Influence of Gut Microbiome on Memory Processes and Its Dysruption byEnvironmental Toxins
DOI: 10.4172/2161-069X.1000652
Abstract
Microbiome research has bloomed over the past few years. Stemming from this field of study has emerged the concept of probiotics and its application in advancing human health. Several links between gut flora, mental health and cognitive processes have been brought to light recently. It has been established that such associations take place due bidirectional interactions between the gut microbiome and central nervous system. However, the exact mechanisms through which microorganisms in the gut influence cognitive processes such as learning and memory are yet to be thoroughly understood. Metabolites like short chain fatty acids (SCFAs) and neurotransmitters, released by microorganisms in the gut have found to influence neuro cognitive conditions. As a consequence of this interrelationship, researchers believe environmental toxins that disrupt the gut likewise may affect cognition. This review strives to provide a comprehensive understanding of existing literature on metabolites and mechanisms by which a healthy, balanced gut microbiota can impact memory processes and its disruption by various environmental toxins.
Keywords: Gut microbiome; Memory; SCFAs; Neurotransmitters; Toxins
Introduction
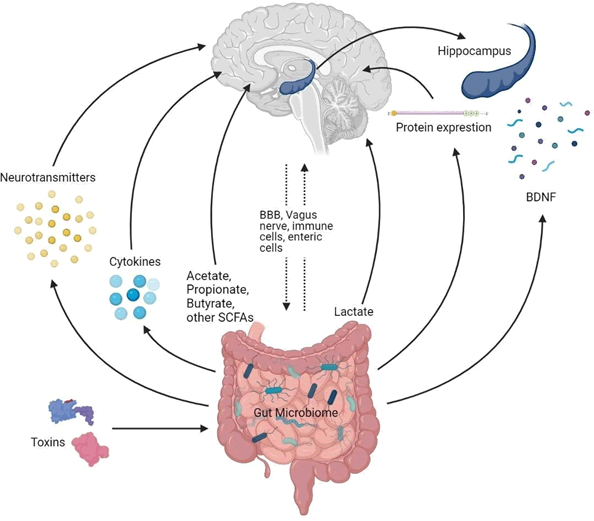
The Gut Microbiome is a distinct community of microorganisms that occupies the gastrointestinal tract of several animals inclusive of insects. The essential function of gastrointestinal (GI) microbiota on diet, health and disease is an intriguing field of research that in the past few years has been centred on with growing interest [1]. The enteric nervous system, known as the second brain of the human body, synthesizes and utilizes more than 30 types of neurotransmitters which are recognized in the central nervous system. Gut microbiota, by product, changes the quantity of neurotransmitters directly or by regulating the associated metabolism by neurotransmitter pathways [2].
Microorganisms are classified as pathogens by the host immune system, which identifies and destroys them. The majority of gut bacteria on the further side is non-pathogenic and live in a symbiotic relationship with enterocytes [3]. The vast majority of these commensals belong to either the gram negative Bacteroides or the gram positive Firmicutes phyla. These bacteria are estimated to have upto 100 times the number of genes as the human genome [4]. Many of these genes have direct effects on host metabolic pathways and provide nutrients that would otherwise be unavailable to the host [5].
The gut microbiota is responsible for various roles in bowel movement, food metabolism, and nutrient absorption as it contains an estimated 1018 microorganisms, the majority of which are anaerobic bacteria [6]. Bacteria in the gut can activate the afferent neurons of the ENS, which have a direct neural connection upon the brain via the vagus nerve [7]. As the brain and the gut communicate in this way, they can influence each other's functions and have a big effect on stress, anxiety, depression, and cognition [8].
In humans, gastrointestinal microbiota produces 300-500 strains of bacteria that could affect brain function through the Gut Brain Axis (GBA), which connects GI and brain activity. Microbiota, through neuro endocrine, neuro metabolic and neuro immunological pathways, regulates the nervous system.
Probiotics are a group of these bacteria that could have convenient effects, beyond gastrointestinal effects, when consumed in the right amount, such as those in the Central Nervous System [9]. The antiinflammatory and antioxidant properties of probiotics, together with bacterial groups like Lactobacillus and Bifidobacterium, have been correlated with mood change, memory, depression and synaptic action [10]. They also have some beneficial impact on the gut brain microbiota axis, averting hyperactivation of the HPA axis (Hypothalamic Pituitary Adrenal) after maternal separation exposure, and minimizing anxiety like responses in a chronic model of dextran sodium sulphate model and stress activated memory dysfunction condition.
Gut Diversity
The microorganisms that reside in the digestive tracts of humans and other species, including insects, are known as gut microbiota, gut flora, or microbiome [11]. The Gut microbiome diversity changes across the gastrointestinal tract. Summarizes common gut flora in various living organisms.
Gut Microbes used as Probiotics
The host characteristics defines the diversity of the gut such as atmosphere, immunity and also the metabolism depth of the population, diet and, in addition, helps in digestion, immunity, by performing different functions for the host. The difference in the gut diversity could be responsible for the dysbiosis, which would have significant implications for both hosts and ecosystems subsequently.
Nevertheless, probiotics are the live microbes that are beneficial to the host when it is given in proper quantities [12].
There are different applications of probiotics that have been reviewed which includes liver injury, metabolic syndrome, radiation induced enteritis, inflammatory bowel disease, etc. To find out the strains which were more immune, that suits the low pH of the stomach and the digestive juices of the duodenum Lactobacillus acidophilus was used in the US during the 1930’s. Likewise, another strain known as Lactobacillus paracasei was introduced in Japan concurrently.
Research studies have shown that L. acidophilus, L. johnsonii, L. fermentum, L. paracasei, L. rhamnosus, L. plantarum, and Bifidobacterium animalis, Bifidobacterium longum are the most familiar probiotic species. Certain species/strains of Lactobacillus and Bifidobacterium are currently the most researched inflammation suppressing taxa of the GI microbiota, and these are also the fractions that are assisted by administering probiotics that selectively stimulate resident Lactobacillus and Bifidobacterium. Bifidobacterium, Faecalibacterium, Lactobacillus, Bacillus, Saccharomyces isolates, among other probiotic members, have shown promise in improving food safety and gut health. Bacillus, Lactobacillus, Enterococcus, Bifidobacterium, and Streptococcus are some of the most common bacterial genera used as probiotics [13].
In order to improve food safety, maintaining good gut health, etc the isolates of certain bacteria like Bifidobacterium, Faecalibacterium, Lactobacillus, Bacillus, Saccharomyces has shown great promise. Other than this E. faecalis, S. thermophilus, Lactobacillus rhamnosus and Saccharomyces boulardii have also been used in various trials. After going through the usefulness of the probiotics, the over usage can also be detrimental. For example over usage of L. rhamnosus has been associated with severe health conditions such as bacteremia, sepsis, and endocarditis especially with the patients suffering from digestive organ inflammation. As a result, it's important to use the right amount of probiotics on individual patients for medicinal purposes [14].
Memory Modulation by Gut Microbiome
Considerable number of studies have purported that the microorganisms present in the gut of the host modulate cognitive processes such as memory through probiotic treatment in model organisms such as mice. One such paper establishes a clear relationship between gut dysbiosis and memory [15]. Mice with high fat diet displayed impaired reflecting memory and low exploratory behaviour than mice with a healthy gut microbiome. Proteobacteria abundance was linked to lower cognition. The authors proffer that such phenomena are a consequence of metabolite production in gut and its influence on cognitive processes.
A similar correlation was found between excess sugar intake and memory function due to changes observed in the gut environment. Early consumption of sugar caused an increase in Parabacteroides which was found to be positively correlated with a decrease in hippocampal dependent memory function tested using the Novel Object in Context (NOIC) task [16].
Furthermore, continuous light exposure disrupts normal gut flora communities in C57BL/6J mice, causing an increase Bacteroidales S24-7 populations and reduction in memory potential at early exposure periods.
A study also found that SPF (specific pathogen free) mice displayed better long term spatial memory, contextual and cued memory and social novelty when compared to germ free mice. The gut microbiome of young SPF mice also seemed to have increased myelination and volume in grey matter regions of a developing brain. As a probiotic Lactobacillus helveticus ROO52 has also presented evidence of alleviate anxiety like behavior and memory dysfunction (Figure 1) [17].
Toxins which Affect the Gut Microbiome and Memory
When exposed to a variety of harmful environmental agents, the human gut microbiome can be disrupted with ease. Environmentally mediated distortions in the gut microbiota are closely linked to the risk of human disease. Increasingly recognized procedures by which environmental chemicals expend their toxic effects are alterations of the functional gut microflora that may have a negative impact on human health [18].
The gut microbiome is responsible for polysaccharide digestion, vitamin and nutrient biosynthesis, colonization resistance, and immune system control, among other items. The arrangement and working of the gut microbiome can be easily affected by an array of intrinsic and extrinsic influences [19].
Insights into the gut brain crosstalk have shown a dynamic communication mechanism that is expected to have numerous effects on affect, motivation, and higher cognitive functions in addition to ensuring proper gastrointestinal homeostasis. The term "gut brain axis" encapsulates the ambiguity of these experiences (GBA). Its job is to keep track of and integrate gut functions, and also to link the brain's emotional and cognitive centers to peripheral intestinal functions and mechanisms including immune activation, intestinal permeability, enteric reflex, and entero endocrine signaling. The enteric microbiota appears to have a significant influence on GBA, interacting not only sectionally with intestinal cells and the ENS, likewise directly with the CNS through neuroendocrine and metabolic pathways [20].
Studies on the impact of gut microbiota manipulation by probiotics and/or antibiotics have further backed up the effect of microbiota on GBA [21]. Microbiota influences anxiety and the HPA system by affecting brain neurochemistry, according to some reports.
The human gut microbiome encodes a greater variety of metabolic enzymes, allowing the human body to perform a wider range of biochemical reactions. Some environmental chemicals may directly affect gut bacteria by disrupting specific metabolic pathways or gene expression, resulting in different selection pressures and thus forming the gut microbial population due to the set's uniqueness (Table 1).
| Toxins | BDNF | Neurotransmitters | Proteins | SCFA’s | Lactate |
|---|---|---|---|---|---|
| Bisphenol A | ¯ | GABA, Glutamate ¯ | ¯ | ¯ | ¯ |
| Methylmercury | ¯ | Dopamine, GABA, Glutamate ¯ | ¯ | ¯ | ¯ |
| Diazinon | - | Acetylcholine ¯ | ¯ | ¯ | ¯ |
| Nicotine | - | Serotonin, Dopamine, GABA, Glutamic Acid, Norepinephrine | ¯ | ¯ | ¯ |
| ¯ | |||||
| Fluoride | ¯ | Serotonin, Dopamine, Norepinephrine, Epinephrine, Acetylcholine ¯ | ¯ | - | ¯ |
Table 1: Provides an overview of the toxins and their effect on the gut microbiome and their metabolites.
Alzheimers Disease
Since the emergence of the Gut Brain Axis hypothesis, researchers have begun to pursue the impact of probiotics and gut diversity on Alzheimer’s disease (AD). This approach may lead to more refined and effective remedies as well as provide better insight on the pathogenesis of the disease [22].
Alzheimer's disease is recognised worldwide as the most prevalent neurodegenerative disease, and many factors contribute to the sporadic type of AD (late-onset), which is more common, which includes age, unhealthy diet, and stress. Two ámain neuropathological markers in ADD are extracellular senile plaque composed of ß-amyloid (Aß) and intracellular neurofibrillary tangles (NFT), composed of hyper phosphorylated tau protein. Microglial cells lose their defensive role after accumulation of Aβ (Aβ1-42) deposits in senile plaque and are unable to clear Aβ, which contributes to the loss of synaptic function, neuron apoptosis, oxidative stress, neuro inflammation and eventually memory loss. It is suspected that inflammatory and oxidative stress disorders caused by Alzheimer's disease could be associated with increased coliform or gram negative bacteria and reduced probiotic to coliform bacteria, which may themselves contribute to the production of oxidative and neuro inflammatory stress through activation of microglia [23-26].
It has been suggested that abnormal gut microbiome composition is partly responsible for memory dysfunction. Supporting evidence was reported by Zhan et al. when the gut environment and memory function of SAMP8 and SAMP1 mice was compared. Differences in the gut microbiota were directly related to the mice’s performance on the behavioural assays [27].
The combination of Bifidobacterium and Lactobacillus bacteria has proven to have a synergistic effect as a probiotic. When a commixture of Lactobacillus acidophilus, Lactobacillus fermentum, Bifidobacterium lactis and Bifidobacterium longum was administered to Aβ (1-42) Injected Wistar rats, Azm et al recorded a considerable reduction in oxidative stress and improvement in spatial memory. Oxidative stress, characterized by superoxide dismutase activity and malondialdehyde serve as a fundamental marker for plaque and tangles formation in AD [28].
APPNL G F mice (APP gene coupled with three mutations) are deficient in memory and cognitive function. Levels of cytokines, amyloid beta and Glial fibrillary acidic protein (GFAP) are higher in these mice than wild type. Supplementation in the form of probiotic treatment augmented memory and reduced amyloid beta aggregates. Probiotics may prove to complement AD therapy via modulation of gut microbiota [29,30].
Conclusion
As a consequence of this interrelationship, researchers believe environmental toxins that disrupt the gut likewise may affect cognition. This review strives to provide a comprehensive understanding of existing literature on metabolites and mechanisms by which a healthy, balanced gut microbiota can impact memory processes and its disruption by various environmental toxins. These results were reported along with considerable alteration in the gut environment in accordance with previous studies on gut brain interactions.
Authors Contributions
Authors Shivani Mandal, Swati Senapati, Adarsh Shankar M contributed to the conception of the study, preparation and design of this review article. All authors read, revised, and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding
There is no funding to report.
Ethical Approvals
This study does not involve experiments on animals or human subjects.
References
- Bhattacharjee S, Lukiw WJ (2013) AlzheimerÂ’s disease and the microbiome. Front Cell Neurosci 7:153.
- Liu T, Feenstra KA, Heringa J, Huang Z (2020) Influence of gut microbiota on mental health via neurotransmitters: A review. J Artif Intell Med Sci 1:1-14.
- Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, et al. (2015) Role of the normal gut microbiota. World J Gastroenterol 21(29):8787-8803.
- MetaHIT Consortium, Qin J, Li R, (2010) A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464(7285):59-65.
- Fukuda S, Ohno H (2014) Gut microbiome and metabolic diseases. Semin Immunopathol 36:103-114.
- Grochowska M, Wojnar M, Radkowski M (2018) The gut microbiota in neuropsychiatric disorders. Acta Neurobiol Exp 78:69-81.
- Forsythe P, Bienenstock J, Kunze WA (2014) Microbial endocrinology: The microbiota gut brain axis in health and disease. Adv Exper Medi Bio 8:115-133.
- Mohajeri MH, La Fata G, Steinert RE, Weber P (2018) Relationship between the gut microbiome and brain function. Nutrition Reviews 76:481-496.
- Zhang X, Zhao Y, Zhang M (2012) Structural changes of gut microbiota during berberine mediated prevention of obesity and insulin resistance in high-fat diet-fed rats. PLoS ONE 7(8):425-429.
- Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, et al. (2010) Effects of the probiotic Bifido bacterium infantis in the maternal separation model of depression. Neuroscience 170:1179-1188.
- Davari S, Talaei SA, Alaei H, salami M (2013) Probiotics treatment improves diabetes-induced impairment of synaptic activity and cognitive function: Behavioral and electrophysiological proofs for microbiome gut brain axis. Neuroscience 240:287-296.
- Ruan Y, Sun J, He J, Chen F, Chen R, et al (2015) Effect of probiotics on glycemic control: A systematic review and meta-analysis of randomized, controlled trials. PLOS ONE 10(7):e0132121.
- Li W, Dowd SE, Scurlock B, Acosta-Martinez V, Lyte M (2009) Memory and learning behavior in mice is temporally associated with diet-induced alterations in gut bacteria. Physiol Behav 96:557-567.
- Tiihonen K, Ouwehand AC, Rautonen N (2010) Human intestinal microbiota and healthy ageing. Ageing Res Rev 9(2):107-116.
- Moszak M, Szulinska M, Bogdanski P (2020) You are what you eat the relationship between diet, microbiota, and metabolic disorders: A Review. Nutrients 12:1096.
- Rajilic-Stojanovic M, de Vos WM (2014) The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol Rev 38:996-1047.
- Almeida A, Mitchell AL, Boland M (2019) A new genomic blueprint of the human gut microbiota. Nature 568:499-504.
- Thursby E, Juge N (2017) Introduction to the human gut microbiota. Biochem J 474(11):1823-1836.
- Quiroga-González C, Cardenas LA, RamÃrez M, Reyes A, González C, et al (2021) Monitoring the variation in the gut microbiota of captive woolly monkeys related to changes in diet during a reintroduction process. Scientific Reports 11(1).
- Khan S, Moore RJ, Stanley D, Chousalkar KK (2020) The gut microbiota of laying hens and its manipulation with prebiotics and probiotics to enhance gut health and food safety. Appl Environ Microbiol 86(13):e00600-20.
- Dong XY, Azzam MMM, Zou XT (2017) Effects of dietary threonine supplementation on intestinal barrier function and gut microbiota of laying hens. Poultry Science 96(10):3654-3663.
- Saxena S, Saxena VK, Tomar S, Sapcota D, Gonmei G (2016) Characterisation of caecum and crop microbiota of Indian indigenous chicken targeting multiple hypervariable regions within 16S rRNA gene. British Poultry Science 57(3):381-389.
- Janczyk P, Halle B, Souffrant WB (2009) Microbial community composition of the crop and ceca contents of laying hens fed diets supplemented with Chlorella vulgaris. Poultry Science 88(11):2324-2332.
- Pandit RJ, Hinsu AT, Patel NV (2018) Microbial diversity and community composition of caecal microbiota in commercial and indigenous Indian chickens determined using 16s rDNA amplicon sequencing. Microbiome 6(1).
- Hugenholtz F, de Vos WM (2018) Mouse models for human intestinal microbiota research: a critical evaluation. Cell Mol Life Sci 75(1):149-160.
- Clavel T, Lagkouvardos I, Blaut M, Stecher B (2011) The mouse gut microbiome revisited: m/86/13/AEM.00600-20.
- Nguyen TLA, Vieira-Silva S, Liston A, Raes J (2015) How informative is the mouse for human gut microbiota research? Disease Models and Mechanisms 8(1):1-16.
- Zhang C, Zhang M, Pang X, Zhao Y, Wang L, et al. (2012) Structural resilience of the gut microbiota in adult mice under high-fat dietary perturbations. ISME J 6(10):1848-1857.
- Murphy EF, Cotter PD, Healy S (2010) Composition and energy harvesting capacity of the gut microbiota: Relationship to diet, obesity and time in mouse models. Gut 59(12):1635-1642.
- Hildebrand F, Nguyen TLA, Brinkman B (2013) Inflammation associated enterotypes, host genotype, cage and inter individual effects drive gut microbiota variation in common laboratory mice. Genome Biol 14(1):R4.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2909
- [From(publication date): 0-2021 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 2242
- PDF downloads: 667