Research Article Open Access
Infra-red Spectral analysis of Taxol Produced by Different Species of Pestalotiopsis
Kathiravan G1*, Sri Raman V2, Rajangam B3 and Rajasekar A11Assistant Professor, Department of Biotechnology, Vel’s University, Chennai, Tamilnadu, India
2Deparment of Chemistry, Vel’s University, Chennai, Tamilnadu, India
3Centre for Advanced studies in Botany, University of Madras, Guindy Campus, Chennai, Tamilnadu, India
- *Corresponding Author:
- Dr. G. Kathiravan
Assistant Professor, Department of Biotechnology
Vel’s University, Chennai-600 117, Tamilnadu, India
Tel: 91-9445105016
E-mail: gkathir72@gmail.com
Received date: September 08, 2014; Accepted date: September 22, 2014; Published date: September 25, 2014
Citation: Kathiravan G, Sri Raman V, Rajangam B, Rajasekar A (2014) Infra-red Spectral analysis of Taxol Produced by Different Species of Pestalotiopsis. J Anal Bioanal Tech 5:205 doi: 10.4172/2155-9872.1000205
Copyright: © 2014 Kathiravan G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Twenty species of Pestolotiopsis were isolated from different hosts and screened for the production of an anticancer drug taxol. Taxol production was confirmed in all the 20 fungi by ultra violet (UV) spectroscopic analysis, and Infra-red Spectrum analysis (IR). The taxol produced by the fungi was compared with that of standard taxol. The taxol from the selected fungi were identical with authentic taxol.
Keywords
Taxol; Anti-cancer; Fungi; Pestalotiopsis; IR – Spectrum
Introduction
Coelomycetes group were mainly isolated from the tropical and temperateregions, less so from the Antartic and Artic but they are capable of growing, reproducing and surviving in a wide variety of ecological situations. They are commonly found and recovered from different cultivated and uncultivated soils of different types, leaf litter and other organic debris from both natural [1]. There are reports of some members of Colemycetes like Pestalotiopsis microspore [2,3] which possess anti cancerous drugs, and Pestalotipsis sp. has been reported to be anti-diabetic [4].
Taxol
Among the various anticancer drugs of plant origin, taxol is considered as the most important chemotherapeutic agent discovered so far. Taxol was approved in 1992 by the US Food and Drug Administration for the treatment of refractory ovarian and breast cancer. It was shown to be active against a variety of cancers such as lung, gastrointestinal, neck and head as well as malignant melanoma [5].
Taxol, the world’s first billion-dollar anticancer drug
The most important member of the clinically useful natural anticancer agent is paclitaxel (Taxol®), which was first extracted from the bark of western yew (Taxus brevifolia) [6]. This compound is the world’s first billion-dollar anticancer drug, and it is used to treat a number of other human tissue-proliferating diseases as well.
Taxol from fungi
By the early 1990’s, however, fungi had been isolated from many of the world’s representative yew species. After several years of effort, the search for novel sources of Taxol has led to the isolation of a novel endophytic fungus (Taxomyces andreanae) colonizing the inner bark of the yew tree (Taxus brevifolia) which is capable of producing Taxol and other taxanes de novo when grown in semi-synthetic liquid medium [7]. However, the yield of taxoids from T. andreanae is very low (24- 50 ng/l). This study also suggests that improved culture techniques, addition of activators / elicitors and application of genetic engineering methods may result in enhanced production of taxoids by this endophytic microbe and permit commercialization of T. andreanae for Taxol production.
Strobel et al. [8] (1996) studied Taxol production from Pestalotiopsis microspora, an endophytic fungus of Taxus wallachiana, and reported that some of the most commonly found endophytes of the world’s yews are Pestalotiopsis spp. Li et al. [9] (1996) also reported that one of the most commonly isolated endophytic species is Pestalotiopsis microspora. Strobel et al. [10] (1996) isolated Pestalotiopsis guepini from extremely rare and previously thought to be extinct, Wollemi Pine (Wollemia nobilis), which has been proved to produce taxol. Subsequently, Li et al. [9] (1996) isolated an endophytic fungus Periconia sp. from Torreya grandifolia. Also, quite surprisingly, a rubiaceous plant – Maguireothamnus speciosus, yielded a novel fungus Seimatoantlerium tepuiense that produces taxol.
In recent years, other investigators have also made observations on taxol production by endophytes. Caruso et al. [11] (2000) detected taxol production from 15 out of 150 fungal strains isolated as endophytes from the internal tissues of woody branches, shoots and leaves of different plants belonging to the genus Taxus spp. The production of taxanes in a few cases reached 50-100 ng/l. Wang et al. [12] (2000) studied taxol production from Tubercularia sp. isolated from Southern Chinese yew (Taxus mairei) in the Fujian province of southeastern China. In addition to that, Shrestha et al. [13] (2001) showed a new report in the production of taxol from three different endophytic fungi isolated from the Himalayan yew Taxus Wallachiana viz., Sporormia minima, Trichothecium sp. and an unidentified dimorphic fungus were confirmed by different analytical and immunoassay methods. It can be inferred that fungi more commonly produce greater quantity of Taxol than higher plants; and the distribution of those fungi producing Taxol is worldwide and not confined to endophytes specific host are from specific geographic location. Thus, it may be thought that taxol had its origin in certain fungi and ultimately, if there is any lateral gene transfer, it may have been in the direction of the microbe to the higher plants [8] (Strobel, 1996). In vitro taxol production from Pestalotiopsis breviseta, Coelomycetous fungi, first reported by Kathiravan and Sriraman (2010) [14,15], was confirmed by Ultra Violet, Infra-red, High performance liquid chromatography, Nuclear magnetic resonance and LC MASS spectroscopy [16]. The presence of Taxol was identical with that of authentic taxol. Similar work was carried out by Kathiravan and Muthumary, 2009 [17]; Kumaran et al., 2008 a, b [18,19]; Kathiravan et al., 2013 [20]; Gowri et al., (2011) [21].
Materials and Methods
The general laboratory techniques followed in the course of the present investigation were as outlined by Booth (1971) [22]. The test fungi used in the present study were grown in 2L Erlenmeyer flasks containing 500 ml MID medium supplemented with 1 g Soytone L-1 for taxol production. Three mycelial agar plugs (0.5 cm) were used as inoculum. The organisms were grown at 24 ± 2°C under static condition for 3-4 weeks [23].
Isolation methods
The samples were placed on suitable agar growth media like PDA and Oatmeal Agar amended with antibiotics. Plates were incubated at a temperature close to that of the collection site. The length of incubation depends on the growth rate of the fungal propagules. Fungal species were isolated when separate colonies appeared. Appendage and sheaths were observed using differential interference or phase contrast microscopy. Measurements of the various structures were made on fresh mounts. Dimensions of these structures were taken from at least 100 individuals. All the cultures were deposited in Madras University Botany Laboratory (MUBL). Microbial Type Culture Collection, Chandigarh (MTCC).
Culture media [8] (Strobel et al., 1996)
MID medium was supplemented with Soytone - 1.0 g, Sucrose - 30.0 g/l, Ammonium tartrate - 5.0 g, Yeast extract - 0.5 g, Ca2 (NO) 3 - 280 mg, KNO3 - 80 mg, KCl - 60 mg, MgSO4 - 360 mg, NaH2PO4 - 20 mg, H3BO3 - 1.4 mg, MnSO4 - 5.0 mg, ZnSO4 - 2.5 mg and KI - 0.7 mg.
Extraction of taxol
Extraction of taxol was performed according to Strobel et al. [8] (1990). After incubating the culture for 3-4 weeks the culture filtrate was passed through four-layered cheesecloth. In order to avoid fatty acid contamination of taxol, 0.25 g of NaCO3 was added to the filtrate. The culture fluid was extracted with two equal volumes of methylene chloride and the organic phase was evaporated to dryness under reduced pressure at 35°C.
Column chromatography
A 1.5 × 30 cm column of silica gel was loaded with the crude sample dissolved in methylene chloride. Elution of the sample was done in a stepwise manner with solvent system as 70 ml of 100% methylene chloride, 20:1 v/v methylene chloride : ethylacetate, 10:1 v/v methylene chloride : ethylacetate, 6:1 v/v methylene chloride : ethylacetate, 3:1 v/v methylene chloride : ethylacetate and 1:1 v/v methylene chloride : ethylacetate. Fractions having the same mobility as that of the authentic Taxol were combined and evaporated to dryness. The residue was subjected to thin layer chromatography.
Thin layer chromatography (TLC) analysis
TLC analysis was carried out on Merck 1 mm (20 × 20 cm) silica gel plate developed with solvent A (Chloroform : Methanol, 7:1 v/v) followed by solvent B (Chloroform : Acetonitrile, 7:3 v/v), solvent C (Ethyl acetate : 2-propanol, 95:5,v/v) solvent D (Methylene chloride : Tetrahydrofuran, 6:2 v/v) and solvent E (Methylene chloride : Methanol : Dimethylformamide, 90:9:1, v/v/v) respectively. The area of the plate containing putative taxol was carefully removed by scraping off the silica at the appropriate Rf and eluted with acetonitrile. Taxol was detected with 1 % w/v vanillin / sulphuric acid reagent after gentle heating. It appeared as a bluish spot that faded to dark grey after 24 h.
Ultra Violet (UV) spectroscopic analysis of taxol
The purified sample of taxol was analyzed by UV absorption, dissolved in 100 % methanol at 273 λmax and compared with authentic taxol.
Infera Red spectroscopic analysis of taxol
The purified TAXOL was ground with IR quality potassium bromide (1:10) pressed into discs under vacuum using spectra lab Pelletiser and the spectrum was recorded (4000– 500 cm−1 nm) in a Burker 17S 85 FTIR Spectrophotometer.
Results and Discussion
Colony morphology
Cultures were growing faster on the Potato dextrose agar , (Plate 1 and Table 1) Czpek Dox agar and Oate meal agar medium and the diameter of colony reached 80 mm after 5 days incubation at 30 ± 2°C. The colony was flat and velvety to woolly and was covered by brown to white hyphal area, short and long. The surface was light brown to white. Reverse side was typically pale brown to black due to melanin production. Out of 20 (a, f, h, and n) which are light brown others produced white colonies.
| S.No | Fungal Name | MUBL NO/ MTCC NO | Growth medium and mycelia growth (mm) | ||
|---|---|---|---|---|---|
| PDA | OMA | CDA | |||
| 1 | Pestalotiopsis acaciae | 453 | 65 | 60 | 54 |
| 2 | P. adusta | 469/4166 | 80 | 78 | 62 |
| 3 | P. breviseta | 470 | 80 | 77 | 61 |
| 4 | P. calabae | 471 | 67 | 58 | 50 |
| 5 | P. clavispora | 455, | 62 | 56 | 52 |
| 6 | P. coangae | 454 | 69 | 60 | 58 |
| 7 | P. coffeae | 472/4241 | 71 | 65 | 62 |
| 8 | P. conigena | 456 | 68 | 60 | 58 |
| 9 | P. eriobotryofolia | 457 | 60 | 52 | 48 |
| 10 | P. foedaris | 459 | 67 | 54 | 48 |
| 11 | P. fibricola | 458/3445 | 69 | 56 | 50 |
| 12 | P. glandicola | 460 | 73 | 68 | 60 |
| 13 | P. japonica | 461 | 80 | 78 | 64 |
| 14 | P. matildae | 462 | 75 | 68 | 60 |
| 15 | P. oleandri | 463 | 72 | 64 | 59 |
| 16 | P. paeoniae | 464 | 73 | 66 | 60 |
| 17 | P. paciseta | 465 | 76 | 68 | 56 |
| 18 | P. taxica | 467 | 74 | 69 | 58 |
| 19 | P. torulosa | 466 | 71 | 64 | 59 |
| 20 | P. zalbrukneriana | 468 | 70 | 66 | 59 |
Table 1: Fungal growth on different media.
Growth rate
Among the three medium Potato Dextrose Agar, Czapek Dox Agar and Oat Meal Agar used. PDA medium was good for producing mycelia, followed by OMA and CDA, respectively. After 5 days of growth, among the twenty species, only three species showed maximum of growth (80 mm) in PDA, 70 mm growth in OMA and CDA was showed minimum growth (Table 1).
Infera Red – Results (Tables 2 & 3)
| Chemical group |
1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
| Methyl group | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Methylene group | + | - | - | - | - | - | - | - | - | - | + | - | + | - | + | + | - | - | + | + |
| Aromatic group | - | - | + | + | + | + | + | + | + | + | + | + | - | + | + | + | + | - | - | - |
| Amide Group |
- | - | - | - | - | - | - | - | - | - | - | - | + | - | + | + | - | - | + | - |
| Alcohol | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Ester | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | + |
| Ketone | + | - | - | - | + | + | - | + | + | - | - | - | - | + | - | - | - | - | - | + |
| Carboxylic acid | + | - | + | + | + | + | + | + | - | + | - | - | - | - | - | - | - | + | - | - |
| Cyclic group Cyclic propane |
- | + | - | - | - | - | - | - | + | - | + | + | - | + | + | + | + | - | + | + |
| C-N Linkage | + | + | + | - | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Combination and Overtone | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | - | - | + | - | + |
Table 2: Infrared – spectroscopy analysis of Chemical groups of 20 Different Pestalotiopsis spp.
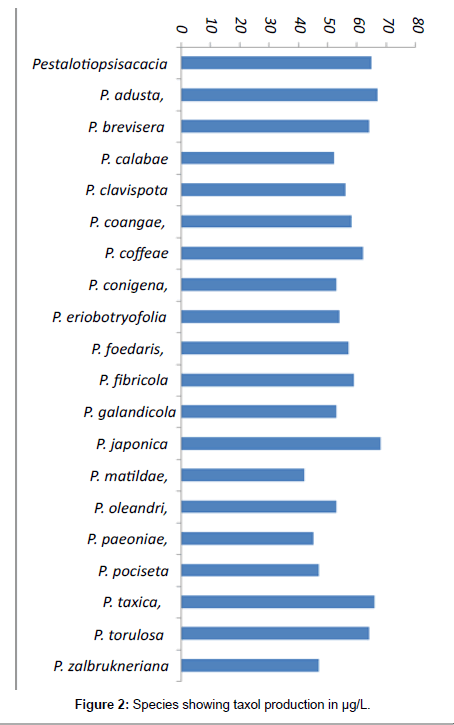
| S.No | Fungal Name | Taxol content μg/L |
|---|---|---|
| 1 | Pestalotiopsis acacia | 65 |
| 2 | P. adusta | 67 |
| 3 | P. breviseta | 64 |
| 4 | P. calabae | 52 |
| 5 | P. clavispora | 56 |
| 6 | P. coangae | 58 |
| 7 | P. coffeae | 62 |
| 8 | P. conigena | 53 |
| 9 | P. eriobotryofolia | 54 |
| 10 | P. foedaris | 57 |
| 11 | P. fibricola | 59 |
| 12 | P. glandicola | 53 |
| 13 | P. japonica | 68 |
| 14 | P. matildae, | 42 |
| 15 | P. oleandri | 53 |
| 16 | P. paeoniae | 45 |
| 17 | P. paciseta | 47 |
| 18 | P. taxica | 66 |
| 19 | P. torulosa | 64 |
| 20 | P. zalbrukneriana | 47 |
Table 3: Species showing taxol production.
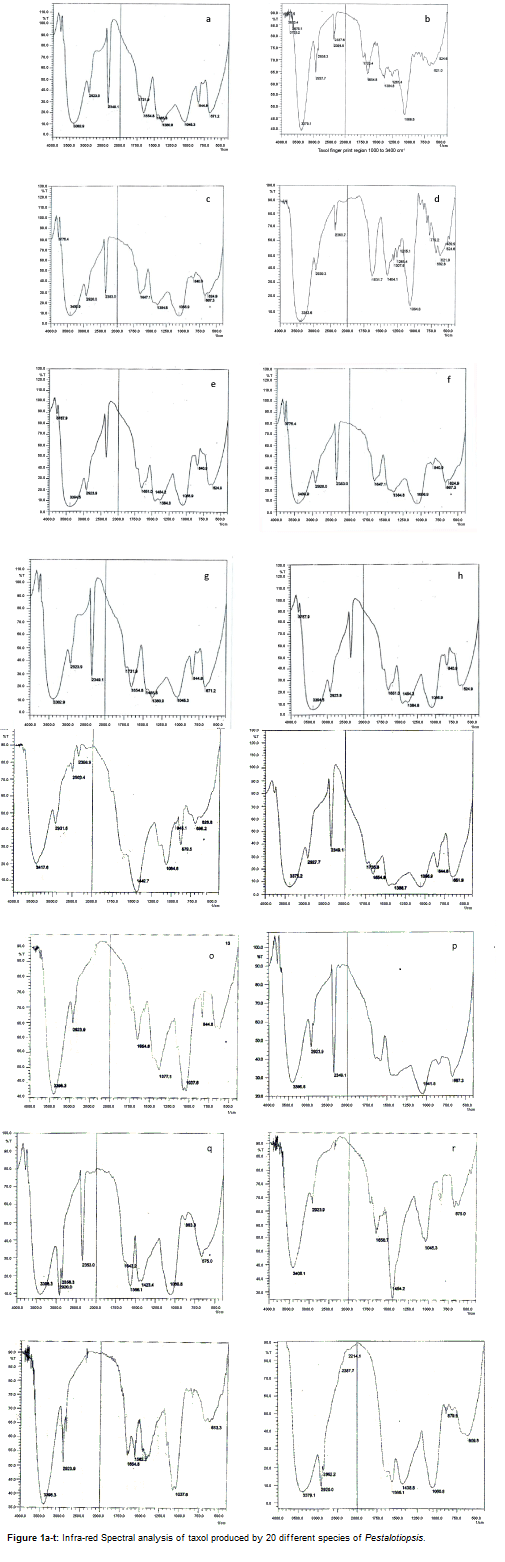
Pestalotiopsis acacia / P. clavispora (Figure 1a-t)
An intense band was observed at 3379.1 cm-1, which is due to the absorption of H-OH. The sharp band at 2927.7 cm-1 is due to the absorption of sp3 hybridized carbon and the band at 2858.3 cm-1 is due to the absorption of symmetric stretching vibration of methylene group. Bands observed at 2364.6 cm-1 and 2337.6 cm-1 regions were due to the absorption of –COOH and enols. The band at 1720.4 cm-1 was due to the absorption of ketone and band at 1654.3 cm-1 was due to the absorption of weak combinations and overtones. Symmetrical stretching of Carboxlic acid gave rise to a band at 1384.8 cm-1 and C-O stretching in esters gave rise to a band at 1261.4 cm-1. An intense at 1068.5 cm-1 due to the unconjugated C-N linkage bands coupled with the stretching of adjacent bands.
P. adusta / P. coangae
A highly intense band was observed at 3363.6 cm-1 which was broad; this band was due to the absorption of H-OH. A peak with less intensity was observed at 2939.3 cm-1, which was due to the absorption of sp3 hybridized carbon. There was no band observed at 2858.3 cm-1 and 1720.4 cm-1. This indicates the absence of methylene group and overtone. Band found at 2360.7 cm-1 was due to –COOH and enols. The bands at 1631.7 cm-1 and 1404.1 cm-1 indicate the presence of weak combinations of overtones and cyclopropane, respectively. The bands at 1307.6 -1, 1261.4 cm-1 and 1215.1 cm-1 were observed due to C-N stretching and C-O stretching of esters and ketone. An intense band at 1064.6 cm-1 was observed which was due to the unconjugated C-N linkage bands coupled with the stretching of adjacent bands.
P. breviseta
A band at 3776.4 cm-1 was observed which increased with increase in intensity. The peak at 3409.9 cm-1 was broad and intense due to the absorption of H-OH. There were no band observed at 2858.3 cm- 1, 2337.6 cm-1, 1720.4 cm-1 and 1261.4 cm-1 indicating the absence of methylene group, ketone group and esters. This intensity of the peak at 2353 cm-1 increased considerably and appeared sharp. The bands at 1647.1 cm-1, 1384.8 cm-1, and 1065.9 cm-1were broad due to the absorption of weak combinations and overtone, symmetrical stretching (carboxylic acid) and uncojucated C-N linkage bands coupled with the stretching of adjacent bands. The band at 2920 cm-1 was due to the absorption of sp3 hybridized carbon. A band at 840.9 cm-1 was due to the aromatic C-H out of plane bending.
P. calabae
There was a band at 3787.9 cm-1 with increase in intensity. A band at 3394.5 cm-1 was broad and intense due to the absorption of H-OH. The band at 2923.9 cm-1 was less intense due to the absorption of sp3 hybridized carbon. There were no bands observed at 2858 cm-1 and 1261 cm-1 indicating the absence of methylene group and esters. There was a sharp band at 2353.0 cm-1. A band at 1651 cm-1 was due to the absorption of weak combinations overtones. Slightly prominent bands were observed at 1454.2 cm-1 and at 1384.8 cm-1 due to the C-H deformation and symmetrical stretching (carboxylic acid), respectively. The band at 840.9 cm-1 was due to the absorption of aromatic C-H out of plane bending.
P. coffeae
The broad intense peak at 3382.9 cm-1 was due to the absorption of H-OH. The band at 2923.9 cm-1with decreased intensity was due to the absorption of sp3 hybridized carbon. There was a slight twist in the peak at 1731.9 cm-1 which was due to the absorption of ketone. The band at 2349.1 cm-1 was highly intense. The band at 1654.8 cm-1 was due to the absorption of weak combinations and overtones. The bands at 1465.8 cm-1 and 1380.9 cm-1 were due to the C-H deformation and symmetrical stretching (carboxylic acid). The band at 1045.3 cm-1 was due to the un-conjugated C-N linkage; coupled with the stretching of adjacent bands. There was a band at 844.8 cm-1due to the absorption of aromatic C-H out of plane bending.
P. conigena
A sharp peak at 3776.4 cm-1(non-H bonded OH stretching which was found to the left of the broad band at 3467.5 c-1. The broad band was intense. A less intense peak at 2935.4 cm-1 was due to the absorption of sp3 hybridized carbon. There was a sharp intense peak at 2350 cm-1 and the prominent peak was found at 1731.9 cm-1. The peak is due to the absorption of ketone. The intensity of the peak at 1654.8 cm-1 has been increased. This peak is due to the absorption of weak combination and overtones. The very prominent peak appears at 1458.1cm-1 due to the C-H deformation. Another peak at 1377.1 cm-1 was observed due to the symmetrical stretching (carboxylic acid). A band at 1050 cm-1was observed due to the unconjugated C-N linkage bands coupled with the stretching of adjacent bands. A peak was observed at 848.8 cm-1 due to C-H aromatic out of plane bending. The peak at 663.5 cm-1was intense due to the absorption of bromide and iodide. This may be due to the solvent effect.
P. eriobotryofolia
A highly intense band at 3409.9 cm-1 was observed due to the absorption of H-OH. The bend at 2925 cm-1 is due to the absorption of sp3 hybridized carbon. The absorption bend at 2350 cm-1was sharp and intense. This is due to the absorption of - COOH and enols. A least prominent band was observed at 1775 cm-1, which is due to the absorption of C=O carboxylic acid. The band at 1654.8 cm-1 is due to the absorption of weak combinations and overtones. The absorption band due to unconjugated C-N linkage bands coupled with the stretching of adjacent bands, at 1045.3 cm-1. A band was observed at 848.8 cm-1 due to C-H aromatic out of plane bending. There was no band observed at 1261 cm-1 indicating the absence of C-O stretching in esters.
P. foedaris
The intensity of broad peak at 3375.2 cm-1 was due to the absorption of H-OH. The peak at 2927.7 cm-1 was less intense due to the absorption of sp3 hybridized carbon. No peak was observed at 2858.3 cm-1 which is due to the absence of symmetric stretching methylene group. The peak at 2349.1 cm-1was sharp and intense. This peak was due to absorption of –COOH and enols. The less intense peak at 1735.8 cm-1 was due to the absorption of acetone and another peak at 1654.8 cm-1 due to absorption of weak combinations and overtone. The band at 1388.7 cm-1 was due to symmetrical stretching (Carboxylic acid). A medium band was observed at 1056.9 cm-1 due to the absorption of unconjugated C-N linkage bands coupled with the stretching of adjacent bands. A band was observed at 844.8 cm-1 due to C-H aromatic out of plane banding. The band at 651.9 cm-1 was intense and appeared due to absorption of bromide and iodide. This may be due to the solvent effect. No band was observed at 1261.4 cm-1 indicating the absence of C-O stretching in esters.
P. fibricola
The band found in the region 3417.6 cm-1 was intense and it was found due to the absorption of H – OH. A less intense band at 2931.6 cm-1 was found due to the absorption of sp3 hybridized carbon. No band was observed at 2858 cm-1 due to the absence of symmetric stretching methylene group. A less intense band at 2503.4 cm-1 , 2356.9 cm-1 were observed due to the stretching of O – H (strongly H- bonded) and often overlaps the C-H absorption. The absorption of bands at 1750 cm-1 and 1650 cm-1 were widened due to the absorption of ketonic group and weak combinations and overtones. The very intense band at 1442.7 cm-1 was due to cyclopropane. This was a strong band. The absorption occurred due to the unconjugated C-N linkage. Bands coupled with the stretching of adjacent bands were found at 1664.6 cm-1. A weak band was observed at 945.1 cm-1due to the absorption of O-H bending (out of plane bending). The absorption band at 879.5 cm-1 was due to C-H aromatic out of plane bending. No peak was observed at 1654 cm-1 indicating the absence of combinations and overtones.
P. glandicola
The intense peak was observed at 3396.3 cm-1 due to the absorption of H-OH. The less intense peak than the previous peak was due to the absorption of sp3 hybridized carbon. No peaks were observed at 2858.3 cm-1, 2364 cm-1 and 2337 cm-1 representing the absence of symmetrical stretching vibration of methylene group. Ketonic group was not present because there was no band absorbed at 1720 cm-1. The band at 1664.8 cm-1 represents the presence of weak combinations and overtones. The band at 1377 cm-1 was due to the absorption of symmetrical stretchiry (Caboxylic acid). No band was observed at 1200 cm-1 indicating the absence of ester. The band at 1037.6 cm-1 occurred due to the absorption of CN linkage bands coupled with stretching of adjacent bands. There is a sharp peak observed at 844.8 cm-1 due to C-H aromatic out of plane bending.
P. japonica
The intense peak at 3396.3 cm-1 was due to the absorption of H-OH. The sharp peak at 2920 cm-1, and 2858.3 cm-1 is recovered back indicating the presence of sp3 hybridized carbon and symmetrically stretching vibration of methylene group respectively. A very sharp peak was observed at 2353.0 cm-1. A less intense peak at 1643.2 cm-1 was observed due to the weak combination and overtones. The band at 1566.1 cm-1 and 1423.4 cm-1 were due to cyclopropane. An intense band observed at 1050.8 cm-1 was due to the absorption of CN linkage bands coupled with the stretching of adjacent bands.
P. matildae
An intense band at 3386.8 cm-1 was observed due to the absorption of H-OH. The less intense band at 2923.9 cm-1 was due to the absorption of sp3 hybridized carbon. A very sharp band observed at 2349.1 cm-1 was prominent variation in the band. A prominent variation in the band was observed at 1200 cm-1 and 1800 cm-1 indicating the presence of cyclopropane and overtones. An intense band at 1041.5 cm-1 was observed due to the absorption of unconjugated C-N linkage bands coupled with the stretching of adjacent bands.
P. oleandri
A highly intense band at 3398.3 cm-1was observed due to the absorption of H-OH. A sharp band observed at 2923.9 cm-1 was observed due to the absorption of sp3 hybridized carbon and there was another sharp band at 2858.3 cm-1 which is due to the absorption of symmetrical stretching vibration of methylene group. The bands at 2364 cm-1 and 2337 cm-1 were much less intense and another band was observed at 1654.8 cm-1. A sharp band at 1562.2 cm-1 was due to the bending of secondary amide. The band at 1037.6 cm-1 was intense which occurred due to the un-conjugated C-N linkage bands coupled with the stretching of adjacent bands. No absorption were observed at 1384 cm-1 and 1261 cm-1indicating (carboxylic acid) and C-O stretching in ester respectively.
P. paeoniae
An intense band at 3406.1 cm-1 was observed due to the absorption of H-OH. The band at 2923.9 cm-1 was found to have the least intensity, which was due to the absorption of sp3 hybridized carbon. The band at 1720.4 cm-1 was due to the absorption of ketone and the band at 1654.8 cm-1 was due to the absorption of weak combinations and overtones. The most intense band in this spectrum was observed at 1454.2 cm-1 which is due to cyclopropane. The band at 1045.3 cm-1 is due to the absorptions of C-N linkage bands coupled with the stretching of adjacent bands. No bands were observed at 1384 cm-1 and 1261 cm-1 indicating the absence of symmetrical stretching (carboxylic acid) and C-O stretching in ester.
P. paciseta
An intense peak was observed at 3396.3 cm-1due to the absorption of H-OH. A well prominent band observed at 2920.0 cm-1 and 2858.3 cm-1 which were due to the absorption of sp3 hybridized carbon and due to the absorption of symmetric stretching vibrations of methylene group, respectively. These peaks were very intense. A sharp band was observed at 2353.0 cm-1. There was no band observed at 1720.4 cm-1 indicating the absence of ketonic group. The band at 1643.2 cm-1 is due to the weak combinations and overtones. An intense band at 1566.1 cm-1 was observed which is due to the bending of secondary amide. The band at 1423.4 cm-1 was observed due to the cyclopropane. An intense band at 1060.8 cm-1 was due to the C-N linkage bands coupled with the stretching of adjacent bands.
P. taxica
An intense band at 3379.1 cm-1 was due to the absorption of H-OH. The bands at 2920.9 cm-1 and 2862.2 cm-1 were observed due to the absorption of sp3 hybridized carbon and symmetrical stretching vibrations of methylene group respectively. In this spectrum the bands at 2387.7 cm-1 and 2212.1 cm-1 have the least intensity. There was no absorption at 1720 cm-1 indicating the absence of ketonic group. The band at 1566.1 cm-1 was due to the bending of secondary amides. A band was observed at 1438 cm-1 due to cyclopropane, and 1060.8 cm-1 due to the C-N linkage bands coupled with the stretching of adjacent bands. There were no absorptions at 1384 cm-1 and 1261 cm-1 representing the absence of symmetrical stretching carboxylic acid and C-O stretching in esters.
P. torulosa
The intense band at 3421.5 cm-1 was due to absorption H-OH. The less intense band at 2935.5 cm-1 is due to sp3 hybridized carbon. There was no peak at 2858.3 cm-1 indicating the absence of symmetrically stretching vibration of methylene group. The band 2506.4 cm-1 is sharp and due to the stretching of O-H and often overlaps the C-H absorption. A very less intense band was observed at 2360.7 cm-1. A very intense band was observed at 1442.7 cm-1 due to cyclopropane. A band was observed at 1276.8 cm-1 which is due to the absorption of C-O stretching in esters. There was another band at 1064.6 cm-1 representing the C-N linkage coupled with the stretching of adjacent bands. A slight sharp peak was observed at 879.5 cm-1 due to the absorption of aromatic C-H out of plane bending. There were no bands observed at 1720 cm-1, 1658 cm-1 and 1384 cm-1 indicating the absence of ketone, weak combinations and overtones, symmetrical stretching (carboxylic acid) respectively.
P. zalbrukneriana
An intense band was observed at 3336.6 cm-1 which is due to the absorption of H-OH. The less intense band at 2939.3 cm-1 is due to sp3 hybridized carbon. There was no band observed at 2858.3 cm-1 indicating the absence of methylene group. There was a very small twist in the band at 2364.6 cm-1 and 2337.6 cm-1 when compared. No bands were observed at 1720 cm-1 and 1260 cm-1 indicating the absence of ketone and ester. The bands at 1635.5 cm-1 and 1384.8 cm-1 indicate the presence of weak combinations, overtones and symmetrical stretching (carboxylic acid) respectively. The band at 1064.6 cm-1 is due to the absorption of C-N linkage band coupled with the stretching of adjacent bands.
Conclusion
Out of 20 species tested for taxol 6 species showed above 60 μg/L of taxol production 9 species produced 50-60 μg/L, and others produced 40-50 μg/L (Figure 2).
Among the three medium Potato Dextrose Agar, Czapek Dox Agar and Oat Meal Agar used. PDA medium was good for producing mycelia, followed by OMA and CDA, respectively.
All the 20 samples were aromatic derivative consisting of sp3 hybridized C-H stretching alkyl substitutions and carbonyl groups as it or amide and few samples as esters. All the 20 samples contain the C-N linkage except Sample 3. Hence, the IR spectra analysis and correlation of 20 different Pestalotiopsis spp., samples clearly indicate the existence of important functional groups in the aromatic derivative which beautifully coincides with the standard pestalotiopsis spp.
From all the 20 different Pestalotiopsis it is evident except for a few samples; all samples show the existence of aromatic system. All 20 samples contain a methyl group and carbon – nitrogen linkage. It can be concluded from IR spectra that the OH group (Alcohol group) is also present in all the 20 test samples. A few samples viz sample 1, 5 6, 8, 9, 14, and 20 show the existence of a keto group. This keto group is present as carboxylic acid in samples 1,5,6,7,8, 10 and 18 respectively. The existence of Carbonyl (C=O) group is present as amide in sample 13, 15, 16 and 19 respectively. The existence of aromatic system is further confirmed by presence of overtones and combination bands in almost all samples expect sample 16, 17, and 19. One or two samples contains methylene group also.
References
- Hudson HJ (1968) The ecology of fungi on plant remains above the soil. New Phytologist 67: 837-874.
- Rao S, Horwitz SB, Ringel I (1992) Direct photoaffinity labeling of tubulin with taxol. J Natl Cancer Inst 84: 785-788.
- Mu JH, Bollon AP, Sidhu RS (1999) Analysis of beta – tublin cDNAs from taxol, Taxol – resistant Pestalotiopsis microspora and taxol – sensitive Pythium ultimum and comparison of the taxol binding properties of their products. Mol Gen Genet 262: 857-868.
- Kiho T, Itahashi S, Sakushima M, Matsunaga T, Usui S, et al. (1997) Polysaccharides in fungi. XXXVIII. Anti-diabetic activity and structural feature of a galactomannan elaborated by Pestalotiopsis species. Biol Pharm Bull 20: 118-121.
- Suffness M, Wall ME (1995) Discovey and development of Taxol. In: Suffness M, Taxol: Science and applications, Volume 22, CRC, Boca Raton F1, 3-25.
- Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT (1971) Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc 93: 2325-2327.
- Stierle A, Strobel G, Stierle D (1993) Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science 260: 214-216.
- Strobel G, Yang X, Sears J, Kramer R, Sidhu RS, et al. (1996) Taxol from Pestalotiopsis microspora, an endophytic fungus of Taxus wallachiana. Microbiology 142: 435-440.
- Li JY, Strobel G, Sidhu R, Hess WM, Ford EJ (1996) Endophytic taxol-producing fungi from bald cypress, Taxodium distichum. Microbiology 142 : 2223-2226.
- Strobel GA, Hess WM, Ford E, Sidhu RS, Yang X (1996) TAXOL from fungal endophytes and the issue of biodiversity. Journal of International Microbiology and Biotechnology 179: 417-423.
- Caruso M, Colombo AL, Crespi-perellino N, Fedeli L, Malyszko J, et al. (2000) Studies on a strain Kitasatospora sp. paclitaxel producer. Anna;s of Microbiology 50: 89-102.
- Wang J, Li G, Lu H, Zheng Z, Huang Y, et al. (2000) Taxol from Tubercularia sp. strain TF5, an endophytic fungus of Taxus mairei. FEMS Microbiol Lett 193: 249-253.
- Shrestha K, Strobel GA, Shrivastava SP, Gewali MB (2001) Evidence for paclitaxel from three new endophytic fungi of Himalayan yew of Nepal. Planta Med 67: 374-376.
- Kathiravan G, Sri Raman V (2010) In vitro TAXOL production, by Pestalotiopsis breviseta--a first report. Fitoterapia 81: 557-564.
- Kathiravan G, Sureban SM (2009) Effect of taxol from Pestalotiopsis mangiferae on A549 cells-In vitro study. J Basic Clin Pharm 1: 1-9.
- Cardellina II JH (1991) HPLC separation of taxol and cephalomannine. J Chrom 14: 659-665.
- Kathiravan G, Muthumary J (2009) Extraction of taxol, an anticancer drug by coelomycetous fungi Pestalotiopsis versicolor and Phyllosticta murrayicola Mycologia Balcanica 6: 69-74.
- Kumaran RS, Muthumary J, Hur BK (2008) Production of taxol from Phyllosticta spinarum, an endophytic fungus of Cupressus sp. Eng Life Sci 8: 438-446.
- Kumaran RS, Muthumary J, Hur BK (2008) Taxol from Phyllosticta citricarpa, a leaf spot fungus of the angiosperm Citrus medica. J Biosci Bioeng 106: 103-106.
- Kathiravan G, Sureban SM, Sree HN, Bhuvaneshwari V, Kramony E (2012) Isolation of anticancer drug TAXOL from Pestalotiopsis breviseta with apoptosis and B-Cell lymphoma protein docking studies. J Basic Clin Pharm 4: 14-19.
- Gowri K, Kathiravan. G, Sukumaran N (2012) Production of taxol by pestalotiopsis breviseta CR01 isolated from the catharanceus roceus and its growth studies. Int J Pharm Bio Sci 3: 1046-1053.
- Booth C (1971) Introduction to general methods. In: Methods in Microbiologym, Volume 4, Academic Press, Landon and New York 1- 47.
- Strobel G, Daisy B (2003) Bioprospecting for microbial endophytes and their natural products. Microbiol Mol Biol Rev 67: 491-502.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14131
- [From(publication date):
September-2014 - Dec 03, 2024] - Breakdown by view type
- HTML page views : 9677
- PDF downloads : 4454