Influence of Physical Parameters on Growth and Bacteriocin Activity by Species of Lactic Acid Bacteria Isolated from Fermenting Foods
Received: 06-Feb-2018 / Accepted Date: 13-Feb-2018 / Published Date: 15-Feb-2018
Abstract
Predominant lactic acid bacteria (LAB) strains of Lactobacillus fermentum (NS9, IMAU62205, KLDS1.0733, IMAU62166), L. plantarum (PON10014, OP, FSHS2, B23) and L. pentosus (NRIC1836, NBRC12011, OP4Dan) previously identified and isolated from fermenting cassava and maize were evaluated for the effect of physical parameters on their growth and bacteriocin production. The optical density (OD) and pH of the LAB strains were measured against time of growth in MRS broth. The effect of time, pH and temperature on the bacteriocin production was determined. The inhibitory activity of the bacteriocin produced by the LAB against some food spoilage microorganisms was evaluated. All the LAB strains showed similar growth pattern. Growth was at its peak at 12 h of incubation. There were variations in the OD of the different strains. The pH decreased with increase in the time of growth from 6.5-7.0 to 4.0-4.3. Optimum bacteriocin production (800-1000 AU/ml) occurred at 18 h of growth, pH 3.0-4.0 and at 40°C. The bacteriocin inhibited (7-12 mm) the test bacteria food pathogens but Candida albicans AT410231 was resistance to the bacteriocin. Time, temperature and pH had a profound effect on the growth and bacteriocin activity of LAB strains in the fermenting foods. The bacteriocin produced was inhibitory to many food spoilage microorganisms. This result is a guide to the control of fermentation needed to achieve safety in spoilage microorganisms. This result is a guide to the control of fermentation needed to achieve safety in traditionally fermented foods.
Keywords: Bacteriocin production; Fermenting foods; Lactic acid bacteria; Growth; pH; Temperature
Introduction
Biological preservation refers to the extension of the shelf-life of food products and improvement of their microbial safety by using two different approaches: The inoculation of the food matrix with microorganisms, defined as protective cultures, with consequent insitu production of inhibitory molecules and/or a competitive effect against pathogen and spoilage bacteria and the use of microbial metabolites in purified form in particular bacteriocins. The use of microorganisms as protective cultures, e.g., bacteriocin producers, may have several advantages, as microorganisms can not only be the source of anti-microbial peptides but also of a wide spectrum of molecules, such as organic acids, carbon dioxide, ethanol, hydrogen peroxide and diacetyl, whose antimicrobial action is well known. Competition of protective cultures with potential pathogens is another way to restrict the growth of undesired microorganisms. Moreover, these microorganisms may have additional functional properties and, in some circumstances, they can be beneficial for the consumers. They can as well contribute to the flavor, texture and nutritional value of the products [1].
Bacteriocins are extracellular peptides or proteins that elicit antimicrobial compounds, which exhibit a bactericidal effect against closely related bacteria. Bacteriocins elicit antimicrobial activity against food spoilage organisms and food-borne pathogens, but do not affect the producing microorganism. Bacteriocins of lactic acid bacteria are considered as safe natural preservatives or bio preservatives. Several types of bacteriocins from food associated lactic acid bacteria have been identified and characterized. Some of the important ones are nisin, bacteriocin, diplococcin, acidophilin, bulgaricin, helveticins, lactacins, plantaricin, reuterin and reutericyclin [2]. Of these, bacteriocin and nisin produced by Lactococcus lactis spp. lactis, have been the most extensively characterized [3,4]. At present, nisin is the only bacteriocin commercially available and marketed [5]. It has been reported that nisin is more active against Gram-positive bacteria, particularly the spore-formers. Other bacteriocins of lactobacilli are effective against closely related species of mesophilic Lactobacillus as such, considered as potential natural food preservatives. Bacteriocins have also been reported to be active against Listeria monocytogenes [6]. Leucosnostoc mesenteroides L124 and L. curvatus L442 isolated from dry fermented sausages, produced bacteriocins that were antagonistic towards closely related species and pathogens [7]. An isolate of Leucosnostoc mesenterioides sub spp. cremoris was also found to produce a bacteriocin-like inhibitory compound against the lactic acid bacteria of wines [8]. The bactericidal activity of bacteriocins is attributable to destabilization of the functions of cytoplasmic membranes of the target cells which include altering the permeability properties of the membrane. Three most important aspects in the study of bacteriocins are their production, characterization and purification. Maximal bacteriocin production could be obtained by supplementing a culture medium with growth limiting factors, such as sugars, vitamins and nitrogen sources by regulating pH or by choosing the best adapted culture medium [9].
Traditionally fermented foods in Nigeria involving the use of roots and tubers basically undergo lactic fermentation. Microorganisms usually involved in these fermentations are lactic acid bacteria which lower the pH of the medium beyond what non-lactics in the fermentation can cope with thereby allowing the lactic acid bacteria to predominate the fermentation. Many antimicrobial substances including bacteriocins are produced in the process and that confer some level of safety to the food products. This is practically evident when fermented cassava products are seen to have longer shelf life compared to non- fermented food products under rural traditional storage conditions. However, the production and efficacy of the antimicrobial substances can be affected by environmental conditions which may be intrinsic or extrinsic. There is paucity of information on how these factors influence the production of antimicrobials during traditional fermentations of foods. This study was aimed at determining how physical parameters affect growth of microorganisms, their bacteriocin production and activity.
Materials and Methods
Bacteriocin activity
Bacteriocin activity was detected by the deferred inhibition assay using lactic acid bacteria strains from fermented cassava and maize as the producing microorganisms. Cultures used for testing bacteriocin production were spotted (5 μl) onto the MRS agar plates and incubated for 24 h at 30°C. Indicator bacteria were inoculated into soft (0.75%) MRS agar which was used to overlay MRS agar plates. Plates were again incubated at 30°C to allow growth of the indicator bacteria as a ‘lawn’ and then examined for zones of bacteriocin activity surrounding the producer colony. Bacteriocin producing cultures were further assessed by confirming the proteinaceous nature of the inhibiting compound in separate experiments. This was determined by transferring 5 μl of proteinase K solution (10 mg/ml in bidistilled water) next to the bacteriocin producing colonies following overnight growth. Plates were thereafter incubated for 3 h at 37°C and over layered with indicator bacteria and incubated overnight as described above. The absence of a zone of inhibition next to the bacteriocin producer on the side of the colony where the proteinase K was spotted indicated the proteinaceous nature of the inhibiting compound.
In order to quantify the bacteriocin activity, supernatant of a bacteriocin producing isolate grown at 30°C for 24 h in MRS broth was adjusted to pH 6.5 (near neutral to avoid acid effect). It was then heated at 94°C for 10 min to inactivate any remaining cells. A double dilution series was made by adding 100 μl of the cell free neutralized supernatant to 100 μl sterile MRS in micro titer plates, and thus continuing to obtain a dilution series of 1:2, 1:4, 1:8 and 1:16 of the cell free neutralized supernatant in MRS broth. The bacteriocin activity was expressed in arbitrary activity units (AU), which is a reciprocal of the last (highest) dilution that showed a clear inhibition zone when 10 μl were spotted onto a plate that was over layered with indicator bacteria and subsequently incubated at 30°C overnight.
Results
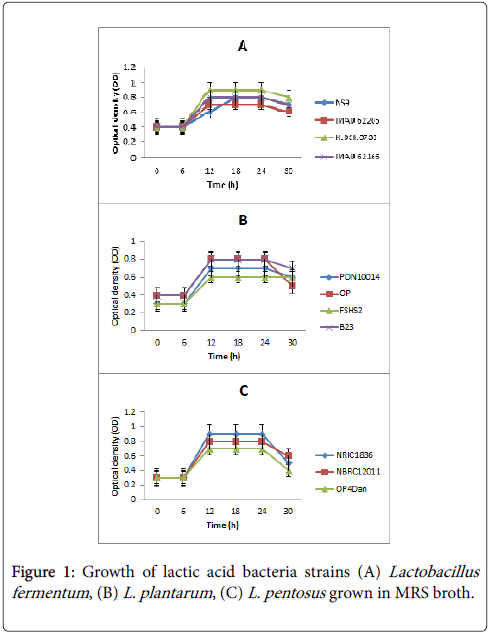
Figure 1 shows the growth of lactic acid bacteria isolates on MRS broth expressed as optical density (OD). Growth temperature was generally at 30°C. The pattern of growth by these isolates indicated a lag phase for 6 h after which growth was exponential from 6-12 h of incubation. Optimal growth was at 12 h. They also exhibited the stationary and decline phases of growth. The isolates however showed some species-specific tendencies in their growth behavior. Hence there was significant difference (p˂0.05) in their growth pattern. Figure 1A is the growth pattern of the Lactobacillus fementum strains. L. fermentum strain KLDS1.0733 showed the highest OD of 0.9 while strain NS9 had the least growth of OD 0.7. This strain however, maintained a longer exponential phase than the others which was relatively complimentary to its slow growth behavior. The L. plantarum strains (Figure 1B) had an OD that ranged from 0.3-0.8 with strain FSHS2 having the least growth but did not indicate decline within the incubation period. The highest growth was shown by strain PON19914 and strain OP. The two strains were very competitive in their growth pattern until after the stationary phase. The OD of the L. pentosus strains is presented in Figure 1C. There were 3 strains in this species and they had OD from 0.6-0.8. The strain with the highest OD was NRIC1836 and OP4Dan the lowest.
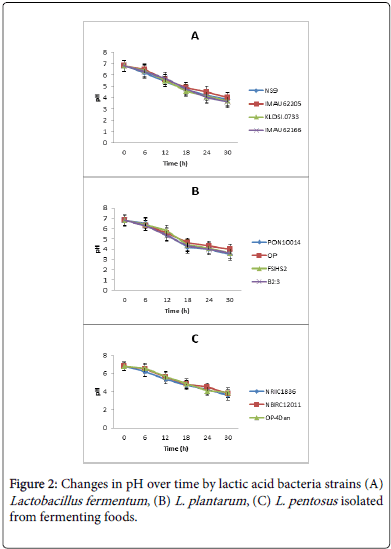
Changes in pH over time by lactic acid bacteria isolates are presented in Figure 2. The pH reduction in all the strains followed a similar pattern over a period of 24-30 hours of incubation. Initial pH was 6.8. There was no significant difference (p˃0.05) in pH values within the strains of a particular species. L. fementum strains had pH reduction up to 3.6-3.8 (Figure 2A). The lowest reduction was achieved by strain IMAU62166. Figure 2B shows the level of pH reduction by L. plantarum strains. The lowest pH reduction here was 3.6 by strains PON10014 and B23. Among the L. pentosus strains (Figure 2C), the three strains were very close in terms of pH production.
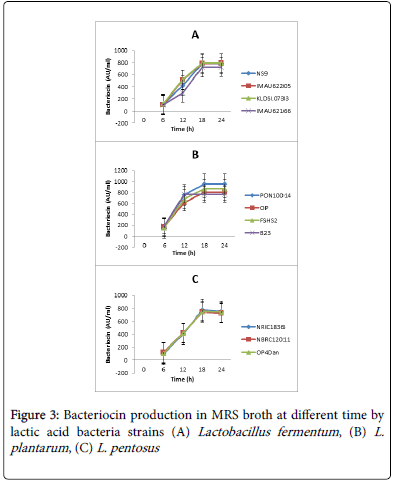
Bacteriocin production over time by the lactic acid bacteria strains is shown in Figure 3. All the lactic acid bacteria strains under study started bacteriocin production after 6 h of growth. High levels of bacteriocins (700-950 AU/ml) were produced. The rate of production differed significantly (p˂0.05) within strains and between species. Production was optimal at 18 h of growth. Figure 3A presents bacteriocins produced by strains of L. fementum , where KLDS1.0733 strains produced the highest quantity of bacteriocins and there was no significant difference in their production levels. Strain IMAU62166 produced the least amount of bacteriocin. Figure 3B shows the bacteriocin production by strains of L. plantarum . Strain PON10014 produced the highest level (950 AU/ml) of bacteriocins in all the lactic acid bacteria strains under study. Strain B23 optimal production was at 12 h and the quantity of bacteriocin produced at that time was 750 AU/ml while the three other strains had optimal production at 18 h. Significant differences in bacteriocin production existed among the strains. Bacteriocin production by L. pentosus strains is shown in Figure 3C. Maximum bacteriocin production by the three strains ranged from 700-750 AU/ml at 18 h. There was no significant difference among the levels of bacteriocins produced by the strains.
Many workers have confirmed the production of bacteriocins by species of Lactobacillus in fermented foods as indicated in these results [10,11]. In another work done by on fermented foods from Senegal, a total of 220 strains of lactic acid bacteria isolated from 32 samples of traditional fermented foods were screened for bacteriocin production [12]. Two bacteriocin producers, Lactococcus lactis subsp. lactis and Enterococcus faecium , were identified from 12 bacteriocin-producing isolates on the basis of phenotypic analyses and 16S rDNA sequence. Both bacteriocins produced by new isolates showed antimicrobial activity against Listeria monocytogenes and Bacillus coagulans whereas only that produced by Lactococcus lactis had an activity against Bacillus cereus. Bacteriocin-producing Lactococcus lactis strains were found in a variety of traditional foods indicating a high potential of growth of this strain in variable ecological complex environment.
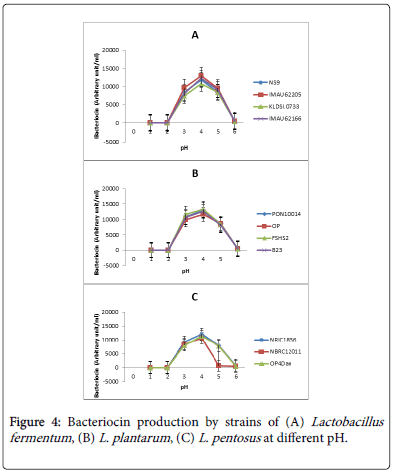
The effect of pH on bacteriocin production by lactic acid bacteria isolates from fermented foods is presented in Figure 4. The pH significantly affected bacteriocin production. All the strains produced bacteriocins between pH 3.6 and 4.0. Among the L. fementum strains (Figure 4A), IMAU62295 had the highest bacteriocin production (13000 AU/ml) at pH 4.0. On the other hand, strain KLDS1.0733 recorded the lowest (10000 AU/ml) production at pH 4.0. The effect of bacteriocin produced by strains of L. plantarum is presented in Figure 4B. Peak values of bacteriocin which ranged from 10000 to 11000 AU/ml were not significant. However, FSHS strain recorded the highest production and strain OP had the lowest. Figure 4C presents the effect of pH on bacteriocin production by strains of L. pentosus . The optimal bacteriocin production here was between 9000 to 11000 AU/ml at pH 4.0. Strain NRIC1836 was the highest bacteriocin producer and strain NBRC12011 was the lowest. Apart from the low production by this strain, it had the most drastic drop to zero production at pH 5.0 as against the other strains that dropped gradually at pH 6.0.
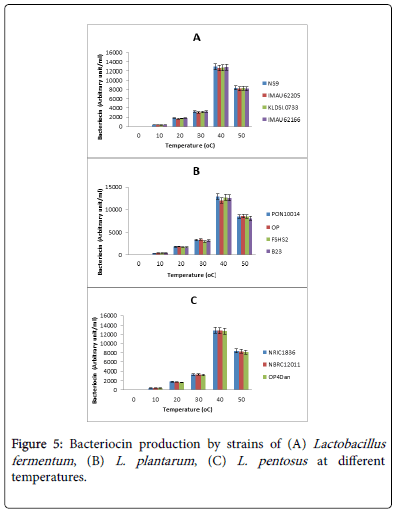
The effect of temperature on bacteriocin production by lactic acid bacteria isolates is shown in Figure 5. Temperature affected bacteriocin production significantly. All the strains produced optimal bacteriocins at 40°C. At 50°C, the production dropped. Levels of production at 40°C differed from one strain to the other. Figure 5A indicates how temperature affected bacteriocin production in strains of L. fementum . At peak levels, strains produced between 12300 to 12500 AU/ml of bacteriocins. There was no significant difference in bacteriocin production levels between the strains. The highest bacteriocin producer was strain NS9.
The effect of temperature on bacteriocin production by strains of L. plantarum is presented in Figure 5B. Optimal bacteriocin production ranged from 12000 AU/ml in strain OP to 14000 AU/ml in strain PON10014. The value of optimal bacteriocin production in strain OP was significantly different from the remaining three strains.
A similar effect on L. pentosus strains is shown in Figure 5C where bacteriocin production among the strains (13100 to 37000 AU/ml) did not differ significantly. Strain NRIC was the highest producer.
Ogunbanwo worked on the characterization of bacteriocin from Lactobacillus spp isolated from Nigerian fermented foods and reported similar results on how pH and temperature affect the production of bacteriocins by lactic acid bacteria [13].
Table 1 presents the inhibitory activity of baceriocins produced by the lactic acid bacteria isolates against pathogenic organisms. The antimicrobial screening for all lactic acid bacteria strains against 9 pathogenic organisms gave average inhibition halos (measured in MRS agar) 6-12 mm. The lactic acid bacteria strains were able to inhibit the selected indicator microorganisms to varying degrees. The bacteriocin activity against the pathogenic microorganisms varied considerably among the isolates. Escherichia coli was greatly inhibited by L. fementum strains IMAU62188 (12 mm), NS9 (10 mm), and 10 mm for each of L. plantarum strain B23 and L. pentosus strain NRIC1836. Staphylococcus aureus was inhibited up to 12 mm by 3 strains and 10 mm by 4 strains. However, antimicrobial property of the lactic acid bacteria isolates did not show any inhibition against Candida albicians ATCC10231-a reference strain.
| Indicator organism | Test organism | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Origin | L. fermentum strains | L. plantarum strains | L. pentosus strains | |||||||||
| NS9 | IMAU62205 | KLDS1.0733 | IMAU62166 | PON10014 | OP | FSHS2 | B23 | NRIC1836 | NBRC12011 | QP4Dan | ||
| Escherichia coli | Milk | 10 | 8 | 7 | 12 | 7 | 8 | 6 | 10 | 10 | 8 | 8 |
| Serratia macescence | Meat | 10 | 12 | 8 | 10 | 8 | 12 | 8 | 10 | 8 | 8 | 10 |
| Pseudomonas aureginosa | Meat | 8 | 10 | 7 | 8 | 12 | 10 | 12 | 8 | 10 | 12 | 8 |
| Lactobacillus delbruckii | Pito | 12 | 8 | 12 | 10 | 9 | 7 | 10 | 10 | 7 | 10 | 12 |
| Lactobacillus bulgaricus | Wine | 7 | 6 | 8 | 8 | 10 | 12 | 7 | 12 | 10 | 8 | 7 |
| Staphylococcus aureus NCTC6571 | Reference strain | 6 | 12 | 10 | 12 | 10 | 8 | 12 | 7 | 9 | 10 | 10 |
| Salmonella typhimurium ATCC13311 | Reference strain | 10 | 8 | 10 | 8 | 7 | 12 | 7 | 10 | 8 | 12 | 7 |
| Candida albicans ATCC10231 | Reference strain | - | - | - | - | - | - | - | - | - | - | - |
| Bacillus cereus | Ugba | 5 | 7 | 10 | 8 | 6 | 12 | 8 | 7 | 9 | 8 | 10 |
Table 1: Inhibitory activity (mm) of bacteriocin produced by lactic acid bacteria from fermenting foods against food spoilage microorganisms.
Similar to these findings, observed varying degrees of inhibition of various food borne pathogens by cell-free filtrates of lactic acid bacteria [14,15]. Showed that antimicrobial producing microorganisms had the ability to inhibit the growth of other bacteria which included both Gram-negative and Gram-positive bacteria [16]. Such antimicrobial activities were also demonstrated in the works of other researchers such as where lactic acid bacteria species were tested against Staphylococcus aureus , Pseudomonas aeruginosa , Candida albicans , Escherichia coli , and Proteus vulgaris [17]. Raccah have demonstrated that the antimicrobial compounds produced by lactic acid bacteria can inhibit the growth of pathogenic bacteria of possible contaminants in fermented products [18-20].
Discussion
There is a growing interest on the technological properties of lactic acid bacteria isolated from traditionally fermented foods with the aim of developing the fermentation process further. In line with this, studied some technological properties of predominant lactic acid bacteria isolated from Nigerian fermented foods with results similar to this study [21].
These properties are significant for their application as starter cultures. Fundamental among these properties are rapid acid production and production of antimicrobial compounds like bacteriocin which is basic to the safety of locally fermented products [22]. Effect of bacteriocin production by lactic acid bacteria on target organisms has been discussed [23].
Bacteriocin production was not limited by the extreme narrow antibacterial spectrum as the case with some lactic acid bacteria. Bacteriocin from the lactic acid bacteria strains was tested against different bacterial pathogens and yeast organism which are pathogens present in foods and can cause various illnesses in human beings. The spectrum of inhibition proved the possibility of using these strains as bio-preservatives or probiotics in the acid fermentation of foods. Bacteriocins have been proved active against many bacterial pathogens, hence its application has been demonstrated [24,25].
Bacteriocin production was strongly dependent on pH and incubation temperature. Previous investigations claim that apart from these factors, nutrient source can also affect bacteriocin production [26,27]. Many physicochemical factors seem to affect bacteriocin production and activity. In this study, the maximum bacteriocin activity at pH 4.0 and temperature 40°C indicated that the bacteriocin can be used in acidic foods. MRS medium appeared to be a suitable medium for growth and bacteriocin production by the lactic acid bacteria strains. The bacteriocin occurred as secondary metabolite, being produced at the stationary growth phase where optimum activity was also from pH 4-5 in strains of L. plantarum and L. pentosus . Similar reports have indicated that maximum bacterium activity was marked at the stationary growth phase suggesting the antimicrobial peptides as a secondary metabolite [28]. Bacteriocin of lactic acid bacteria is particularly necessary because of the important role the lactic acid bacteria play in the majority of fermented foods. Nisin is the first food preservative agent approved by Food and Drug Administration (FDA) to be used in processed cheese in 1988 as prescribed by Roseland [29]. The bacteriocins in this study have the potential to develop probiotic and bio-preservative qualities.
There are several reports of the ability of bactericins produced by lactic acid bacteria on pathogenic microorganisms in foods [11]. The ability to inhibit other microorganisms is due to the fact that lactic acid bacteria produce substances which are injurious to the indicator organisms depending on the concentration or quantity produced. These substances serve as competitive advantage to lactic acid bacteria when in mixed culture especially during fermentation and hence the dominance of lactic acid bacteria during fermentation of cassava, cereals and vegetables. Wakil and Osamwonyi indicated that lactic acid bacteria isolates showing antimicrobial activity were discovered to produce antimicrobial substances like lactic acid, hydrogen peroxide, and diacetyl, showing that the ability to inhibit other microorganisms was directly related to the ability of these microorganisms to produce these substances [30]. Daeschel reported the ability of lactic acid bacteria to produce lactic acid, thereby reducing the pH of the fermenting medium [31]. The lactic acid produced serves to reduce the pH of the medium, thereby making it acidic which is not conducive for the survival of spoilage bacteria that may have found their way into the fermenting substrate during spontaneous fermentation. Lactic acid is a natural preservative that inhibits putrefying bacteria and is responsible for the improved microbiological stability and safety of the food. The acidity also leads to the souring of the final product which is characteristic of fermented products.
Conclusion
The high level of bacteriocins produced by lactic acid bacteria isolated from cassava fermentation indicates a measure of safety on the fermented food. Physical parameters affected the growth and bacteriocin production and activity by the isolates from the cassava fermentation. Bacteriocin production was optimal from 18-24 h of growth, pH 3.5-4.0 and temperature at 40°C. Bacteriocin produced by the test organisms was reasonably inhibitory to the indicator organisms of food spoilage except Candida albicans strain ATCC10231- a reference strain. Some of these LAB strains with desired characteristics can be genetically modified to serve as starter culture for cassava fufu production.
References
- Gaggia F, Di Gioia D, Baffoni L, Biavati B (2011) The role of protective and probiotic cultures in food and feed and their impact in food safety. Trends in Food Science and Technology 1: 58-66.
- Leroy F, De Vuyst L (2004) Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends in Food Science and Technology 15: 67-78.
- Buchman GW, Banerjee S, Hansen JN (1988) Structural expression and evolution of a gene encoding the precursor nisin a small protein antibiotic. Journal of Biological Chemistry 263: 16260-16266.
- Liu W, Hansen JN (1990) some chemical and physical properties of nisin, a small-protein antibiotic produced by Lactococcus lactis. Applied Environmental Microbiology 56: 2551-2558.
- Balasubramanyam BV, Varadaraj MC (1998) Cultural conditions for the production of bacteriocin by a native isolate of lactobacillus delbruecki spp bulgaricus CFR 2008 in milk medium. Journal of Applied Microbiology 84: 97-102.
- Vignolo G, Fadda S, De Kairuz MN, de Ruiz Holgado AAP, Oliver G (1996) Control of Listeria monocytogenes in ground beef by Lactocin 705: a bacteriocin produced by L casei CRL 705. International Journal of Food Microbiology 27: 397-402.
- Mataragas M, Metaxopoulos J, Drosinos EH (2002) Characterization of two bacteriocins produced by Leuconostoc mesenteroides L124 and Lactobacillus curvatus L442, isolated from dry fermented sausages. World Journal of Microbiology and Biotechnology 18: 847-856.
- Yardugul S, Bozogla F (2002) Studies on an inhibitor produced by Lactic acid bacteria of wines on the control of malo lactic fermentation. Eur Food Res Technol 215: 38-41.
- Vignolos GM, de Kairuz MN, de Ruiz Holgado AAP, Oliver G (1995) Influence of growth conditions on the production of lactocin 705, a bacteriocin produced by Lactobacillus casei CRL 705. Journal of Applied Bacteriology 78: 5-11.
- Tatsadjieu NL, Mjintarg YN, Sonfack TK, Daoudou B, Mbofurg CMF (2009) Characterization of lactic acid bacteria producing bacteriocins against chicken Salmonella enteric and Escherichia coli. African Journal of Microbiology Research 3: 220-227.
- Jagadeeswari S, Vidya P, Mukeshkumai DJ, Balakumarah MD (2010) Isolation and Characterization of bacteriocin producing Lactobacillus sp from traditional fermented foods. Electronic Journal of Environmental, Agricultural and Food Chemistry 9: 575-581.
- Diop MB, Dubois Dauphin R, Tine E, Ngom A, Desfoin J, et al. (2007) Bacteriocin Products from Traditional Food Products. Biotechnology, Agronomy SOC and Environment 11: 275-281.
- Ogunbanwo ST, Sanni AI, Onilude AA (2003) Characterization of bacteriocin produced by Lactobacillus plantarum F1 and Lactobacillus brevis OG1. African Journal of Biotechnology 2: 219-227.
- Kivanc M (1990) Antagonistic action of lactic cultures toward spoilage and pathogenic microorganisms in food. DieNahrung 34: 273-277.
- Tadesse G, Ephraim E, Ashenafi M (2005) Assessment of the antimicrobial activity of lactic acid bacteria isolated from Borde and Shamita traditional Ethiopian fermented beverages, on some foodborne pathogens and effect of growth medium on the inhibitory activity. Internet Journal of Food Safety 5: 13-20.
- Afolabi OR, Bankole OM, Olaitan OJ (2008) Production and characterization of antimicrobial agents by lactic acid bacteria isolated from fermented foods. The Internet Journal of Microbiology 4: 2.
- Adesokan IA, Odetoyinbo BB, Olubamiwa AO (2008) Biopreservative activity of lactic acid bacteria on suya produced from poultry meat. African Journal of Biotechnology 7: 3799-3803.
- Raccah M, Baker RC, Degenstein JM, Mulnix EJ (1979) Potential application of microbial antagonism to extend storage ability of a flesh type food. Journal of Food Science 44: 43-46.
- Smith JL, Palumbo SA (1983) Use of starter cultures in meat. Journal of Food Protection 46: 997-1006
- Cintas LM, Casaus P, Holo H, Hernandez PE, Nes IF, et al. (1998) Enterocins L50A and L50B, two novel bacteriocins from Enterococcus faecium L50, are related to staphylococcal hemolysins. Journal of Bacteriology 180: 1988-1994
- Olasupo NA, Schilinger U, Holzapfel WH (2001) Studies on some technological properties of predominant lactic acid bacteria isolated from Nigerian fermented foods. Food Biotechnology 15: 157-167.
- Olanrewaju OO, Victor OO, Titilayo TA (2009) Safety of Small Scale Food Fermentations in Developing Countries. Internet Journal of Food Safety 11: 29-34
- Elhag NB, Bahiker ERB, Mahdi AA (2014) Effect of bacteriocins producing lactic acid bacteria on target microorganism. Journal of Agriculture, Food and Applied Sciences 2: 155-162
- Flythe MD, Russell JB (2004) The effect of pH and a bacteriocin (bovicin HCS) on Clostridium sporogenes MD1, a bacterium that has the ability to degrade amino acids in ensiled plant materials. FEMS Microbial Ecology 47: 215-222.
- De Vuyst L, Leroy F (2007) Bacteriocins from lactic acid bacteria: production, purification and applications. Journal of Molecular Microbiology and Biotechnology 13: 194-199.
- Todorov SD, Dick S (2004) Comparison of 2 methods for purification of plantaricin S731, a bacteriocin produced by Lactobacillus plantarum S731. Enzyme and Microbial Technology 36: 318-326.
- Karthikeyao V, Santhush SW (2009) Study of bacteriocin as a food preservative and the L. acidophilus strain as probiotic. Pakistan Journal of Nutrition 8: 335-340.
- Lisboa MP, Bonatto D, Bizani D, Hanques JA, Brandelli A (2006) Characterization of a bacteriocin-like substance produced by Bacillus amyloliquifaciens isolated from Brazilian atlantic forest. International Microbiology 9: 111-118.
- Roseland ET, Langsoud T, Granum PE, Sorhaug T (2005) Production of antimicrobials by strains of Lactobacillus or Lactococcus co-cultured with Bacillus cereus in milk. International Journal of Food Microbiology 88: 83-100.
- Wakil SM, Osamwonyi UO (2012) Isolation and screening of antimicrobial producing lactic acid bacteria from fermenting millet gruel. International Research Journal of Microbiology 3: 72-79.
- Daeschel MA (1993) Applications and interactions of bacteriocins from lactic acid bacteria in foods and beverages. In: Bacteriocins of Lactic Acid Bacteria, Academic Press, New York, USA, pp: 63-91.
Citation: Ukwuru MU, Ohaegbu CG (2018) Influence of Physical Parameters on Growth and Bacteriocin Activity by Species of Lactic Acid Bacteria Isolated from Fermenting Foods. J Biochem Microb Toxicol 1: 104.
Copyright: © 2018 Ukwuru MU, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 4977
- [From(publication date): 0-2018 - Sep 23, 2025]
- Breakdown by view type
- HTML page views: 4047
- PDF downloads: 930