Research Article Open Access
Influence of Housing Density and Grazing on Heat Shock Protein 27 Expression in Skeletal Muscle of Beef Cattle
Masahiro Shibata*, Yasuko Hikino, Kazunori Matsumoto and Naoyuki Yamamoto
Livestock Production and Wildlife Management Research Division, NARO Western Region Agricultural Research Center, Ohda, Shimane 694-0013, Japan
- *Corresponding Author:
- Masahiro Shibata
Livestock Production and Wildlife Management Research Division
NARO Western Region Agricultural Research Center
Ohda, Shimane 694-0013, Japan
Tel: +81-854-82-0144
Fax: +81-854-82-2280
E-mail: shibatam@affrc.go.jp
Received Date: August 05, 2014; Accepted Date: September 01, 2014; Published Date: September 06, 2014
Citation: Shibata M, Hikino Y, Matsumoto K, Yamamoto N (2014) Influence of Housing Density and Grazing on Heat Shock Protein 27 Expression in Skeletal Muscle of Beef Cattle. J Fisheries Livest Prod 2:117. doi: 10.4172/2332-2608.1000117
Copyright: © 2014 Shibata M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Fisheries & Livestock Production
Abstract
Heat shock proteins (HSPs) are used as a stress biomarker in several studies as well as other stress biomarker, but the influence of different rearing environment on HSPs expression with other stress biomarker in cattle is unclear. To clear this point, two experiments were conducted to investigate the influence of rearing environment on HSP 27 in steer muscle. In experiment 1, 10 Japanese black steers were divided into 2 groups for a housing density stress experiment: a high-density (HD) group and freedom (FR) group. IgG levels and the neutrophil-to-lymphocyte (N/L) ratio in blood was analyzed as a stress marker. IgG levels and N/L ratios in the HD group were higher than those in the FR group. HSP 27 expression in the semitendinosus (ST) muscle of the HD group was higher than that of the FR group. These results suggest that HSP 27 expression may reflect the influence of the stress response with IgG levels and N/L ratios. In experiment 2, 8 Japanese Black steers were divided into 2 groups for a feeding experiment: a grazing (GR) group and concentrate-fed (CT) group. Blood IgG levels in the GR group after grazing were lower than those in the CT group. Expressions of the HSP 27 gene and its protein in the GR group were decreased in the longissimus lumborum and ST muscles compared with those in the CT group at the end of grazing. HSP 27 gene expression in the ST muscle of the GR group was lower after grazing than before grazing, but there was no significant difference in its expression in the CT group before and after grazing. The present study suggests that HSP 27 probably reflects not only differences in the rearing systems, but also the influence of the stress reaction.
Keywords
Beef cattle; Grazing; Heat shock protein 27; Housing density; IgG; Stress
Introduction
Several stressors have influences on growth, reproduction, and meat quality in livestock production. Evaluation of various physiological and psychological stressors, such as housing density [1], transport [2], weaning [3], heat [4], examination [5], and job strain [6], have been studied in several animals using biomarkers. Cows grazed on native grassland are stressed more easily than those housed in group pens by exposure to high temperatures, high humidity, and high solar radiation in the hot season [7]. Our previous study shows that decrease in expression of heat shock protein (HSP) 27 occurred in grazed cattle by skeletal muscle proteome analysis [8]. HSPs play an important role in regulating protein folding and coping with proteins affected by heat and other stresses [9]. HSPs behave as molecular chaperones by binding to other cellular proteins, assisting intracellular transport, and folding into adequate secondary structures, thus preventing condensation of protein during stress [10,11]. HSPs have a homeostatic function in living tissues, stabilizing unfolded proteins, assisting with refolding of denatured proteins and preventing protein aggregation [12]. Tissues and cells up-regulate expression of HSP in respond to stress. Feasson et al. [13] indicates that an increase in the HSP 27 protein in skeletal muscle was observed after eccentric exercise stress compared with that before the stress. Another study reports that transport-stressed pigs show a higher level of HSP 70 in the heart and kidney than do control pigs [14]. Thus, although HSPs are used as a stress biomarker in several studies as well as other stress biomarker, the influence of different rearing environment on HSPs expression with other stress biomarker in cattle is unclear.
The aim of the present study was to investigate the expression of well-known stress biomarkers and HSP 27 in beef cattle under various feeding conditions. Whether expression of HSP 27 reflects differences in the rearing system of cattle is unknown. We hypothesized that expression of HSP 27 alters by the difference in the feeding environment of cattle, and moreover, its expression would be employed as a stress biomarker of cattle. To verify this hypothesis, we investigated whether HSP 27 expression occurs with changes in the expression of known stress biomarkers under stress conditions by high-density housing. Furthermore, to examine changes in the expression of HSP 27 in different rearing conditions, we measured HSP 27 expression in outdoor-grazed and indoor concentrate-fed steers.
Materials and Methods
Animal management
Management of steers and all procedures were performed according to the Animal Experimental Guidelines of the NARO Western Region Agricultural Research Center (NARO/WARC), Japan.
Experiment design and sample collection
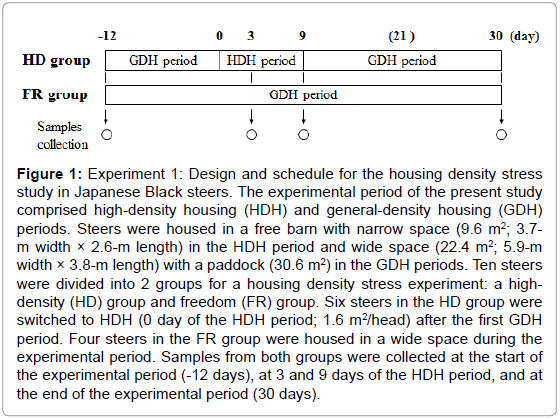
In experiment 1, 10 Japanese Black steers of 8 months of age that had been bred at NARO/WARC were randomly selected and divided into 2 groups for a housing density stress experiment: a high-density (HD, 261 ± 12.3 kg, n = 6) group as the stress test group and a freedom (FR, 252 ± 6.26 kg, n = 4) group. Figure 1 shows details of the experimental design and schedule. All steers were housed in a free barn and fed a commercial concentrate diet (flaked corn, grain sorghum, corn gluten feed, wheat bran, and soybean meal; 17% CP and 70% TDN, Nishi-nihon Kumiai Shiryou, Hyogo, Japan) at 3.0 to 3.6 kg/head/day according to the Japanese feeding standard for beef cattle and grass hay (7.2% CP and 59% TDN on a dry matter basis) ad libitum, and all had free access to water. Blood samples were collected from both groups at the start of the experiment (-12 days), at 3 and 9 days after high-density housing, and at the end of the experimental period (30 days) (Figure 1) to measure immunoglobulin G (IgG) levels and neutrophil-to-lymphocyte (N/L) ratios. A portion of the blood samples were separated to obtain serum by centrifugation and the serum samples were stored at -20°C until analysis. Skeletal muscle tissues from the longissimus lumborum (LL) and semitendinosus (ST) muscles were obtained by biopsy at the time of the blood sample [15]. These muscle samples were rapidly frozen in liquid nitrogen and stored at -80°C until RNA extraction.
Figure 1: Experiment 1: Design and schedule for the housing density stress study in Japanese Black steers. The experimental period of the present study comprised high-density housing (HDH) and general-density housing (GDH) periods. Steers were housed in a free barn with narrow space (9.6 m2; 3.7- m width × 2.6-m length) in the HDH period and wide space (22.4 m2; 5.9-m width × 3.8-m length) with a paddock (30.6 m2) in the GDH periods. Ten steers were divided into 2 groups for a housing density stress experiment: a highdensity (HD) group and freedom (FR) group. Six steers in the HD group were switched to HDH (0 day of the HDH period; 1.6 m2/head) after the first GDH period. Four steers in the FR group were housed in a wide space during the experimental period. Samples from both groups were collected at the start of the experimental period (-12 days), at 3 and 9 days of the HDH period, and at the end of the experimental period (30 days).
In experiment 2, 8 Japanese Black steers of 22 months of age that had been bred at NARO/WARC were randomly selected and divided into 2 groups: a grazing (GR, 654 ± 24.3 kg, n = 3) group and concentrate-fed (CT, 620 ± 22.8 kg, n = 5) group. They were housed individually in a tie stall barn and fed concentrate diet (flaked corn, flaked barley, wheat bran, and soybean meal; 11% CP and 73% TDN) ad libitum and grass hay (7.2% CP and 59% TDN on a dry matter basis) at 1.5 kg/day from 10 to 26 months of age. After this control period, the 3 steers of the GR group were placed on an outdoor pasture until 30 months of age, while the 5 steers of the CT group were continued on the concentrate and grass hay diet in the tie stall barn. Skeletal muscle tissues from the LL and ST muscles of both groups were obtained by biopsy at 22 months of age and at the end of fattening. These muscle samples were stored as described above. Blood samples were collected from both groups at the time of the muscle biopsy sample to measure IgG levels. The grass mass and chemical compositions of grass during the grazing period were analyzed by an outside laboratory (Table 1) (Agricultural Chemistry Research Institute, Hokkaido, Japan).
| Period 1 | Grass mass (kg of DM / ha) |
TDN (% of DM) |
CP (% of DM) |
ADF (% of DM) |
NDF (% of DM) |
|---|---|---|---|---|---|
| Initial Mid End |
1,746 2,524 3,902 |
71.2 69.7 72.2 |
14.2 17.1 7.8 |
20.0 21.6 18.0 |
36.4 42.4 36.4 |
1Initial, Mid, and End refer to samples taken at the beginning of grazing, at the middle of grazing, and at the end of grazing, respectively, during the experimental period.
Table 1: Grass mass and chemical compositions (% of DM) of grass during grazing.
Blood sample analyses
Serum samples were homogenized in SDS-PAGE buffer (pH 6.8) containing 50 mM Tris-HCl, 5% SDS, 10% (v/v) glycerol, and 5% (v/v) β-mercaptoethanol. Serum protein was separated by SDS-PAGE and transferred onto polyvinylidene difluoride membranes (PVDF; GE Healthcare, Fairfield, CT) by electroblotting. After blotting, the membranes were stained (Ponceau S; Sigma, St. Louis, MO) to verify equal loadings. The blotting membranes were blocked in Trisbuffered saline with Tween-20 (TBS-T) (pH 7.6) comprising 20 mM Tris-HCl, 137 mM NaCl, and 0.1% (v/v) Tween-20 with 2% BSA (Sigma) for 1 h, then incubated with a primary antibody specific for target protein for 1 h at room temperature. The following primary antibody was used for immunoblotting: 1:10,000 dilution of rabbit polyclonal antibody to bovine IgG (701-401-002; Rockland Inc., Gilbertsville, PA). The membrane was washed with TBS-T and further incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit IgG, 1:5,000 dilution (GE Healthcare) secondary antibody for 1 h at room temperature. The HRP activity was detected using an enhanced chemiluminescence (ECL) plus detection kit (GE Healthcare), and films were then scanned. The optical densities of proteins were analyzed using software (Diversity Database Ver.1.1; pdi, Huntington Station, NY). Whole blood was analyzed for the differential white blood cell counts by an outside laboratory (Japan Clinical Laboratories, Kyoto, Japan).
Muscle sample analyses
Total RNA was extracted from muscle tissues using TRIZOL reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. The first-strand cDNA was synthesized from 3 μg of total RNA using SuperScript II RNase H– reverse transcriptase (Invitrogen) with oligo(dT) primer (Amersham Pharmacia Biotech, Piscataway, NJ). After reverse transcription, the gene expression of HSP 27 was performed by real-time PCR using an ABI 7500 detection system (Applied Biosystems, Foster City, CA). The first-strand cDNA was diluted with deionized water and amplified using SYBR Green PCR Master Mix (Applied Biosystems) with gene-specific primers by realtime PCR (Table 2). Every pair of oligonucleotide primers was designed to amplify a region including at least 1 intron. The real-time PCR reaction was carried out initially for 2 min at 50°C, then for 10 min at 95°C, then 50 cycles for 15 s at 95°C, and then for 1 min at 60°C. The housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a normalizing control. The primers were designed using Primer Express (Applied Biosystems).
| Gene 2 | GenBank Accession No. | Forward primer (5’ to 3’) | Reverse primer (5’ to 3’) | Product size, bp |
|---|---|---|---|---|
| HSP 27 GAPDH |
NM001025569 U85042 |
TCCCTGGACGTCAACCACTT TGACCCCTTCATTGACCTTCA |
GGTGACGGGAATGGTGATCT ACCCCAGTGGACTCCACTACAT |
261 201 |
1All sequence data were from bovine sequences.
2HSP 27, heat shock protein, 27 kDa; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Table 2: Sequences of real-time PCR primers used in this study1.
Pulverized muscle tissue was homogenized in SDS-PAGE buffer (pH 6.8) as described above, then centrifuged at 15,000 × g for 10 min at 4°C. The supernatant fraction of each sample was taken, and the protein concentration was determined using a protein assay kit (RC DC; Bio-Rad Laboratories, Hercules, CA). Total protein was separated by SDS-PAGE and transferred onto PVDF membranes (GE Healthcare) by electroblotting. After blotting, the membranes were stained with Ponceau S (Sigma) to verify equal loadings. HSP 27 expression of the blotted membrane was detected using a 1:5,000 dilution of mouse monoclonal HSP 27 antibody (clone 8A7, Rockland Inc.). The following procedures were then carried out according to the method described above.
Statistical analysis
Gene expression and blood component data were represented as means. The relationships between the groups were analyzed using one-way analysis of variance (ANOVA) and a post-hoc Fisher test. The relationships between results before and after the treatment were analyzed using one-way ANOVA and a post-hoc Fisher test. A P value of <0.05 was considered statistically significant.
Results
Experiment 1
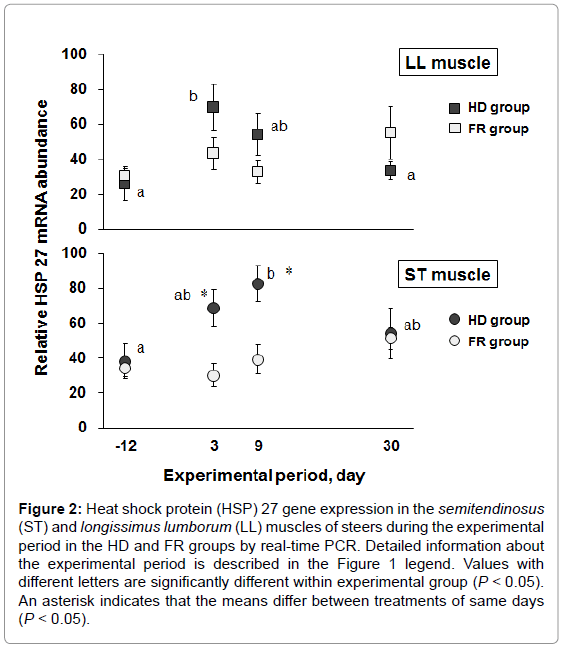
To investigate whether expression of HSP 27 varies with stress, we designed a steer stress model using different housing densities and measured IgG levels and N/L ratios, which are well-known stress biomarkers, and HSP 27 expression. Six steers in the HD group were housed in a free barn with narrow space (1.6 m2/head) for 9 days of the high-density period as the stress test group. In contrast, 4 steers of the FR group were housed in a wide space (5.6 m2/head) throughout the experimental period. Body weight was not significantly different at the end of the experimental period (30 days) between the HD and FR groups (281 ± 10.8 kg and 281 ± 5.12 kg, respectively). Table 3 and Table 4 show the IgG levels and N/L ratios in the blood of the HD and FR groups during the experimental period. The approximate molecular weight of IgG was detected at 25 kDa by western blot analysis. The IgG levels of the HD group were significantly higher than those of the FR group at 9 days of the high-density period (P<0.05). The IgG levels of the HD group were significantly higher during the high-density period (3 and 9 days) than at the start (-12 days) and end (30 days) of the experimental period (P<0.05), but there were no significant differences in the FR group during the experimental period. The N/L ratio was calculated from the neutrophil and lymphocyte compositions in the blood. The N/L ratios at 3 days of the high-density period were significantly higher in the HD group than in the FR group (P<0.05). The N/L ratios of the HD group were significantly higher at 3, 9, and 30 days than at -12 days (P<0.05). The N/L ratio of the FR group was the highest at 9 days during the experimental period. Figure 2 shows the expression of the HSP 27 gene in the skeletal muscles during the experimental period. The HSP 27 gene expression in the LL muscle of the HD group was significantly greater at 3 days of the high-density period than before the high-density period (P<0.05), and thereafter returned to base level. Expression of the HSP 27 gene in the ST muscle at 9 days of the high-density period was significantly greater than that before the high-density period (P<0.05), and thereafter tended to return to base level. At 3 and 9 days of the high-density period, HSP 27 expression was significantly increased in the ST muscle of the HD group compared with that of the FR group (P<0.05). Expression of HSP 27 occurred in synchrony with increases in IgG levels and N/L ratios under the stress condition.
| HD group 1 | FR group 2 | Inter-group differences | |
|---|---|---|---|
| Experimental days 3 -12 days 3 days 9 days 30 days |
6.65 ± 0.89 a 10.3 ± 0.90bc 12.6 ± 1.06 *, b 8.43 ± 0.82ac |
7.50 ± 1.87 7.38 ± 1.96 7.60 ± 1.63 6.96 ± 0.77 |
n.s. n.s. *P < 0.05 n.s. |
| Intra-group Differences |
a VS. b VS. c P< 0.05 |
n.s. |
1Values are expressed as means ± SEM (n = 6).
2Values are expressed as means ± SEM (n = 4).
3Experimental days of both groups at the start of this experiment (-12 days), at 3 and 9 days after housing at high density, and at the end of the experimental period (30 days). Detailed information is provided in the legend of Figure 1.
Table 3: Blood IgG levels in steers of the high-density (HD) group and freedom(FR) group during the experimental period.
| HD group1 | FR group2 | Inter-group differences | |
|---|---|---|---|
| Experimental days3 -12 days 3 days 9 days 30 days |
0.300 ± 0.04a 0.487 ± 0.04*,b 0.548 ± 0.07 b 0.559 ± 0.05 b |
0.290 ± 0.06a 0.344 ± 0.04ac 0.798 ± 0.11b 0.545 ± 0.05c |
n.s. *P < 0.05 n.s. n.s. |
| Intra-group Differences |
a VS. b P< 0.05 |
a VS. b VS. c P< 0.05 |
1Values are expressed as means ± SEM (n = 6).
2Values are expressed as means ± SEM (n = 4).
3Experimental days of both groups at the start of this experiment (-12 days), at 3
and 9 days after housing at high density, and at the end of the experimental period
(30 days). Detailed information is provided in the legend of Figure 1.
Table 4: Blood neutrophil-to-lymphocyte (N/L) ratio in steers of the high-density (HD) group and freedom (FR) group during the experimental period.
Figure 2: Heat shock protein (HSP) 27 gene expression in the semitendinosus (ST) and longissimus lumborum (LL) muscles of steers during the experimental period in the HD and FR groups by real-time PCR. Detailed information about the experimental period is described in the Figure 1 legend. Values with different letters are significantly different within experimental group (P < 0.05). An asterisk indicates that the means differ between treatments of same days (P < 0.05).
Experiment 2
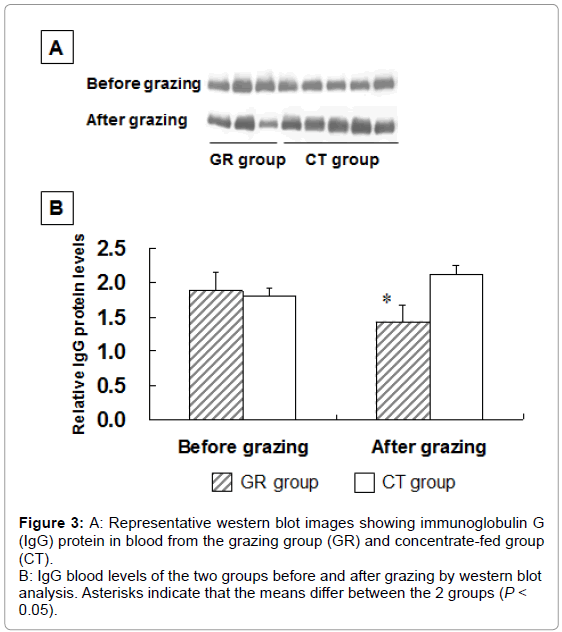
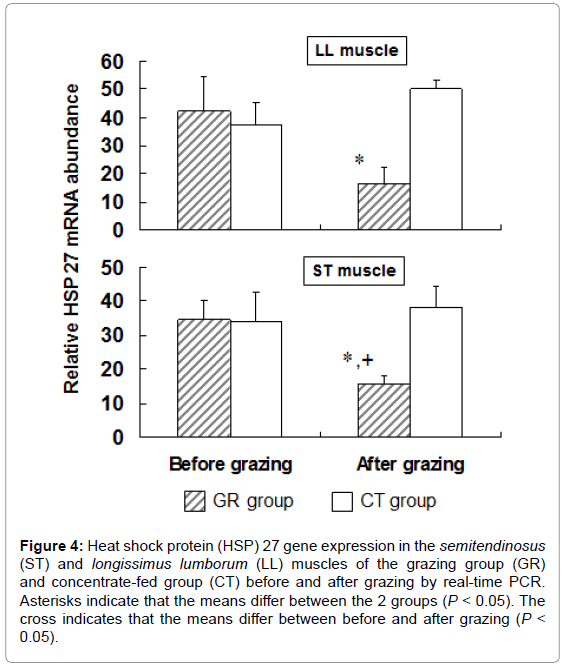
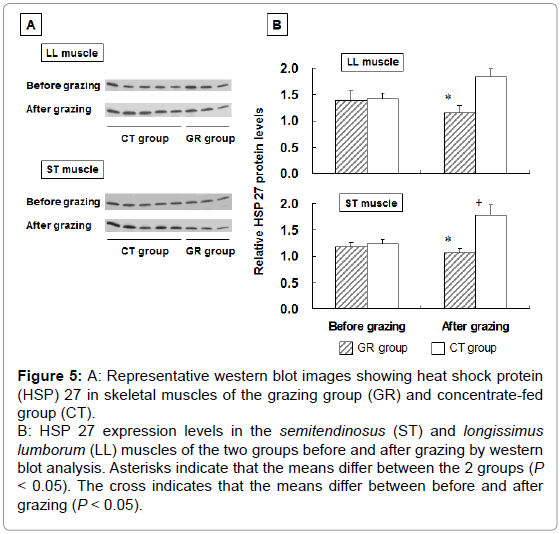
To examine the influence of grazing on HSP 27 expression, we measured HSP 27 expression in outdoor-grazed and indoor concentratefed steers. Steers were outdoor-grazed for 3 months during the latter fattening period. Body weight at the end of the fattening period was not significantly different between the GR and CT groups (697 ± 12.9 kg and 707 ± 25.8 kg, respectively). Figure 3A, 3B shows that the blood IgG levels in the GR group after grazing were significantly lower than those in the CT group by western blot analysis (P<0.05). This result suggests that a stress response occurred in the CT group compared with the GR group. Figure 4 shows the HSP 27 gene expression in the skeletal muscles of the GR and CT groups before and after grazing. Expression of the HSP 27 gene in the GR group was significantly decreased by 67% and 59% in the LL and ST muscles, respectively, compared with that in the CT group after grazing (P<0.05). Expression of the HSP 27 gene in the ST muscle of the GR group was significantly lower after grazing than before grazing (P<0.05), but there was no significant difference in its expression in the CT group during the experimental period. The approximate molecular weight of HSP 27 was 27 kDa as detected by western blot analysis (Figure 5A). The HSP 27 protein expression in the GR group after grazing was significantly decreased by 37.5% and 40.3% in the LL and ST muscles, respectively, compared with that in the CT group (P<0.05) (Figure 5B). Expression of the HSP 27 protein in the ST muscle of the CT group was significantly higher after grazing than before grazing (P<0.05). In skeletal muscle, expression of HSP 27 protein corresponded to that of HSP 27 mRNA. A difference in the stress response was recognized between the GR and CT groups based on the change in IgG and HSP 27 expressions.
Figure 3: A: Representative western blot images showing immunoglobulin G
(IgG) protein in blood from the grazing group (GR) and concentrate-fed group
(CT).
B: IgG blood levels of the two groups before and after grazing by western blot
analysis. Asterisks indicate that the means differ between the 2 groups (P <
0.05).
Figure 4: Heat shock protein (HSP) 27 gene expression in the semitendinosus (ST) and longissimus lumborum (LL) muscles of the grazing group (GR) and concentrate-fed group (CT) before and after grazing by real-time PCR. Asterisks indicate that the means differ between the 2 groups (P < 0.05). The cross indicates that the means differ between before and after grazing (P < 0.05).
Figure 5: A: Representative western blot images showing heat shock protein
(HSP) 27 in skeletal muscles of the grazing group (GR) and concentrate-fed
group (CT).
B: HSP 27 expression levels in the semitendinosus (ST) and longissimus
lumborum (LL) muscles of the two groups before and after grazing by western
blot analysis. Asterisks indicate that the means differ between the 2 groups (P < 0.05). The cross indicates that the means differ between before and after
grazing (P < 0.05).
Discussion
The aim of the present study was to investigate IgG levels and N/L ratios, which are well-known stress biomarkers, and expression of HSP 27 in beef cattle under various feeding conditions. We investigated whether HSP 27 expression occurs with changes in IgG levels and N/L ratios under stress conditions. Furthermore, to examine changes in the expression of HSP 27 in different rearing conditions, we measured HSP 27 expression in outdoor-grazed and indoor concentrate-fed steers.
Serum biomarkers associated with stress have been detected in pigs housed at high density using biochemical and proteome analysis [1]. To create a steer stress model, we designed high- and low-density housing systems and measured various stress indices. Immunoglobulins have been utilized as stress markers in several studies. Psychological stress by academic examination induces an increase in serum IgA, IgG, and IgM in students with high stress perceptions [16]. IgA, IgG, and IgM titers in saliva during exams are higher in students with symptoms of psychological stress [5]. In one study, the serum IgG concentration in the high job strain group was higher than that in the low job strain group, but there was no difference in IgA and IgM between the 2 groups [6]. These studies indicated that Ig has been detected in several stress condition as stress marker. In the present study, the IgG levels at 9 days of the high-density period were higher in the HD group than in the FR group. Furthermore, increase in the IgG levels in the HD group was shown during the high-density period compared with the levels at the start and end of the experimental period. These results suggest that a stress response occurred in the HD group.
Furthermore, the N/L ratio expresses the severity of afflictions such as surgical stress [17]. It is well established that weaning stress of calves causes an increase in the N/L ratio with a reduction in lymphocytes [3]. In addition, transported cattle show a stress response with higher N/L ratios [2]. The N/L ratio of grazed cows on native grassland is higher than that of cows housed in group pens in mid-summer [7]. The N/L ratio has been used as a stress marker in many studies and was employed the present study as a stress marker. The N/L ratio of the HD group was higher than that of the FR group at 3 days of the high-density period. In addition, the N/L ratios in the HD group were the lowest before the high-density housing period. Increases in IgG levels and N/L ratios of the HD group indicate that a stress response occurred in the HD group compared with the FR group.
We examined whether changes in the expression of HSP 27 occur in the high-density conditions, because changes in the IgG levels and N/L ratios were confirmed in the steers housed in high-density conditions. HSPs have been utilized in several studies as stress markers. For instance, increased HSP 70 in some species, such as fish, amphibians, arthropods, and plants, is a biomarker for detection of heat stress [18]. High ambient temperature induces an additional accumulation of HSP 27 mRNA and protein in leukocytes compared with the exerciseinduced expression at low temperatures [4]. In addition, an increase in the HSP 27 protein level in skeletal muscle was observed at 1 and 14 days after eccentric exercise stress compared with that before the stress [13]. Transport-stressed pigs show a higher level of HSP 70 in the heart and kidney than do control pigs [14]. An increase in HSP 90 in the white blood cells of lactating beef cows occurred with long-term caloric stress induced by feeding a low-energy diet [19]. Previous reports have indicated that expression of HSPs depends not only on the ambient temperature, but also on several other stress factors such as exercise, transport, and low-calorie diets. In the present study, the expression of HSP 27 mRNA in the HD group increased in the LL and ST muscles of the high-density period compared with that before the high-density period. Moreover, HSP 27 expression was increased in the ST muscle of the HD group compared with that of the FR group. Results in the experiment 1 indicate that expression of HSP 27 occurred in synchrony with increases in IgG levels and N/L ratios under stress conditions in steers. These results suggest that HSP 27 expression may also reflect the influence of the stress response.
Our previous study indicated that decreases in expression of HSP 27 occurred in grazed cattle compared with indoor concentrate-fed cattle in a stall barn by skeletal muscle proteome analysis [8]. In the experiment 1, we confirmed that HSP 27 expression takes place in synchrony with increases in stress biomarkers under stress conditions in steers. To verify whether expression of HSP 27 as a stress biomarker reflects differences in rearing systems, we analyzed blood IgG levels and HSP 27 expression in skeletal muscle when steers were outdoor-grazed and indoor concentrate-fed. At the end of grazing, expression of the HSP 27 gene and its protein in the GR group was decreased in the LL and ST muscles compared with that in the CT group. Furthermore, HSP 27 gene expression in the ST muscle of the GR group was lower after grazing than before grazing. Results in the experiment 2 indicate that expression of HSP 27 reflected a difference between outdoor grazing and indoor concentrate-feeding. Furthermore, decreases in the IgG levels of the GR group after grazing indicate that the stress response was suppressed in the GR group compared with the CT group. These results suggest that HSP 27 may be employed to detect the stress response under the differences in rearing environment as a stress biomarker.
In terms of the effect of HSP on meat quality, Bernard et al. [20] reported that low expression of HSP 40 in muscle was associated with elevated beef tenderness. Although the molecular classes of HSP in the present study are different from the previous report [20], decreased HSP 27 expression might also affect beef tenderness. Further studies are needed to clarify the correlation between HSP 27 and beef quality, including tenderness, taste or nutrients.
The present study confirmed that expression of both HSP 27 protein and mRNA levels were present in skeletal muscle. Apart from skeletal muscle, HSP 27 protein expression will be also identified in the dressed carcass and retail meat. This procedure may be useful for retail beef to determine whether beef cattle encountered a stressful environment. Moreover, to aid stress evaluation in the beef cattle production field by HSP 27, further studies should determine whether HSP 27 can be measured in other samples, such as blood, saliva and urine.
Conclusion
In the present study, we confirmed that HSP 27 gene expression may reflect the influence of the stress response because its expression took place in synchrony with increases in the IgG level and N/L ratio under a high-density housing stress condition in steers (experiment 1). In addition, we revealed that decreases in both HSP 27 expression and IgG levels occur during outdoor grazing compared with indoor concentrate-feeding in steers (experiment 2). These results indicate that outdoor grazing would be less stressful than indoor concentratefeeding because increases in both HSP 27 expression and IgG levels occurred under the stress condition of experiment 1 in the present study. The present study suggests that HSP 27 probably reflects not only the difference in the rearing system, but also the influence of the stress reaction.
Acknowledgements
The authors appreciate the help of the technical staff at the WeNARC. This work was supported in part by a Grant-in-Aid from the Ministry of Agriculture, Forestry, and Fisheries of Japan and by a Grant-in-Aid (No. 25450402) for Scientific Research from Ministry of Education, Science, Sports and Culture of Japan.
References
- Ramell AM, Pato R, Pena R, Saco R, Manteca X, et al. (2011) Identification of serum stress biomarkers in pigs housed at different stocking densities. Vet J 190: e66-e71.
- Chacon G, Garcia BS, Villarroel M, Maria GA (2005) Effect of transport stress on physiological responses of male bovines. DtschTierarztlWochenschr, 112: 465-469.
- Kim MH, Yang JY, Upadhaya SD, Lee HJ, Yuu CH, et al.(2011) The stress of weaning influences serum levels of acute-phase proteins, iron-binding proteins, inflammatory cytokines, cortisol, and leukocyte subsets in Holstein calves. J Vet Sci 12: 151-157.
- Fehrenbach E, Veith R, Schmid M, Dickhuth HH, Northoff H, et al. (2003) Inverse response of leukocyte heat shock proteins and DNA damage to exercise and heat. Free Radic Res 37: 975-982.
- Matos-Gomes N, Katsurayama M, Makimoto FH, Santana LL, Paredes-Garcia E, et al. (2010) Psychological stress and its influence on salivary flow rate, total protein concentration and IgA, IgG and IgM titers. Neuroimmunomodulation 17: 396-404.
- Nakata A, Araki S, Tanigawa T, Miki A, Sakurai S, et al. (2000) Decrease of suppressor-inducer (CD4+ CD45RA) T lymphocytes and increase of serum immunoglobulin G due to perceived job stress in Japanese nuclear electric power plant workers. J Occup Environ Med 42: 143-150.
- Kiyoshi K, Yamashita C, Yayota M, Ohtani S, Ohashi H (2010) Nutritional status and physiological stress responses in Japanese Black Cow grazing on a native pasture. Res Bull Aichi Agric Res Cent 42: 65-72.
- Shibata M, Matsumoto K, Oe M, Kameyama OM, Ojima K, et al. (2009) Differential expression of the skeletal muscle proteome in grazed cattle. J AnimSci 87: 2700-2708.
- Gething MJ, Sambrook J (1992) Protein folding in cell. Nature 355: 33-35.
- Chirico WJ, Waters MG, Blobel G (1988) 70K heat shock related protein stimulate protein translocation into microsomes. Nature 332: 805-810.
- Hartl FU (1996) Molecular chaperones in cellular protein folding. Nature 381: 571-580.
- Liu Y, Steinacker JM (2001) Changes in skeletal muscle heat shock proteins: pathological significance. Front Biosci 6D: 12-25.
- Feasson L, Stockholm D, Freyssenet D, Richard I, Beckmann JS, Denis C (2002) Molecular adaptations of neuromuscular disease-associated proteins in response to eccentric exercise in human skeletal muscle. J Physiol 543: 297-306.
- Yu H, Bao ED, Zhao RQ, Lv QX (2007) Effect of transportation stress on heat shock protein 70 concentration and mRNA expression in heart and kidney tissues and serum enzyme activities and hormone concentrations of pigs. Am J Vet Res 68: 1145-1150.
- Shibata M, Matsumoto K, Aikawa K, Muramoto T, Fujimura S, Kadowaki M (2006) Gene expression of myostatin during development and regeneration of skeletal muscle in Japanese Black Cattle. J AnimSci 84: 2983-2989.
- Maes M, Hendriks D, Gastel A, Demedts P, Wauters A, et al. (1997) Effects of psychological stress on serum immunoglobulin, complement and acute phase protein concentrations in normal volunteers. Psychoneuroendcrinology 22: 397-409.
- Zahorec R (2001) Ratio of neutrophil to lymphocyte counts—rapid and simple parameter of systemic inflammation and stress in critically ill. BratislLekListy 102: 5-14.
- Dunlap DY, Matsumura F (1997) Development of broad spectrum antibodies to heat shock protein 70s as biomarkers for detection of multiple stress by pollutants and environmental factors. Ecotoxicol Environ Saf 37: 238-244.
- Eitam H, Brosh A, Orlov A, Izhaki I, Shabtay A (2009) Caloric stress alters fat characteristics and Hsp 70 expression in milk somatic cells of lactating beef cows. Cell Stress Chaperones 14: 173-182.
- Bernard C, Cassar-Malek I, Cunff ML, Dubroeucq H, Renard G, et al. (2007) New indicators of beef sensory quality revealed by expression of specific genes. J Agric Food Chem 55: 5229-5237.
Relevant Topics
- Acoustic Survey
- Animal Husbandry
- Aquaculture Developement
- Bioacoustics
- Biological Diversity
- Dropline
- Fisheries
- Fisheries Management
- Fishing Vessel
- Gillnet
- Jigging
- Livestock Nutrition
- Livestock Production
- Marine
- Marine Fish
- Maritime Policy
- Pelagic Fish
- Poultry
- Sustainable fishery
- Sustainable Fishing
- Trawling
Recommended Journals
Article Tools
Article Usage
- Total views: 14439
- [From(publication date):
November-2014 - Jul 04, 2025] - Breakdown by view type
- HTML page views : 9814
- PDF downloads : 4625