4℃. This solution was diluted to the desired concentration with physiological saline prior to use.
Chromatographic Analysis of EESF
EESF was dissolved in 10 mg/mL 50% methanol; its phytochemical composition was analyzed using high-performance liquid chromatography (HPLC) with an Agilent 1260 series HPLC instrument (Agilent Technologies, San Jose, CA, USA) and an Agilent Extend-C18 column (250 × 4.6 mm). The column was operated in gradient mode with a mixture of 0.1% formic acid in water and acetonitrile as solvents (eluent B: 5-95% in 55 min), a flow rate of 1 mL/min, and an injection volume of 10 μL. The chromatograms were recorded at 320 nm, each peak was in the UV/visible spectrum (200-400 nm).
Liquid Chromatography-Tandem Mass Spectroscopy (LCMS/ MS) Analysis of EESF
An EESF sample was dissolved in 0.1 mg/mL 50% methanol (100 ppm) and analyzed by LC-MS/MS using an AB Sciex QTrap® 4500 system (Sciex, Redwood, CA, USA) coupled to an ultra-performance liquid chromatography system (Shimadzu, Japan) with photodiode array and mass detectors. A Luna Omega Polar C18 column (2.1×150 mm; 1.6 μm; Phenomenex, Torrance, CA, USA) was used as the stationary phase, and 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B) were used as the mobile phase (gradient mode; eluent B: 5-95% in 16 min), with a flow rate of 0.3 mL/min and an injection volume of 2 μL. Components were identified at 320 nm. MS with an electrospray ionization (ESI) source in negative mode was used with the following optimized parameters: curtain gas, 35℃; temperature, 500℃ for gas source 1 and 40℃ for gas source 2; ion spray voltage, 4.5 kV for negative ion mode; delisting potential, 135 V; scan range, m/z 200-1000.
Cell Culture
The mouse fibroblast cell line 3T3-L1 pre-adipocytes used in this study was obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). 3T3-L1 pre-adipocytes were cultured using growth medium containing 90% Dulbecco's Modified Eagle’s Medium (DMEM, Gibco BRL, Grand Island, NY, USA), 10% bovine calf serum (BCS, Gibco BRL, Grand Island, NY, USA) and 1% penicillin and streptomycin (Gibco BRL) at 37℃ in an atmosphere of 5% CO₂. The growth medium was replaced every 48 h to prevent overgrowth of cells.
3T3-L1 Pre-adipocyte Differentiation Induction and Morphological Observation
Prior to differentiation, 3T3-L1 pre-adipocytes were maintained in a growth medium containing 10% BCS and 1% penicillin and streptomycin until they reached confluency. Confluent cells were then cultured using differentiation medium containing 10% fetal bovine serum (FBS, Gibco BRL) and 1% penicillin and streptomycin for a further two days, after which the medium was replaced with the one containing 10 μg/mL insulin, 1 μM dexamethasone, and 0.5 μM IBMX (MDI) for a further two days. Afterwards, the medium was replaced with one containing 10 μg/mL insulin every two days. In addition, to assess inhibition of 3T3-L1 pre-adipocyte differentiation, cells were treated with EESF by replacing the medium with one containing insulin and MDI. The 3T3-L1 pre-adipocytes whose differentiation had been induced as described, were subsequently used in various experimental analyses. To examine the effects of EESF on the morphological changes caused by differentiation of 3T3-L1 pre-adipocytes into adipocytes, the same methods described above were used for differentiation and EESF treatment, after which the cells were observed under ×200 magnification using an inverted microscope (Carl Zeiss, Gottingen, Germany). Images were taken using the Axio Vision program.
Cell Viability Measurement
To examine the effects of EESF on the cell viability of 3T3-L1 preadipocytes, the cells were plated into a 6-well culture plate and cultured until confluent. The confluent cells were then treated with EESF. After 72hrs, the supernatant was removed, and the cells were treated with 0.05% trypsin-EDTA to allow for their detachment from the plate. Next, phosphate-buffered saline (PBS) and a 0.5% trypan blue solution (Gibco BRL) were added to each well. After approximately two minutes, a hemocytometer was used to count the live cells in the sample under an inverted microscope and the relative cell counts were compared. As an alternative way to measure cell viability, the cells were prepared as described above, then after removing the medium, 0.5 mg/mL tetrazolium bromide salt (MTT, Amresco, Solon, OH, USA) was added and the cells were cultured in complete darkness in a CO₂ incubator. After 3 h, the MTT reagent was removed and DMSO was added to the wells to completely dissolve the formazan produced. The dissolved formazan (200 μL) was then transferred to a 96-well plate and the OD at 540 nm measured using ELISA reader (Molecular Devices, Sunnyvale, CA, USA).
Oil Red O Staining and Triglyceride Quantification
Oil red O staining is a dye binding method used to quantify total lipids. The lipid-specific binding allows for a quantitative analysis of the lipids produced by adipocytes. Oil red O staining was carried out to examine the effects of EESF on the production of lipid droplets inside 3T3-L1 adipocytes following induction of differentiation. For both control and 3T3-L1 adipocytes treated with varying concentrations of EESF, the medium was removed and the cells were washed with PBS then fixed for 1 h using 3.7% formalin. After fixing, the cells were washed with 60% isopropanol and treated with the Oil Red O solution at room temperature for 20min to stain the lipid droplets. After the completion of staining, the Oil Red O solution was removed and the cells were washed three times with distilled water. The stained cells were then observed under an inverted microscope, and images were taken using the Axio Vision program. In addition, for a quantitative analysis of the level of inhibition on EESF-induced triglyceride production, the cells were treated with 100% isopropanol to dissolve the Oil Red O and 200 μL of the dissolved solution was transferred to a 96-well plate after which the OD was measured at 500 nm using an ELISA reader.
Western blot Analysis
A western blot analysis was used to examine the effects of EESF on the expression of adipogenic transcription factors and adipocytespecific genes. 3T3-L1 adipocytes were prepared as previously described, after which lysis buffer [25] mM Tris-Cl (pH 7.5), 250 mM NaCl, 5 mM EDTA, 1% NP-40, 1 mM phenymethylsulfonyl fluoride (PMSF), 5 mM dithiothreitol (DTT)] was added and lysis was allowed to proceed at 4℃ for 1 h. The cell lysate was prepared by centrifugation at 14,000 rpm for 30 min. The concentration of proteins in the supernatant was quantified using the Bio-Rad protein quantification agent (Bio-Rad, Hercules, CA, USA) according to the manufacturer’s instructions. Lysate was mixed with an equal volume of Laemmli sample buffer (Bio- Rad) prior to electrophoresis on a sodium dodecyl sulphate (SDS)- polyacrylamide gel. The separated proteins were then transferred to a nitrocellulose membrane (Schleicher and Schuell, Keene, NH, USA) by electroblotting. The nitrocellulose membrane was then blocked with 5% skimmed milk prior to incubation with the appropriate primary antibody at 4℃ for 12 h. The membranes were then washed with PBS-T, and then incubated with a secondary antibody appropriate for each of the primary antibodies at room temperature for 1 h. Immunoreactive bands were detected using an Enhanced Chemiluminescence (ECL) reagent (Amersham Life Science Corp.) and exposure to an X-ray film to allow for an analysis of the expression levels of specific proteins.
Statistical Analysis
All experiments were replicated in three independent experiments. All data were expressed as the mean ± SD and analyzed using the GraphPad Prism software (version 5.03; GraphPad Software, Inc., La Jolla, CA, USA). ANOVA with Bonferroni multiple comparison test was used to confirm significant differences among the group means. A value of p < 0.05 was considered to represent a statistically significant difference.
Results
Chemical Characterization of EESF
We used HPLC and LC-MS/MS with ESI to characterize the EESF. Each major peaks were identified in the HPLC profile of the EESF. As shown in Figure 1A and 1B, EESF Peaks 1, 2, 3, and 4 were tentatively identified as Neophesperidin, Myricetin-3-rhamnoside, Sophoricoside and Genistein, respectively (Figure 1).
Effects of EESF on the Growth of 3T3-L1 Pre-adipocytes
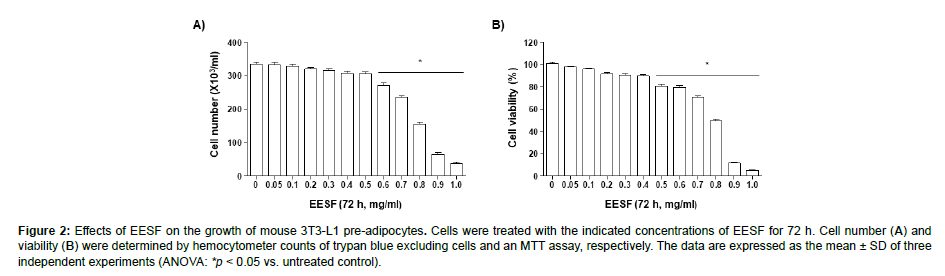
To determine the cytotoxic effects of EESF on 3T3-L1 preadipocytes, the growth and the cell viability of 3T3-L1 pre-adipocytes were examined following EESF treatment. First, to assess growth inhibition by EESF, the cultured cells were treated with varying concentrations of EESF for 72 h, and live cell numbers, determined using a hemocytometer, were compared (Figure 2A). As shown, no significant growth inhibition was apparent up to 0.5 mg/mL EESF, but a dose-dependent increase in growth inhibition was observed above 0.6 mg/mL. An MTT assay was used to determine the effects of EESF on the viability of 3T3-L1 pre-adipocytes under the same conditions described above (Figure 2B). EESF did not induce a significant change in cell viability up to 0.4 mg/mL EESF but a strong inhibitory effect on cell survival was observed above 0.5 mg/mL. Based on these results, EESF was determined to exhibit negligible cytotoxicity up to 0.4 mg/ mL; hence, subsequent experiments were performed with EESF concentrations up to 0.4 mg/mL (Figure 2).
Figure 2: Effects of EESF on the growth of mouse 3T3-L1 pre-adipocytes. Cells were treated with the indicated concentrations of EESF for 72 h. Cell number (A) and viability (B) were determined by hemocytometer counts of trypan blue excluding cells and an MTT assay, respectively. The data are expressed as the mean ± SD of three independent experiments (ANOVA: *p < 0.05 vs. untreated control).
Effects of EESF on the Production of Lipid Droplets
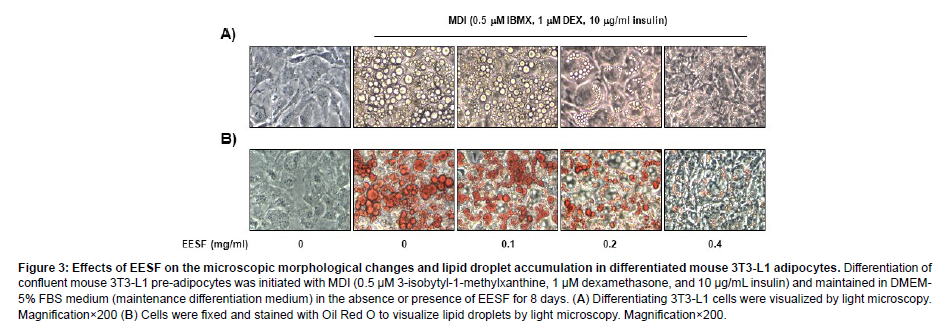
To examine the effects of EESF on the production of lipid droplets during adipogenesis, 3T3-L1 pre-adipocytes which had been induced to differentiate were treated with EESF and the extent of intracellular production of lipid droplets was observed under an inverted microscope before and after Oil Red O staining. As shown in Figure 3, lipid droplets were not produced when differentiation was not induced, but following MDI treatment to induce differentiation, the intracellular production of lipid droplets was readily apparent. The number of lipid droplets was substantially inhibited in a dose-dependent manner upon EESF treatment. These data indicate that EESF inhibits the differentiation of 3T3-L1 pre-adipocytes into adipocytes (Figure 3).
Figure 3: Effects of EESF on the microscopic morphological changes and lipid droplet accumulation in differentiated mouse 3T3-L1 adipocytes. Differentiation of confluent mouse 3T3-L1 pre-adipocytes was initiated with MDI (0.5 μM 3-isobytyl-1-methylxanthine, 1 μM dexamethasone, and 10 μg/mL insulin) and maintained in DMEM- 5% FBS medium (maintenance differentiation medium) in the absence or presence of EESF for 8 days. (A) Differentiating 3T3-L1 cells were visualized by light microscopy. Magnification×200 (B) Cells were fixed and stained with Oil Red O to visualize lipid droplets by light microscopy. Magnification×200.
Effects of EESF on the Production of Triglycerides
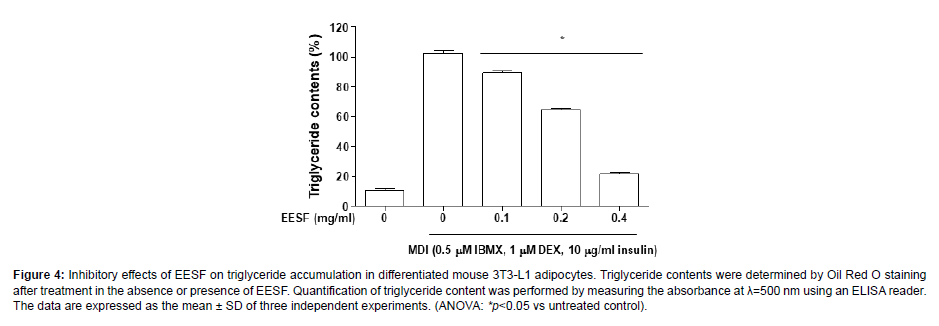
To determine the effects of EESF on the production of triglycerides, the Oil Red O-stained lipid droplets were extracted using isopropanol and the triglyceride content was determined as shown in Figure 4. During the process of MDI-induced differentiation into adipocytes, triglyceride production markedly increased; however, EESF treatment led to a gradual decrease in triglyceride production, with the maximum concentration 0.4 mg/mL EESF producing an approximately 80% inhibition of triglyceride production. The result indicates that there is an inhibitory effect of EESF on the production of triglycerides, and this is correlated with the inhibition of lipid droplet formation (Figure 4).
Figure 4: Inhibitory effects of EESF on triglyceride accumulation in differentiated mouse 3T3-L1 adipocytes. Triglyceride contents were determined by Oil Red O staining after treatment in the absence or presence of EESF. Quantification of triglyceride content was performed by measuring the absorbance at λ=500 nm using an ELISA reader. The data are expressed as the mean ± SD of three independent experiments. (ANOVA: *p<0.05 vs untreated control).
Effects of EESF on the Expression of Adipogenic Transcription Factors and Adipocyte-Specific Genes
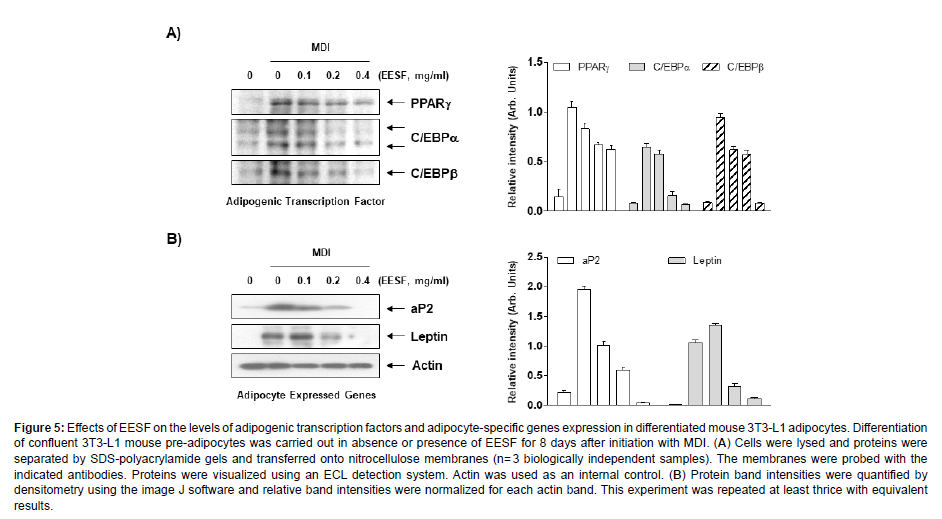
We examined the effects of EESF on the expression of adipogenic transcription factors and adipocyte-specific proteins. As shown in Figure 5A, substantially increased expression levels of PPARγ, C/EBPα and C/EBPβ were observed when the differentiation was induced without EESF treatment; however, upon EESF treatment, a dosedependent decrease in expression levels was observed. EESF treatment also significantly decreased the expression levels of the adipocytespecific proteins aP2 and leptin, as shown in Figure 5B. Collectively, these results indicate that EESF leads to the inhibition of adipogenesis by inhibiting the expression of adipogenic transcription factors, leading to a reduction in the production of lipid droplets and triglycerides and the expression of adipocyte-specific proteins (Figure 5).
Figure 5: Effects of EESF on the levels of adipogenic transcription factors and adipocyte-specific genes expression in differentiated mouse 3T3-L1 adipocytes. Differentiation of confluent 3T3-L1 mouse pre-adipocytes was carried out in absence or presence of EESF for 8 days after initiation with MDI. (A) Cells were lysed and proteins were separated by SDS-polyacrylamide gels and transferred onto nitrocellulose membranes (n= 3 biologically independent samples). The membranes were probed with the indicated antibodies. Proteins were visualized using an ECL detection system. Actin was used as an internal control. (B) Protein band intensities were quantified by densitometry using the image J software and relative band intensities were normalized for each actin band. This experiment was repeated at least thrice with equivalent results.
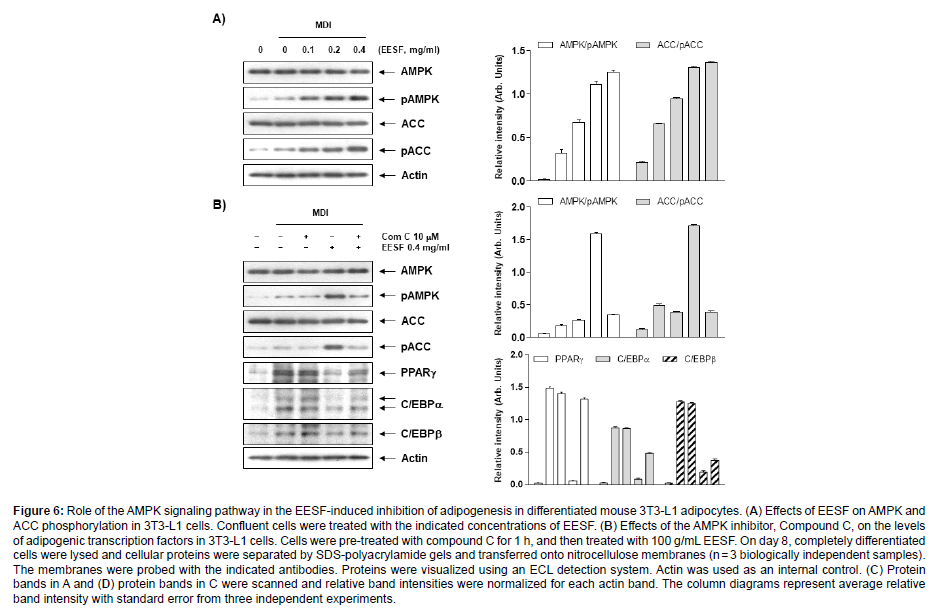
Effects of EESF on the Inhibition of Adipogenesis through the AMPK Signaling Pathway
Finally, we examined whether the AMPK signaling pathway was involved in the EESF-induced inhibition of adipogenesis. First, the expression of ACC acting on AMPK and the step downstream was examined for changes, and as shown in Figure 6A and C, the phosphorylation of AMPK and ACC substantially increased. These results suggest a role for AMPK activation in the EESF-induced inhibition of adipogenesis inhibition. To address this further, the AMPK pathway was suppressed by pretreatment with compound C, an AMPK inhibitor, and this led to an inhibition of the EESF-induced phosphorylation of AMPK and ACC, as shown in Figure 6B and D, while the expression of the adipogenic transcription factors PPARγ, C/EBPα, C/EBPβ and SREBP1c were shown to have increased. Taken together, these data indicate that there is a crucial role played by AMPK activation and the subsequent reduced expression of adipogenic transcription factors in the EESF-induced inhibition of adipogenesis (Figure 6).
Figure 6: Role of the AMPK signaling pathway in the EESF-induced inhibition of adipogenesis in differentiated mouse 3T3-L1 adipocytes. (A) Effects of EESF on AMPK and ACC phosphorylation in 3T3-L1 cells. Confluent cells were treated with the indicated concentrations of EESF. (B) Effects of the AMPK inhibitor, Compound C, on the levels of adipogenic transcription factors in 3T3-L1 cells. Cells were pre-treated with compound C for 1 h, and then treated with 100 g/mL EESF. On day 8, completely differentiated cells were lysed and cellular proteins were separated by SDS-polyacrylamide gels and transferred onto nitrocellulose membranes (n = 3 biologically independent samples). The membranes were probed with the indicated antibodies. Proteins were visualized using an ECL detection system. Actin was used as an internal control. (C) Protein bands in A and (D) protein bands in C were scanned and relative band intensities were normalized for each actin band. The column diagrams represent average relative band intensity with standard error from three independent experiments.
Discussion
The aim of this study was to investigated the anti-adipogenesis effect of EESF using 3T3-L1 preadipocytes. According to the present results of this study, the lipid accumulation and Triglyceride content in the cytoplasm of differentiated 3T3-L1 cells was actively increased and was significantly inhibited by EESF treatment in a concentrationdependent manner. Adipocyte differentiation is accompanied by lipid and Triglyceride accumulation; therefore, the results indicate that EESF treatment significantly suppressed the differentiation of 3T3-L1 preadipocytes.
A previous study reported that the lipid droplets produced during the process of differentiation into adipocytes are in a vesicle surrounded by a phospholipid monolayer. They begin to form when triglyceride and cholesterol ester accumulate in the bilayer of the endoplasmic reticulum so that the layers start to separate [26]. The lipid droplets in mature adipocytes are known to be controlled by the influx of triglycerides due to the action of lipoprotein lipase (LPL) and release of triglycerides mediated by adipose triglyceride lipase (ATGL) and hormone sensitive lipase (HSL). These lipid droplets have been reported to participate in the induction of obesity as well as in diseases such as cancer, arteriosclerosis, and type II diabetes [27,28]. Also, Triglycerides are composed of one glycerol molecule connected to three fatty acid molecules via ester bonds. They account for over 95% of fat ingested as food, and along with glucose, they are used as an important energy source by cells [29]. However, when an excess amount of triglycerides is absorbed and stored by adipocytes they can contribute to obesity and thereby cause various diseases [30]. Triglyceride storage by adipocytes is known to be mediated by triglyceride-rich lipoproteins including chylomicrons and very low density lipoprotein (VLDL) [31]. Therefore, we suggest that there is an inhibitory effect of EESF on the production of triglycerides, and this is correlated with the inhibition of lipid droplet formation.
The differentiation of preadipocytes into adipocytes is regulated by a complicated process that is sequentially coordinated by various transcription factors. Among them, C/EBPβ is induced in the early stages of differentiation and is known to induce the expression of PPARγ and C/EBPα, major regulators of adipogenesis and lipogenesis in the late stage differentiation. In addition, SREBP-1c is involved in lipid metabolism and is a further regulator of fatty acid synthesis enzymes. This transcription factor also enhances the differentiation of preadipocytes and the expression of down-stream genes associated with fatty acid metabolism [32-35]. In addition to the activation of adipogenic transcription factors, trans activating adipocyte-specific genes such as aP2 and leptin are also critically important for the differentiation of pre adipocytes into mature adipocytes [36,37]. Therefore, the inhibition of adipogenic transcription and adipocyte-specific factors would decrease the adipocyte differentiation associated biosynthesis of fatty acids and Triglycerides. In order to investigate the role of EESF in regulating the expression level of the adipogenic transcription factors during adipogenesis of 3T3-L1 cells, we compared their expression levels in the presence and absence of EESF. In addition, EESF also reduced the expression of adipocyte markers aP2 and leptin on the 3T3-L1 cells. aP2 is a carrier protein that can trigger the accumulation of lipid droplets in the cytoplasm of differentiating adipocytes [38,39], and leptin up regulates the adipocyte genes involved in lipid oxidation, enhancing lipid accumulation in the adipocytes [40,41]; therefore, our results also suggest that EESF strongly suppress the de novo synthesis of Triglyceride and differentiation of adipocytes.
Accumulating evidence suggests that the AMPK signaling pathway is a target for the energy balance and metabolic disorders involved in the maintenance of lipid and cholesterol homeostasis. During preadipocyte differentiation and adipogenesis, AMPK is inactivated by lower phosphorylation levels. In addition, AMPK activation by phosphorylation can reduce the degree of obesity by inhibiting adipocyte differentiation by changing the expression and activity of the enzymes and proteins involved in lipid metabolism [42-44]. Furthermore, ACC, a downstream substrate of AMPK, is a rate-limiting enzyme that limits the critical rates in fatty acid synthesis and oxidation, reducing the fatty acid and lipid synthesis to inhibit the onset and progression of obesity [45]. Therefore, AMPK signaling has gained the attention of researchers as a molecular target for fighting obesity. The present study showed EESF markedly elevates the phosphorylation level of AMPK in a dosedependent manner. The ACC phosphorylation was also increased, indicating that the AMPK signaling pathway was activated following EESF administration. In addition, the elevated phosphorylation of ACC and AMPK was inhibited simultaneously by treatment with compound C, as well as the EESF-induced reduction in the expression of transcription factors such as PPARγ, C/EBP members, and SREBP- 1c was reversed by the suppression of AMPK activity by compound C. The results collectively indicate that EESF stimulated AMPK activity, suppressing the adipocyte differentiation regulators and consequently their target lipogenic enzymes and proteins, ultimately resulting in reduced lipid accumulation.
Conclusion
In conclusion, our results indicate that EESF reduced adipocyte differentiation and adipogenesis in the 3T3-L1 pre-adipocytes by suppressing the adipogenic transcriptional factors and their downstream target genes without cytotoxicity. Further, EESF also increased the phosphorylation of AMPK and ACC; however, the artificial blockage of AMPK activity suppressed the inhibitory effects of EESF on the expressions of adipogenic transcriptional factors, demonstrating that EESF has significant anti-adipogenic effect that function via the AMPactivated protein kinase pathway. These findings suggested the possible use of EESF as a therapeutic substance or as a lead in the development of therapeutic substances for the prevention and management of obesity.
Acknowledgement
This work was supported by a grant from the Honam National Institute of Biological Resources (HNIBR) / Nakdonggang National Institute of Biological Resources (NNIBR), funded by the Ministry of Environment (MOE) of the Korea (HNIBR202102119 / NNIBR202202110) and Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (IPET) through Agri-Bio industry Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (IPET121041021HD050).
Conflict of Interest
The authors have no conflicts of interest to disclose.
References
- Jung UJ, Choi MS (2014) Obesity and Its Metabolic Complications: The Role of Adipokines and the Relationship between Obesity, Inflammation, Insulin Resistance, Dyslipidemia and Nonalcoholic Fatty Liver Disease. Int J Mol Sci 15: 6184-6223.
- Longo M, Zatterale F, Naderi J, Parrillo L, Formisano P, et al. (2019) Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int J Mol Sci 20: 2358.
- Gugliucci A (2022) Biomarkers of dysfunctional visceral fat. Adv Clin Chem 109: 1-30.
- Kumari B, Yadav UCS (2018) Adipokine Visfatin's role in pathogenesis of Diabesity and related metabolic derangements. Curr Mol Med 18: 116-125.
- Spiegelman BM, Flier JS (2001) Obesity and the regulation of energy balance. Cell 104: 531-543.
- Nam GE, Kim Y-H, Han KD, Jung J-H, Rhee E-J, et al. (2020) Obesity Fact Sheet in Korea, 2019: Prevalence of Obesity and Abdominal Obesity from 2009 to 2018 and Social Factors. J Obes Metab Syndr 29: 124-132.
- World Health Organization (2022) WHO European Regional Obesity Report 2022. World Health Organization. Regional Office for Europe.
- Joo JK, Lee KS (2014) Pharmacotherapy for Obesity. J Menopausal Med 20: 90-96.
- Ji SY, Jeon KY, Jeong JW, Hong SH, Huh MK, et al. (2017) Ethanol extracts of Mori folium inhibit adipogenesis through activation of AMPK signaling pathway in 3T3-L1 preadipocytes. J Life Sci 27: 155-163.
- Gesta S, Tseng YH, Kahn CR (2007) Developmental origin of fat: tracking obesity to its source. Cell 131: 242-256.
- Alkaladi A, Ali H, Abdelazim AM, Afifi M, Baeshen M, et al. (2020) Raspberry ketone attenuates high-fat diet-induced obesity by improving metabolic homeostasis in rats. Asian Pac J Trop Biomed 10: 18-22.
- Siersbæk R, Baek S, Rabiee A, Nielsen R, Traynor S, et al. (2014) Molecular architecture of transcription factor hotspots in early adipogenesis. Cell Rep 7: 1434-1442.
- Vishvakarma R, Mishra A (2020) Effect of protease inhibitor from Agaricus bisporus on glucose uptake and oxidative stress in 3T3-L1 adipocytes. Asian Pac J Trop Biomed 10: 136-146.
- White UA, Stephens JM (2010) Transcriptional factors that promote formation of white adipose tissue. Mol Cell Endocrinol 318: 10-14.
- Ji S, Doumit ME, Hill RA (2015) Regulation of Adipogenesis and Key Adipogenic Gene Expression by 1, 25-Dihydroxyvitamin D in 3T3-L1 Cells. PLoS One 10: e0126142.
- Yuan HD, Piao GC (2011) An active part of Artemisia sacrorum Ledeb. inhibits adipogenesis via the AMPK signaling pathway in 3T3-L1 adipocytes. Int J Mol Med 27: 531-536.
- Kang SY, Kim KH, Yook HS (2021) Antioxidant Activities of Fermented Sophorae fructus, and Inhibitiory Actions on Tyrosinase and Elastase. J Korean Soc Food Sci Nutr 50: 254-263.
- Gan T, Liu YD, Wang Y, Yang J (2010) Traditional Chinese Medicine herbs for stopping bleeding from haemorrhoids. Cochrane Database Syst Rev 6: CD006791.
- Joo SS, Kang HC, Lee MW, Choi YW, Lee DI (2003) Inhibition of IL-1beta and IL-6 in osteoblast-like cell by isoflavones extracted from Sophorae fructus. Arch Pharm Res 26: 1029-1035.
- Joo SS, Won TJ, Kang HC, Lee DI (2004) Isoflavones extracted from Sophorae fructus upregulate IGF-I and TGF-beta and inhibit osteoclastogenesis in rat bone marrow cells. Arch Pharm Res 27: 99-105.
- Joo SS, Kwon SH, Hwang KW, Lee DI (2005) Improvement of menopausal signs by isoflavones derived from Sophorae fructus in ovariectomized female rats and the antioxidant potentials in BV2 cells. Arch Pharm Res 28: 566-572.
- Lau FY, Chui CH, Gambari R, Kok SH, Kan KL, et al. (2005) Antiproliferative and apoptosis-inducing activity of Brucea javanica extract on human carcinoma cells. Int J Mol Med 16: 1157-1162.
- Lee J, Kim KW, Kim HK, Chae SW, Jung JC, et al. (2010) The effect of Rexflavone (Sophorae fructus extract) on menopausal symptoms in postmenopausal women: a randomized double-blind placebo controlled clinical trial. Arch Pharm Res 33: 523-530.
- Shim JG, Yeom SH, Kim HJ, Choi YW, Lee DI, et al. (2005) Bone loss preventing effect of Sophorae Fructus on ovariectomized rats. Arch Pharm Res 28: 106-110.
- Wang JR, Song XH, Li LY, Gao SJ, Shang FH, et al. (2022) Metabolomic analysis reveals dynamic changes in secondary metabolites of Sophora japonica L. during flower maturation. Front Plant Sci 13: 916410.
- Teresita PB, Cristina VD, Meytha MM, Federico CM, Walid KH (2016) Lipogenic enzymes complexes and cytoplasmic lipid droplet formation during adipogenesis. J Cell Biochem 117: 2315-2326.
- Le Lay S, Dugail I (2009) Connecting lipid droplet biology and the metabolic syndrome. Prog Lipid Res 48: 191-195.
- Yang X, Heckmann BL, Zhang X, Smas CM, Liu J (2013) Distinct mechanisms regulate ATGL-mediated adipocyte lipolysis by lipid droplet coat proteins. Mol Endocrinol 27: 116-126.
- Chen HC, Farese RV Jr (2000) DGAT and triglyceride synthesis: a new target for obesity treatment?. Trends Cardiovasc Med 10: 188-192.
- Goldberg IJ (2012) Triglyceride: one molecule at the center of health and disease. Biochim Biophys Acta 1821: 719-720.
- Park C, Hwang Y, Hwang BS, Shin SY, Cho PY, et al. (2019) Trapa japonica inhibits adipocyte differentiation and adipogenesis through AMPK signaling pathway in 3T3-L1 pre-adipocytes. J Obes Weight Loss Ther 12: 513.
- Lee IS, Kim JP, Ryoo IJ, Kim YH, Choo SJ, et al. (2010) Lanostane triterpenes from Ganoderma lucidum suppress the adipogenesis in 3T3-L1 cells through down-regulation of SREBP-1c. Bioorg Med Chem Lett 20: 5577-5581.
- Liang YC, Yang MT, Lin CJ, Chang CL, Yang WC (2016) Bidens pilosa and its active compound inhibit adipogenesis and lipid accumulation via down-modulation of the C/EBP and PPARγ pathways. Sci Rep 6: 24285.
- Xu X, So JS, Park JG, Lee AH (2013) Transcriptional control of hepatic lipid metabolism by SREBP and ChREBP. Semin Liver Dis 33: 301-311.
- Zhou X, Xu J, Shi Y, Ye JM (2015) Discovery of novel antidiabetic drugs by targeting lipid metabolism. Curr Drug Targets 16: 1372-1380.
- Evans M, Park Y, Pariza M, Curtis L, Kuebler B, et al. (2001) Trans-10, Cis-12 conjugated linoleic acid reduces triglyceride content while differentially affecting peroxisome proliferator activated receptor gamma2 and aP2 expression in 3T3-L1 preadipocytes. Lipids 36: 1223-1232.
- Dong J, Ishimori N, Paigen B, Tsutsui H, Fujii S (2008) Role of modulator recognition factor 2 in adipogenesis and leptin expression in 3T3-L1 cells. Biochem Biophys Res Commun 366: 551-555.
- Tian X, Kang DS, Benovic JL (2014) β-arrestins and G proteincoupled receptor trafficking. Handb Exp Pharmacol 219:173-186.
- Hotamisligil GS, Bernlohr DA (2015) Metabolic functions of FABPs-mechanisms and therapeutic implications. Nat Rev Endocrinol 11: 592-605.
- Harris RB (2014) Direct and indirect effects of leptin on adipocyte metabolism. Biochim Biophys Acta 1842: 414-423.
- Ma X, Lee P, Chisholm DJ, James DE (2015) Control of adipocyte differentiation in different fat depots; implications for pathophysiology or therapy. Front Endocrinol (Lausanne) 6: 1-8.
- He Y, Li Y, Zhao T, Wang Y, Sun C (2013) Ursolic acid inhibits adipogenesis in 3T3-L1 adipocytes through LKB1/AMPK pathway. PLoS One 8: e70135.
- Mitsuhashi K, Senmaru T, Fukuda T, Yamazaki M, Shinomiya K, et al. (2016) Testosterone stimulates glucose uptake and GLUT4 translocation through LKB1/AMPK signaling in 3T3-L1 adipocytes. Endocrine 51: 174-184.
- Pang JS, Choi YS, Park TS (2008) Ilex paraguariensis extract ameliorates obesity induced by high-fat diet: potential role of AMPK in the visceral adipose tissue. Arch Biochem Biophys 476: 178-185.
- Coles CA (2016) Adipokines in healthy skeletal muscle and metabolic disease. Adv Exp Med Biol 900: 133-160.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Lee YK, Oh YT, Seong SH, Kim BR, Im S (2022) Inhibition of Adipocyte Differentiation and Adipogenesis by the Extract from Sophora japonica Fruit. J Obes Weight Loss Ther 12: 531. DOI: 10.4172/2165-7904.1000531
Copyright: © 2022 Lee YK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2667
- [From(publication date): 0-2022 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 2317
- PDF downloads: 350