Research Article Open Access
Inherited Risk Factors for Hemorrhagic Complications in the I Trimester of Pregnancy
Kuznetsova NB2,3 , Donnikov AE1, Bushtyreva IO1,2,3 and Barinova VV2,3*
1Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia, Russia
2Rostov State Medical University, Ministry of Health of Russia, Russia
3State Budget Establishment of Rostov Region Perinatal Center, Russia
- *Corresponding Author:
- Barinova VV
Rostov State Medical University
Ministry of Health of Russia, Russia
Tel: 79289095568
E-mail: victoria-barinova@yandex.ru
Received date: May 26, 2017; Accepted date: May 29, 2017; Published date: May 31, 2017
Citation: Donnikov AE, Kuznetsova NB, Bushtyreva IO, Barinova VV (2017) Inherited Risk Factors for Hemorrhagic Complications in the I Trimester of Pregnancy. J Preg Child Health 4:328. doi:10.4172/2376-127X.1000328
Copyright: © 2017 Donnikov AE et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Pregnancy and Child Health
Abstract
Aim: The purpose of the study was to assess the role of genetic risk factors in the development of retrochorial hematoma. Materials and methods: Genotyping of four polymorphisms of the folate cycle (MTHFR C677T, MTHFR A1298C, MTR A2756G, MTRR A66G) and eight polymorphisms of hemostasis system (F2 G20210A, F5 G1691A, F7 G10976A, F13 G103T, FGB G-455A, ITGA2 C807T, ITGB3 T1565S, SERPINE1-675 5G/4G) was performed to identify genetic risk factors of retrochorial hematoma accompanied by bleeding in the I trimester of pregnancy. The study was conducted among 238 pregnant women with retrochorial hematoma and 67 pregnant women without retrochorial hematoma. Results: The risk of retrochorial hematoma increases in the presence of rare alleles of polymorphic loci of proconvertin F7 gene (sensitivity 62,61 (56,12-68,77), specificity 16,42 (8,49-27,48), PPV 72,68 (66,04-78,66)) and fibrin stabilizing factor F13 gene (sensitivity 73,11 (67-78,63), specificity 5,97 (1,65-14,59), PPV 73,42 (67,31-78,93)), while the presence of polymorphic alleles of these genes in homozygous state is the most unfavorable combination. The chance of developing a retrochorial hematoma increases 5.5 times with the combination of F7 G10976A (genotype G/A and A/A) and F 13 G103T (genotype G/T and T/T). Conclusion: Since the genotype G/A or A/A of G10976A F7 gene polymorphism and genotype G/T or T/T of G103T F13 polymorphism are associated with a predisposition to hypocoagulation.
Keywords
Retrochorial hematoma; Gene polymorphism; Genes of folate cycle; Genes of hemostasis system
Introduction
Retrochorial hematoma (RCH)-is a hemorrhage due to detachment of the chorion surrounding the embryo from the endometrium [1]. RCH is one of the most frequent complications of the first trimester of pregnancy [2].
The conducted studies and meta-analyzes have shown that pregnant women with RCH have higher risk of spontaneous abortion. Moreover, in case of the prolongation of pregnancy, patients with RCH have higher risk of maternal and neonatal complications: Fetal growth restriction, stillbirth, premature rupture of membranes, premature detachment of placenta, premature birth, fetal distress, meconium aspiration [3-7].
The current approach to the prevention and treatment of the above mentioned gestational complications is a symptomatic treatment, due to insufficient knowledge of the RCH pathogenesis. However, new trends of medical genetics, in particular - genomics, that is developing rapidly and engaged in studies of genome structure, the identification of genes, studies of mutations and polymorphisms, offer opportunities for primary, presymptomatic diagnosis of hereditary predisposition to the disease and, consequently, for its early prevention [8].
The polymorphisms of factor V Leiden (F5 G1691A), prothrombin gene (F2 G20210A), protein C and protein S deficiency, polymorphisms of methylenetetrahydrofolate reductase (MTHFR) and antithrombin III deficiency [9] are the most studied. However, involvement of other genetic factors in the pathogenesis of RCH is also of great interest.
Materials and Methods
The study included 305 pregnant women who were examined and treated in the State Budget Establishment of Rostov Region "Perinatal Center" from 2011 to 2016. All the pregnant women were divided into 2 groups: the first group consisted of 238 pregnant women with RCH, the second (control) group consisted of 67 pregnant women with clinically normal pregnancy without RCH according to the ultrasound results in the I trimester. The average age of pregnant women with RCH was 30 ± 4.8 years and 29.4 ± 5.4 years in the control group (Ucriteria of Mann-Whitney r ≥ 0,05), groups were comparable.
All the pregnant women previously gave informed consent to carrying out this research.
The ultrasound examination was performed on the Philips HD 11 ultrasound device. Coccyx-parietal size, heart rate, yolk sac, its medium-internal diameter, chorion localization and its structure, structural features of the uterus and ovaries were evaluated; we precisely evaluated the size and the volume of RCH, its localization and stage of development. Localization of RCH was classified as corporeal (located in the bottom of the uterus) and supracervical (over internal os). The volume of RCH in pregnant women, whom molecular genetic genotyping was performed, varied from 0.022 to 4.86 cm3, median -0.29 cm3, interquartile range (25-75 percentile) -0.05-1.09 cm3.
In order to identify the genetic markers that determine the development of chorionic detachment in the I trimester of pregnancy we performed genotyping of four polymorphisms of the folate cycle (MTHFR (5,10 - methylenetetrahydrofolate reductase) C677T (rs 1801133), MTHFR (5,10 - methylenetetrahydrofolate) A1298C (rs 1801131), MTR (vitamin B12-dependent methionine synthase) A2756G (rs 1805087), MTRR (metionin synthase reductase) A66G (rs 1801394) and eight polymorphisms of hemostasis system (F2 (prothrombin co-factor II) G20210A (rs 1799963), F5 (proaccelerin, labile factor, co-factor V) G1691A (Leiden mutation) (rs 6025), F7 (proconvertin co-factor VII) G10976A (rs 6046), F13 (fibrin stabilizing factor, co-factor XIII) G103T (rs 5985), FGB (β-chain of fibrinogen) G-455A (rs 1800790), ITGA2 (α-2-integrin) C807T (rs 1126643), ITGB3 (platelet glycoprotein IIIA) T1565S (rs 5918), SERPINE1 (PAI-1, plasminogen activator inhibitor 1) - 675 5G/4G (rs 1799768)). To determine the genetic polymorphisms PCR method with the detection of the results in real time, melting curve analysis, qualitative analysis using a kit of reagents "Genetics folate metabolism" and "CardioGenetika Thrombophilia" were applied. Peripheral blood was used as a material for the study.
Tests for compliance with the Hardy-Weinberg’s equilibrium (with p>0,05 balance is carried out) and the identification of the associations between the occurrence of detachment of chorion and genotype were performed with the help of χ2 test (Pearson criteria or Fisher criteria). Differences were considered statistically significant at the achieved level of significance p<0.05.
Results
Genotype distribution for the polymorphic loci of genes MTHFR C677T, MTHFR A1298C, MTR A2756C, MTRR A66G, F2 G20210A, F5 G1691A, F7 G10976A, F13 G103T, FGB G-455A, ITGA2 C807T, ITGB3 T1565C, SERPINE1 - 675 5G/4G corresponded to the expected ones under Hardy-Weinberg equilibrium in the group with chorion detachment and in the control group.
Pragmatism of the studied polymorphisms and their combinations were evaluated by using a multivariate analysis and logistic regression. Statistically significant results were obtained for factors F7 G10976A (genotype G/A or A/A), and F13 G103T (genotype G/T or T/T) (Table 1) among all the studied polymorphisms of genes of hemostasis system and folate cycle.
| Quality indicators | F7 G10976A (genotype G/A or ?/?) | F13 G103T (genotype GT or TT) |
|---|---|---|
| Sensitivity, CI 95% | 62.61 (56.12-68.77) | 73.11 (67-78.63) |
| Specificity, CI 95% | 16.42 (8.49-27.48) | 5.97 (1.65-14.59) |
| Positive Predictive Value (PPV), CI | 72.68 (66.04-78.66) | 73.42 (67.31-78.93) |
| Negative Predictive Value (NPV), CI | 11 (5.62-18.83) | 5.88 (1.63-14.38) |
| Overall Fraction Correct, CI | 52.46 (46.69-58.18) | 58.36 (52.61-63.95) |
| Likelihood ratios for positive test results LR+ | 0.74 | 0.77 |
| Likelihood ratios for negative test results LR- | 2.27 | 4.5 |
Table 1: Hereditary risk factors for retrochorial hematoma.
High sensitivity, low specificity and high positive predictive value were detected for the polymorphism of proconvertin gene F7 G10976A (genotype G/A or A/A) and fibrin stabilizing factor F13 G103T (GT or TT genotype).
To determine the role of possible combinations of polymorphisms in the genesis of RCH (polymorphic variants of different genes encoding proteins of hemostatic system and folate cycle) we conducted the multivariate analysis using logistic regression. The analysis included the polymorphic variants of hemostatic system genes and the genes of folate cycle.
Statistically significant results were obtained only for a combination of F7 G10976A (genotype G/A or A/A) and F13 G103T (GT or TT genotype) among all combinations of polymorphisms. The chance of RCH development increased 5.5 times in combination of F7 G10976A (genotype G/A and A/A) and F13 G103T (genotype GT and TT).
Based on the study of hemostasis system genes polymorphisms, we have developed a mathematical model to assess the risk of RCH development. The analysis included all the studied genetic markers except the least significant. At the final stage of the analysis there were only two variables: the number of rare alleles in the patient's genotype at polymorphic loci G10976A of F7 gene and G103T of F13 gene. The part of dispersion explainable with this model is 36.9% (calculated with the help of Nadelkerkes’ method). The regression equation was:
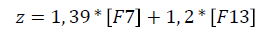
where z: is a classifying discriminant function,
[F7]: the number of A alleles at position 10976 of F7 gene in the patient's genotype,
[F13]: the number of T alleles at position 103 of F13 gene in the patient's genotype.
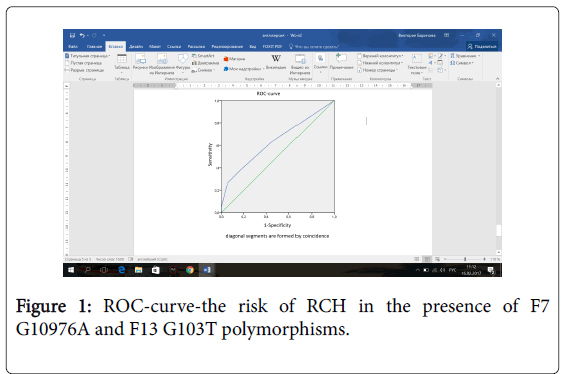
The area under the ROC-curve was AUC=0.64 (0.58-0.71), p=0.035 (Figure 1). Sensitivity and specificity of the model at the point of maximum sensitivity and specificity was 37% and 84%, respectively.
To calculate the probability of RCH development on the basis of the proposed model the following regression equation (logit transformation) can be used:
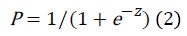
Where, P: is the probability of belonging to a particular class with the resulting value of the function z
E: the base of natural logarithms
Z: the classifying discriminant function
Since the logit transform solves the problem of restrictions on the values for the original dependent variable (probability), then these converted values can be used in a conventional linear regression equation. Based on the fact that the value of the function is the probability that the initial value belongs to a particular class, if the pvalue less than 0.5, then it is judged that the event is not likely to occur; otherwise the event is expected to occur.
The hypocoagulation changes of plasma hemostasis, mainly due to changes in the external pathway of blood coagulation, occur in the development of chorionic detachment. We detected an increase in prothrombin time (PT) and international normalized ratio (INR) (Table 2).
| Indicators of hemostasis | 1st group (with RCH), n=468 | 2d group (control), n=79 |
|---|---|---|
| Fibrinogen, g/l | 3.43 ± 0.88 | 2.96 ± 0.52 |
| (1.6-7.26) | (2.1-5.2) | |
| Thrombin time (TT), s | 18.64 ± 1.40 | 18.86 ± 1.84 |
| (13.0-25.7) | (14.0-22.4) | |
| Prothrombin time (PT), s | 12.5 ± 0.58* | 11.5 ± 0.73 |
| (9.8-15.6) | (10.5-13.5) | |
| Prothrombin index, % | 85.4 ± 4.85* | 93.7 ± 6.9 |
| (71-126) | (83-121) | |
| International normalized ratio (INR) | 1.06 ± 0.05* | 0.98 ± 0.06 |
| (0.84-1.44) | (0.88-1.24) | |
| Activated partial thromboplastin time (APTT), sec | 33.26 ± 3.26 | 31.37 ± 2.94 |
| (17.3-51.9) | (25.0-43.0) | |
| Soluble fibrin monomer complexes, mg% | 4.49 ± 1.63* | 3.3 ± 0.59 |
| (3.0-14.0) | (2.0-4.5) | |
| D-dimer, ng/ml | 739.4 ± 444.1* | 360.5 ± 179.0 |
| (205.0-3696.0) | (92.0-753.0) | |
| Factor VII activity, % | 99.83 ± 5.67** | 124.6 ± 2.75 |
| (84.5-117.9) | (104.7-138.1) | |
| Antithrombin III, % | 90.37 ± 26.39 | 83.34 ± 8.98 |
| (54-208) | (62-99) |
Table 2: Indicators of hemostasis in pregnant women with RCH and in women of the control group in terms of 6-11.6 weeks (Note: The comparison of the groups was performed by using the Mann-Whitney test, r?0,05 *, ** p<0.01).
The activity of factor VII in pregnant women with RCH (99.83 ± 5.67) was significantly lower compared with the activity of factor VII in pregnant women of control group (124.6 ± 2.75).
Discussion
In our research, we have studied polymorphisms of folate cycle genes and hemostatic system genes and also have assessed the state of plasma hemostasis in pregnant women with RCH.
We have not found the association between the polymorphisms of folate cycle genes and RCH development, in contrast to the results of other authors, confirming the link between the C677T polymorphism of MTHFR gene and RCH development [10,11].
Association with chorionic detachment was obtained for gene polymorphism F7 G10976A (genotype G/A or A/A), and F13 G103T (genotype G/T or T/T) among all studied polymorphisms of the hemostatic system (F2 G20210A, F5 G1691A, F7 G10976A, F13 G103T, FGB G-455A, ITGA2 C807T, ITGB3 T1565C, SERPINE1-675 5G/4G).
There are no publications on the relationship of the G10976A polymorphism of the F7 gene to the RCH, but there are data on both the protective role of the G7976A polymorphism of the F7 gene in miscarriages [12] and, conversely, the absence of any connection between the G10976A polymorphism of the F7 gene and the outcome of pregnancy [13].
The effect of the polymorphism F13 G103T (Val34Leu) on the state of blood coagulation system, and its ability to cause hemorrhages, can be explained by the fact that the decrease of the active subunit A of the F13 factor due to the mutation leads to the formation of less stable thrombi, that increases the bleeding risk [14]. We have not met any data on the role of F13 polymorphism G103T in the genesis of bleeding in the first trimester of pregnancy.
Thus, the results of our study suggest that the presence of polymorphic loci of proconvertin genes F7 G10976A and fibrin stabilizing factor F13 G103T, is a hereditary risk factor for bleeding in pregnant women, since the polymorphic variants of these genes affect the systemic hemostasis leading to hypocoagulation state, prolonging the time of formation of prothrombinase (proconvertin polymorphism) and forming less stable thrombi (fibrinase polymorphism). Hypocoagulation by external mechanism was confirmed in our study by the changes in the coagulogram in pregnant women with RCH. Speaking about the hereditary predisposition to bleeding, of course, one should take into account the bleeding of mucous membranes, bleeding during operations and the duration of bleeding in physiological processes, for example, the duration of menstruation. Bleeding of the examined patients with RCH can be considered as a manifestation of the systemic effect at the local level (at the level of the uterine-chorial region).
Conclusion
According to the results of this research, we can state that the presence of rare alleles of polymorphic loci of the genes proconvertin (F7) and fibrin stabilizing factor (F13) in the homozygous state is the most unfavorable combination for the development of RCH in pregnant women. Since these genotypes are associated with a predisposition to hypocoagulation, there is reason to claim that a genetically determined tendency to bleeding is a significant risk factor for the development of RCH.
These combinations of hemostasis system genes polymorphisms (G10976A of the F7 gene and G103T of the F13 gene), in the presence of which, pregnant women are at increased risk of RCH developing, should be considered as an important finding.
The above data, i.e., the presence of polymorphic loci of genes responsible for the development of hypocoagulation and their combination, confirm that bleeding in pregnant women with RCH is hereditary and prove the genetic predisposition to hypocoagulation in pregnant women with RCH.
References
- Pri-paz SM (2012) Placenta previa, obstetric maging. Elsevier-Saunders.
- Asato K, Mekaru K, Heshiki C (2014) Subchorionic hematoma occurs more frequently in in vitro fertilization pregnancy. Eur J Obstet Gynecol Reprod Biol 181: 41-44.
- Tuuli MG, Norman SM, Odibo AO (2011) Perinatal outcomes in women with subchorionic hematoma: A systematic review and meta?analysis. Obstet Gynecol 117: 1205-1212.
- Kyser KL (2012) Meta-analysis of subchorionic hemorrhage and adverse pregnancy outcomes. Proc Obstet Gynecol 2: 4.
- Chhabra A, Lin EC (2014) Subchorionic hemorrhage imaging.
- McPherson JA, Odibo AO, Shanks AL (2013) Adverse outcomes in twin pregnancies complicated by early vaginal bleeding. Am J Obstet Gynecol 208: 56.
- Palatnik A, Grobman WA, Palatnik A (2015) The relationship between first-trimester sub chorionic hematoma, cervical length and preterm birth. Am J Obstet Gynecol 213: 1-4.
- Baranov VS (2009) Genetic passport-the basis of individual and predictive medicine. Saint-petersburg, p: 528.
- Kutteh WH, Davenport WB (2014) Inherited thrombophilias and adverse pregnancy outcomes: A review of screening patterns and recommendations. Obstet Gynecol Clin North Am 41: 133-144.
- Panfilova OY (2012) Clinical significance of the detection of thrombophilia, markers of inflammation and endotheliopathy for the prediction and prevention of repeated premature detachment of the normally located placenta and chorion detachment. Obstet Gynecol Sci 59: 233-237.
- Heller DS, Rush D, Heller DS (2003) Sub chorionic hematoma associated with thrombophilia: Report of three cases. Baergen Pediatr Dev Pathol 6: 261-264.
- Seremak-Mrozikiewicz A, Drews K, Kurzawi?ska G (2009) The connection between Arg353Gln polymorphism of coagulation factor VII and recurrent miscarriages. Ginekol Pol 80: 8-13.
- Zonouzi P, Chaparzadeh N, Ghorbian S (2013) The association between thrombophilic gene mutations and recurrent pregnancy loss. J Assist Reprod Genet 30: 1353-1359.
- Wells PS, Anderson JL, Scarvelis DK, Doucette SP, Gagnon F (2006) Factor XIII Val34Leu variant is protective against venous thromboembolism: A huge review and meta-analysis. Am J Epidemiol 164: 101-109.
--
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 5519
- [From(publication date):
June-2017 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 4632
- PDF downloads : 887