Influence of Media Type and Carbon Source on Callus Induction and Regeneration Response of Different Indica Rice Genotypes
Received: 16-May-2019 / Accepted Date: 29-May-2019 / Published Date: 07-Jun-2019 DOI: 10.4172/2329-8863.1000425
Abstract
Suitable carbon source and an ideal media type are essential pre-requisites for rice tissue culture and transformation experiments. Maltose is found to be the best carbon source and MS media is found to be the ideal media type for the results obtained from callus induction and regeneration capabilities of seven indica rice genotypes taken under study. Varied tissue culture response of selected genotypes is found conspicuous at different sugar concentrations.
Keywords: Carbon source; Media type; Rice tissue culture; Indica rice genotypes
Introduction
Indica rice varieties are known to be recalcitrant to culture and several attempts are made throughout the years to improve tissue culture protocols for effective callus induction and regeneration- in vitro [1-3]. Several factors of the culture media play a critical role in tissue culture response of the various rice genotypes. Types of the media used, and carbon sources are two vital factors among them. Moreover, genotypic differences do exist in response to the culture media [4-7]. In this study attempts were made to find the suitable media for rice tissue culture between the two widely used media viz ., MS and N6. Response of the cultivars towards varied carbon sources and concentrations is studied. Browning of callus in long standing rice tissue cultures is one of the major concerns which hamper the regeneration capacities of the rice cultivars [8,9]. This is observed in rice cultures grown on sucrose, compared to maltose. This study envisaged to find out the ideal concentrations of sucrose and maltose to be used for ideal green plant regeneration. This study holds importance because the choice of best responding variety to suitable sugar concentration in an amenable media is an essential pre-requisite for gene transformation experiments and efficient regeneration of the successful transformants. Results obtained were tabulated and the consequences are discussed.
Materials and Methods
Genotypes
The rice genotypes selected for the study were elite indica rice cultivars Swarna, Gayatri, Samba Mahsuri, Pooja, Tapaswini, Sahabhagidhan and Anjali. Swarna, a widely grown variety in eleven states of India, is highly popular with a yield potential of 8.0 t/ha [10]. It is also being widely grown in Bangladesh and Myanmar suggesting its wide adaptability [11]. Gayatri, a high yielding cultivar released from NRRI, is widely grown in shallow and medium low land ecology in Eastern India. Samba Mahsuri (BPT 5204) is one of the India’s most popular and highly prized rice varieties because of its high yield and excellent cooking quality [12].
Pooja, a high yielding cultivar released from NRRI, is widely grown in shallow and medium low land ecology in Eastern India [13]. Shabhagidhan is a popular variety suitable for upland, rainfed direct seeded as well as transplanted conditions. It is released for cultivation in states of Jharkhand and Odisha. It bears golden husked long bold grains and has an average productivity of 3.8-4.5 t/ha [14]. Tapaswini is an elite indica rice variety with a yield potential of 5.0 t/ha [15,16]. Anjali is an upland early maturing rice variety released from NRRI [17]. Characteristic features of seven different genotypes taken in study are given in Table 1 [18].
| S. No | Name of Variety | Duration (in days) | Grain type Q/ha. | Yield | Year of notification | Parentage |
|---|---|---|---|---|---|---|
| 1 | Swarna | 150 | Short bold | 72 | 1983 | Vasista × Mahsuri |
| 2 | Gayatri | 160 | short bold | 46 | 1988 | Pankaj/Jagannath |
| 3 | Samba Mahsuri | 145 | medium slender | 55 | 1979 | GEB-24 × T(N)1 × Mahsuri |
| 4 | Pooja | 150 | medium slender | 46 | 1990 | Vijaya × T.141 |
| 5 | Tapaswini | 135 | medium slender | 55 | 1996 | Jagannath × Mahsuri |
| 6 | Shabhagidhan | 100 | long bold | 41 | 2009 | IR 55419-04*2. Way Rarem (IR 55419-04 (IR 12979-24-1 (Brown)/UPRLRi5 |
| 7 | Anjali | 90 | short bold | 32 | 2003 | RR-19-2 × RR-149-1129 |
Table 1: Characteristic features of seven different genotypes taken in study.
Mature dehusked grains of the selected genotypes were washed with sterile distilled water and were surface sterilized successively with, 70% ethanol for two min, sodium hypochlorite (contains 4% (v/v) active chlorine) for 15 min and with 0.1% (w/v) aqueous mercuric chloride solution for 5 min with intermittent repeated washings with sterile distilled water [19].
The kernels were inoculated in culture tubes in two separate experiments containing semisolid callus induction (CI) medium in two separate media namely MS [20] and N6 [21], each supplemented with 2,4-D (2.0 mg l-1) and Kn (0.5 mg l-1) were evaluated for their potential to support callus induction and subsequent green plant regeneration, of the callus induced on these two media from different genotypes. Calli developed on these media were transferred onto MS regeneration medium supplemented with phytohormones [NAA (0.5 mg l-1)+Kn (0.5 mg l-1)+BAP (1.5 mg l-1)]. Callus induction (CI) and regeneration frequencies (RF) were recorded and statistical analyses were performed using SAS software [6].
Results and Discussion
Influence of media types on callus induction and regeneration response of different rice genotypes
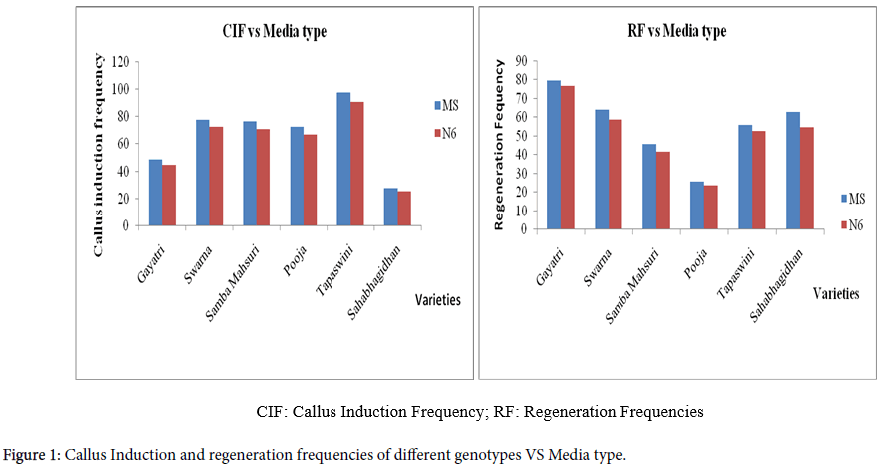
This experiment was conducted with an objective of identifying suitable medium for raising the somatic cell cultures in indica rice so that the identified medium can be effectively used for rice tissue culture and transformation purposes. The genotypes showed varied response on both the media with respect to callus induction (Table 2).
| S. No | Genotype | CI (%) | RE (%) | |||
|---|---|---|---|---|---|---|
| MS | N6 | MS | N6 | |||
| 1 | Gayatri | F | 48.6 | 44.5 | 79.6 | 76.4 |
| SD | 1.2 | 0.9 | 0.9 | 1.2 | ||
| SE | 0.6 | 0.5 | 0.5 | 0.6 | ||
| 2 | Swarna | F | 77.6 | 72.53 | 63.76 | 58.56 |
| SD | 0.8 | 1.23 | 1 | 0.85 | ||
| SE | 0.4 | 0.7 | 0.58 | 0.49 | ||
| 3 | Samba Mahsuri | F | 76.3 | 70.76 | 45.5 | 41.73 |
| SD | 0.9 | 1.05 | 0.79 | 1.15 | ||
| SE | 0.5 | 0.61 | 0.45 | 0.66 | ||
| 4 | Pooja | F | 72.5 | 66.67 | 25.76 | 23.43 |
| SD | 0.9 | 1.16 | 0.96 | 1.26 | ||
| SE | 0.5 | 0.67 | 0.55 | 0.73 | ||
| 5 | Tapaswini | F | 97.4 | 90.73 | 55.6 | 52.6 |
| SD | 1.1 | 0.9 | 1.11 | 0.91 | ||
| SE | 0.6 | 0.52 | 0.64 | 0.52 | ||
| 6 | Sahabhagidhan | F | 27.5 | 25.46 | 62.63 | 54.6 |
| SD | 1 | 0.7 | 0.97 | 1.15 | ||
| SE | 0.6 | 0.4 | 0.56 | 0.66 | ||
Table 2: Influence of media type on callus induction and regeneration response of indica rice varieties.
MS medium, in general, proved to be better for early callus induction and calli induced on this medium showed good levels of green plant regeneration in all the genotypes tested compared to N6 medium (Figure 1).
Even though, compositions of the both the media are distinctive, ratio of nitrogen between nitrate form and ammonium form during plant utilization in the media was found to play a critical role in somatic embryogenesis of monocots [22,23] and the results showed that rice genotypes responded better on MS medium compared to N6 medium.
Most desirable strategy to obtain better regenerati on is to use the most appropriate medium [24]. MS medium is the best suitable medium as per the results obtained. The analysis of variance conducted with the six varieties suggests that the differences between media, genotypes and interaction were significant for both callus induction and green plant regeneration. (Table 3).
| A. Callus induction | |||||
|---|---|---|---|---|---|
| Source of Variation | % of total variation | p-value | p-value summary | Significant? | p-value |
| Interaction | 0.1153 | 0.011 | * | Yes | -- |
| Media | 1.24 | <0.0001 | **** | Yes | -- |
| varieties | 98.5 | <0.0001 | **** | Yes | -- |
| ANOVA table | SS | DF | MS | F (DFn, DFd) | p-value |
| Interaction | 20.09 | 5 | 4.019 | F (5,24)=3.813 | P=0.0110 |
| Media | 216.1 | 1 | 216.1 | F (1,24)=205.0 | P<0.0001 |
| varieties | 17164 | 5 | 3433 | F (5,24)=3257 | P<0.0001 |
| Residual | 25.29 | 24 | 1.054 | ||
| B. Regeneration | |||||
| Source of Variation | % of total variation | p-value | p-value summary | Significant? | p-value |
| Interaction | 0.3267 | 0.0009 | *** | Yes | -- |
| Media | 1.648 | <0.0001 | **** | Yes | -- |
| Varieties | 97.77 | <0.0001 | **** | Yes | -- |
| ANOVA table | SS | DF | MS | F (DFn, DFd) | p-value |
| Interaction | 32.48 | 5 | 6.495 | F (5,24)=6.078 | P=0.0009 |
| Media | 163.8 | 1 | 163.8 | F (1,24)=153.3 | P<0.0001 |
| Varieties | 9720 | 5 | 1944 | F (5, 24)=1819 | P<0.0001 |
| Residual | 25.65 | 24 | 1.069 | ||
Table 3: Analysis of variance on the media type for in vitro response.
Effect of carbon sources on callus induction and plant regeneration
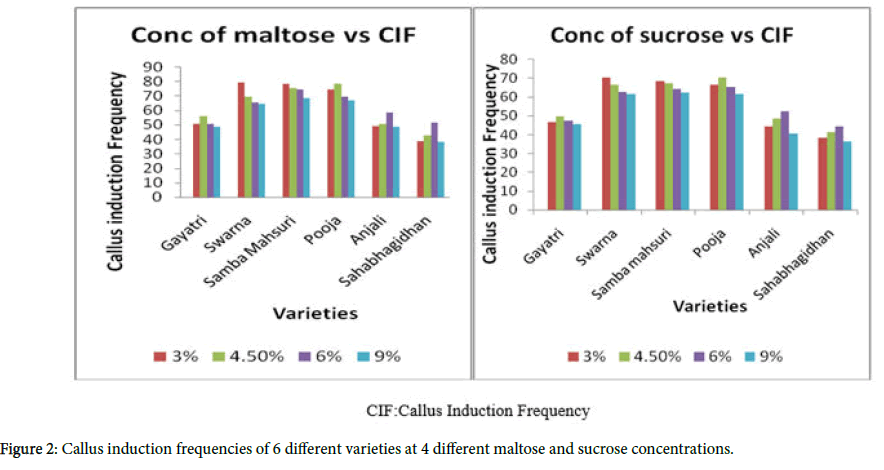
This experiment was conducted with the objective of identifying the ideal carbon source for rice tissue culture which can be employed later for transformation purposes. Two sugars i.e., sucrose and maltose were tested at four different levels as the carbon sources in the MS callus induction medium and the responses of six genotypes, Gayatri, Swarna, Samba Mahsuri, Pooja, Anjali, and Sahabhagidhan were studied.
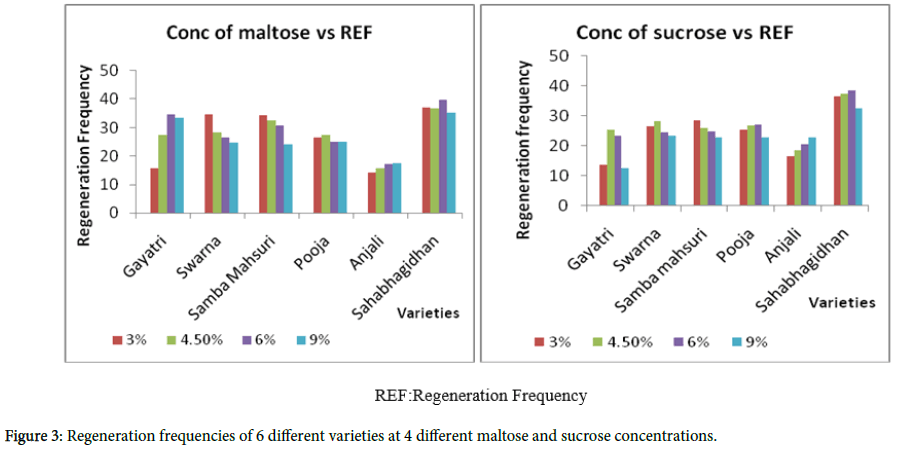
Genotypic differences were observed for both callus induction (Figures 2 and 3) and regeneration (Table 4) in response to different concentrations of sucrose and maltose in the callus induction medium.
| a) Callus induction | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S. No | Varieties | % | Carbon source | |||||||
| Maltose | Sucrose | |||||||||
| 3% | 4.50% | 6% | 9% | 3% | 4.50% | 6% | 9% | |||
| 1 | Gayatri | CIF | 50.7 | 56.4 | 50.6 | 48.56 | 46.63 | 49.8 | 47.36 | 45.36 |
| SD | 2.05 | 2.2 | 2.2 | 1.72 | 3.85 | 3.9 | 4.05 | 4.1 | ||
| SE | 1.18 | 1.27 | 1.27 | 0.99 | 2.22 | 2.25 | 2.34 | 2.36 | ||
| 2 | Swarna | CIF | 79.8 | 69.4 | 65.73 | 64.53 | 70.5 | 66.6 | 62.7 | 61.63 |
| SD | 2.05 | 2.1 | 1.33 | 1.92 | 3.75 | 3.85 | 4.05 | 3.65 | ||
| SE | 1.18 | 1.2 | 0.76 | 1.1 | 2.16 | 2.22 | 2.33 | 2.1 | ||
| 3 | Samba Mahsuri | CIF | 78.5 | 75.56 | 74.66 | 68.53 | 68.53 | 67.43 | 64.4 | 62.46 |
| SD | 2.26 | 1.82 | 1.97 | 1.85 | 4.35 | 3.85 | 4.3 | 4.26 | ||
| SE | 1.3 | 1.05 | 1.14 | 1.06 | 2.51 | 2.22 | 2.48 | 2.46 | ||
| 4 | Pooja | CIF | 74.36 | 78.53 | 69.6 | 67.36 | 66.67 | 70.53 | 65.46 | 61.6 |
| SD | 2.05 | 1.7 | 2.35 | 2.35 | 4.152 | 3.8 | 4.3 | 3.91 | ||
| SE | 1.18 | 0.98 | 1.35 | 1.35 | 2.39 | 2.19 | 2.48 | 2.26 | ||
| 5 | Anjali | CIF | 49.4 | 50.66 | 58.53 | 48.7 | 44.34 | 48.56 | 52.6 | 40.6 |
| SD | 2.1 | 1.97 | 1.7 | 2.2 | 3.75 | 4.1 | 3.9 | 4.05 | ||
| SE | 1.21 | 1.14 | 0.98 | 1.27 | 2.16 | 2.37 | 2.25 | 2.33 | ||
| 6 | Sahabhagidhan | CIF | 38.56 | 42.8 | 51.6 | 38.43 | 38.4 | 41.53 | 44.56 | 36.46 |
| SD | 1.8 | 1.95 | 2.15 | 1.88 | 4.2 | 4.11 | 4.25 | 3.91 | ||
| SE | 1.03 | 1.12 | 1.24 | 1.08 | 2.42 | 2.37 | 2.45 | 2.25 | ||
| CIF: Callus induction frequency; SD: Standard Deviation; SE: Standard Error | ||||||||||
| b) Regeneration | ||||||||||
| S. No | Varieties | Carbon source | ||||||||
| Maltose | Sucrose | |||||||||
| 3 | 4.5 | 6 | 9 | 3 | 4.5 | 6 | 9 | |||
| 1 | Gayatri | REF | 15.57 | 27.26 | 34.63 | 33.56 | 13.8 | 25.53 | 23.4 | 12.6 |
| SD | 2.15 | 2.57 | 2.31 | 1.94 | 2.56 | 2.6 | 3.02 | 2.2 | ||
| SE | 1.24 | 1.48 | 1.34 | 1.12 | 1.47 | 1.52 | 1.74 | 1.27 | ||
| 2 | Swarna | REF | 34.63 | 28.43 | 26.53 | 24.8 | 26.57 | 28.37 | 24.57 | 23.57 |
| SD | 2.15 | 1.75 | 1.76 | 2.05 | 1.91 | 1.95 | 2.11 | 1.87 | ||
| SE | 1.24 | 1.01 | 1.02 | 1.18 | 1.1 | 1.12 | 1.21 | 1.08 | ||
| 3 | Samba Mahsuri | REF | 34.46 | 32.46 | 30.7 | 24.13 | 28.6 | 26.13 | 24.76 | 22.76 |
| SD | 1.88 | 1.7 | 1.9 | 3.19 | 2.61 | 2.11 | 1.95 | 2.1 | ||
| SE | 1.08 | 0.98 | 1.1 | 1.84 | 1.51 | 1.22 | 1.12 | 1.21 | ||
| 4 | Pooja | REF | 26.53 | 27.26 | 25.13 | 24.86 | 25.5 | 26.73 | 27.1 | 22.76 |
| SD | 2.15 | 2.04 | 2.05 | 2.45 | 2.16 | 1.85 | 1.75 | 2.05 | ||
| SE | 1.24 | 1.17 | 1.18 | 1.41 | 1.25 | 1.06 | 1.01 | 1.18 | ||
| 5 | Anjali | REF | 14.1 | 15.56 | 17.16 | 17.6 | 16.63 | 18.56 | 20.66 | 22.73 |
| SD | 2.21 | 2.21 | 2 | 3.75 | 2 | 1.8 | 2 | 2.15 | ||
| SE | 1.27 | 1.27 | 1.15 | 2.17 | 1.18 | 1.06 | 1.18 | 1.24 | ||
| 6 | Sahabhagidhan | REF | 37 | 36.6 | 39.83 | 35.3 | 36.7 | 37.46 | 38.7 | 32.53 |
| SD | 1.96 | 4.35 | 1.79 | 2.38 | 2.05 | 2.05 | 1.96 | 2.3 | ||
| SE | 1.13 | 2.51 | 1.03 | 1.37 | 1.18 | 1.18 | 1.13 | 1.32 | ||
Table 4: Effect of sugars on in vitro response in different indica rice varieties.
Addition of higher levels (>6%) of sucrose to the callus induction media did not show any significant enhancement of callus induction when the pooled data of all the six genotypes was taken into consideration.
The analysis of variance of the data suggests that the differences between various concentrations of sucrose and maltose and genotypes were significant for both callus induction, and green plant regeneration (Table 5).
| Callus induction with maltose | |||||
|---|---|---|---|---|---|
| Source of Variation | % of total variation | p-value | p-value summary | Significant? | |
| Interaction | 7.863 | <0.0001 | **** | Yes | |
| % maltose | 3.896 | <0.0001 | **** | Yes | |
| Varieties | 86.68 | <0.0001 | **** | Yes | |
| ANOVA table | SS | DF | MS | F (DFn,DFd) | p-value |
| Interaction | 967.2 | 15 | 64.48 | F (15,48)=16.08 | P<0.0001 |
| % maltose | 479.2 | 3 | 159.7 | F (3,48)=39.83 | P<0.0001 |
| Varieties | 10661 | 5 | 2132 | F (5,48)=531.6 | P<0.0001 |
| Residual | 192.5 | 48 | 4.011 | ||
| Callus induction with sucrose | |||||
| Source of Variation | % of total variation | p-value | p-value summary | Significant? | |
| Interaction | 3.538 | 0.1717 | ns | No | |
| Sucrose % | 3.858 | 0.0002 | *** | Yes | |
| Varieties | 84.69 | <0.0001 | **** | Yes | |
| ANOVA table | SS | DF | MS | F (DFn, DFd) | p-value |
| Interaction | 347.5 | 15 | 23.17 | F (15, 48)=1.431 | P=0.1717 |
| Sucrose % | 378.9 | 3 | 126.3 | F (3,48)=7.800 | P=0.0002 |
| Varieties | 8319 | 5 | 1664 | F (5,48)=102.7 | P<0.0001 |
| Residual | 777.3 | 48 | 16.19 | ||
| Regeneration with maltose | |||||
| Source of Variation | % of total variation | p-value | p-value summary | Significant? | |
| Interaction | 24.91 | <0.0001 | **** | Yes | |
| Maltose % | 1.346 | 0.0262 | * | Yes | |
| Varieties | 67.33 | <0.0001 | **** | Yes | |
| ANOVA table | SS | DF | MS | F (DFn,DFd) | p-value |
| Interaction | 1047 | 15 | 69.77 | F (15,48)=12.44 | P<0.0001 |
| Maltose % | 56.56 | 3 | 18.85 | F (3,48)=3.361 | P=0.0262 |
| Varieties | 2828 | 5 | 565.7 | F (5,48)=100.8 | P<0.0001 |
| Residual | 269.2 | 48 | 5.609 | ||
| Regeneration with sucrose | |||||
| Source of Variation | % of total variation | p-value | p-value summary | Significant? | |
| Interaction | 13.7 | <0.0001 | **** | Yes | |
| Sucrose % | 6.402 | <0.0001 | **** | Yes | |
| Varieties | 72.95 | <0.0001 | **** | Yes | |
| ANOVA table | SS | DF | MS | F (DFn,DFd) | p-value |
| Interaction | 441.8 | 15 | 29.45 | F (15,48)=6.312 | P<0.0001 |
| Sucrose % | 206.4 | 3 | 68.79 | F (3,48)=14.74 | P<0.0001 |
| Varieties | 2352 | 5 | 470.3 | F (5,48)=100.8 | P<0.0001 |
| Residual | 224 | 48 | 4.666 | ||
Table 5: Anova on response of genotypes to different levels of sugars.
A level of maltose at 3% was found to be ideal for genotypes like Swarna and Samba Mahsuri for obtaining high rates of callus induction and regeneration, where as 4.5% of maltose was ideal in case of Gayatri and Pooja for inducing a high rate of callus induction, but the result varied with respect to regeneration i.e., Gayatri showed good regeneration at 9.0% sucrose while Pooja showed good regeneration at 6% maltose. For genotypes like Anjali and Sahabagidhan, 6% maltose was better for callus induction while 9% of sucrose yielded higher levels of regeneration in case of Anjali and 6% maltose in case of Sahabhagidhan. As compared to sucrose, maltose proved to be better, for the callus induction, although genotypic differences were conspicuous.
This can be attributed to the reduced rate of hydrolysis of the maltose to glucose and glucose-1-phosphate [25-27] thereby providing a readily metabolized carbon source over a long period in culture and improvement of osmotic stability of the culture medium in comparison to sucrose [28]. This is in congruence with earlier research works of Kumria et al. [29], Zaidi et al. [5] and Bagheri et al. [30].
There was significant influence regarding the level of sucrose or maltose in the callusing media [31] and also on the regeneration potential of the calli depending on the genotypes (Table 4). However, browning of the callus is observed due to the ethylene produced under the influence of sucrose on regeneration of calli. This is in congruence with the research studies of Hidekazu Kobayashi and Hitoshi Saka [32]. This is one of the reasons why maltose is preferred over sucrose [33].
Conclusion
Ideal carbon source and an amenable media are essential prerequisites for best tissue culture response of selected indica rice genotypes. The results of this study showed that MS media and maltose are best sources for tissue culture response. However, variation in concentrations of the sugar source is to be judged in response to type of genotype chosen. This study holds importance in improvement of protocols for rice tissue culture and future transformation experiments for crop improvement.
Acknowledgements
The authors are thankful to Director, NRRI for the facilities and encouragement. The authors Sai Krishna Repalli and Chaitanya Kumar Geda are thankful to ICAR (NPTC) for providing them Fellowship.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Aldemita RR, Hodges TK (1996) Agrobacterium tumefaciens-mediated transformation of japonica and indica rice varieties. Planta 199: 612-617.
- Cho MJ, Yano H, Okamoto D, Kim HK, Jung HR, et al. (2004) Stable transformation of rice (Oryza sativa L.) via microprojectile bombardment of highly regenerative, green tissues derived from mature seed. Plant Cell Reports 22: 483-489.
- Afolabi AS, Oyebanji O, Odusanya O, Abo ME, Misra M, et al. (2008) Regeneration of plants from rice caryopsis derived callus culture of Nigerian local cv. Suakoko 8 and a NERICA cv. FARO 55. African Journal of Plant Science 2: 109-112.
- Khanna HK, Raina SK (2002) Elite indica transgenic rice plants expressing modified Cry1Ac endotoxin of Bacillus thuringiensis show enhanced resistance to yellow stem borer (Scirpophaga incertulas). Transgenic Research 11: 411-423.
- Zaidi MA, Narayanan M, Sardana R, Taga I, Postel S, et al. (2006) Optimizing tissue culture media for efficient transformation of different indica rice genotypes. Agron Res 4: 563-575.
- Chaitanya KG, Krishna RS, Dev TM, Rao GJN (2013) Genetic variation in in vitro response of elite aromatic and non-aromatic rice varieties. ORYZA-An International Journal on Rice 50: 329-333.
- Repalli SK, Ananata MB, Prasanta KD (2018) In vitro responses of ten indica rice genotypes for callus induction. Ann Agric Res 39: 26-31.
- Maeda E, Sato T, Suzuki K (2002) Microtopography and shoot-bud formation of rice (Oryza sativa) callus. Plant Biotechnology 19: 69-80.
- Qian H, Zhang X, Xue Q (2004) Factors affecting the callus induction and gus transient expression in indica rice Pei;ai 64s. Pakistan Journal of Biological Sciences 7: 615-619.
- Rao VR, Reddy PS, Murthy N, Rao I, Rao PS, et al. (1983) Swarna (MTU 7029)-A new stable hybrid with wide adaptation. Oryza 20: 240-242.
- Baisakh N, Datta K, Oliva N, Ona I, Rao GJN, et al. (2001) Rapid development of homozygous transgenic rice using anther culture harboring rice chitinase gene for enhanced sheath blight resistance. Plant Biotechnology 18: 101-108.
- Reddy MV, Ssndb P, Reddy BM, Rao LVS (1979) BPT 5204-A new rice variety for kharif season for coastal districts of Andhra Pradesh [India]. Note. Andhra Agricultural Journal.
- Diwakar MC (2014) Status paper on rice, Directorate of Rice Development. Patliputra Colony, Patna.
- Panda AR (2000) Growing Rice Variety Tapaswini-Technical Bulliten, CRRI Cuttack, Odisha.
- Dokku P, Das KM, Rao GJN (2013) Pyramiding of four resistance genes of bacterial blight in Tapaswini, an elite rice cultivar, through marker-assisted selection. Euphytica 192: 87-96.
- Sinha PK (2002) Upland rice package and practices-Variety Anjali- CRRI Technical Bulliten-18, CRRI, Cuttack, Odisha.
- Vijayachandra K, Palanichelvam K, Veluthambi K (1995) Rice scutellum induces Agrobacterium tumefaciens vir genes and T-strand generation. Plant Molecular Biology 29: 125-133.
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum 15: 473-497.
- Chu CC (1978) The N_6 medium and its applications to anther culture of cereal crops. In Proceedings of Symposium on Plant Tissue Culture, pp: 43-50.
- Ge X, Chu Z, Lin Y, Wang S (2006) A tissue culture system for different germplasms of indica rice. Plant Cell Reports 25: 392-402.
- Geng P, La H, Wang H, Stevens EJ (2008) Effect of sorbitol concentration on regeneration of embryogenic calli in upland rice varieties (Oryza sativa L.). Plant Cell, Tissue and Organ Culture 92: 303-313.
- Wanichananan P, Teerakathiti T, Roytrakul S, Kirdmanee C, Peyachoknagul S (2010) A highly efficient method for Agrobacterium mediated transformation in elite rice varieties (Oryza sativa L. spp. indica). African Journal of Biotechnology 9: 5488-5495.
- Sopory SK (1979) Effect of sucrose, hormones, and metabolic inhibitors on the development of pollen embryoids in anther cultures of dihaploid Solanum tuberosum. Canadian Journal of Botany 57: 2691-2694.
- Li XQ, Liu CN, Ritchie SW, Peng JY, Gelvin SB, et al. (1992) Factors influencing Agrobacterium-mediated transient expression of gusA in rice. Plant Molecular Biology 20: 1037-1048.
- Lentini Z, Reyes P, MartÃnez CP, Roca WM (1995) Androgenesis of highly recalcitrant rice genotypes with maltose and silver nitrate. Plant Science 110: 127-138.
- Last DI, Brettell RI (1990) Embryo yield in wheat anther culture is influenced by the choice of sugar in the culture medium. Plant Cell Reports 9: 14-16.
- Kumria R, Waie B, Rajam MV (2001) Plant regeneration from transformed embryogenic callus of an elite indica rice via Agrobacterium. Plant Cell, Tissue and Organ Culture 67: 63-71.
- Bagheri N, Babaeian-Jelodar N, Ghanbari A (2009) Evaluation of effective factors in anther culture of Iranian rice (Oryza sativa L.) cultivars. Biharean Biologist 3: 119-124.
- Mostafiz BS, Wagiran A (2018) Efficient callus induction and regeneration in selected indica rice. Agronomy 8: 77.
- Kobayashi H, Saka H (2000) Relationship between ethylene evolution and sucrose content in excised leaf blades of rice. Plant Production Science 3: 398-403.
- Darachai P, Chutipaijit S, Sompornpailin K (2004) Carbon sources and supporting materials in callus induction effects on regeneration of indica Rice (Oryza sativa L. cv. RD6 and RD15). In Proc. of The 8th International Symposium on Biocontrol and Biotechnology pp: 4-6.
Citation: Repalli SK, Geda CK, NSN P, Rao GJN (2019) Influence of Media Type and Carbon Source on Callus Induction and Regeneration Response of Different Indica Rice Genotypes. Adv Crop Sci Tech 7: 425. DOI: 10.4172/2329-8863.1000425
Copyright: © 2019 Repalli SK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3614
- [From(publication date): 0-2019 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 2786
- PDF downloads: 828