Influence of Bone Marrow Stem Cell Transplantation on Cardiac Function in Remodeled Left Ventricle of Rats after AMI
Received: 14-Mar-2016 / Accepted Date: 10-Jun-2016 / Published Date: 17-Jun-2016
Abstract
Objective: To explore the effect of allogenic bone marrow transplant on cardiac function in rats after AMI.
Material and methods: 50 Male, Wistar rats were randomly divided into transplant group and control 1, 2, 3 groups. Acute myocardial infarction (AMI) model was designed by ligation of the left anterior descending (LAD) coronary artery. Transplant group; prepared allogeneic marrow mononuclear cells (BM-MNCs) suspension were implanted in the epicardial area around the infarcted region. Control group 1; simple thoracotomy without ligation of coronary arteries. Control group 2; only AMI model was established. Control group 3; culture medium was table implanted into the subepicardioial and peri-infarction area. 4 weeks after transplantation, echocardiography and cardiac catheterization assessment of left ventricular morphology as well as function were performed. Morphological characteristics and organization of transplanted BM-MNCs within the infarcted area and its periphery was observed.
Results: Compared with control group 1, left ventricular systolic diameter (LVEDs), left ventricular end-diastolic diameter (LVEDd) and left ventricular end-diastolic pressure (LVEDP) were significantly increased (P <0.05), while left ventricular ejection fraction (LVEF), left ventricular fractional shortening (LVFS), left ventricular systolic pressure (LVSP), maximal rate of left ventricular pressure (+ dp/dt max) and maximal decrease rate left ventricular pressure (-dp / dtmax) were significantly lower (P <0.05) in control group 2, 3 and transplantation group; LVEDs, LVEDd and LVEDP in transplantation group were significantly lower (all P <0.05); while LVEF, LVFS, + dp/dt max and -dp/dt max were significantly higher than the control group 2 and 3 (P <0.05). Immunohistochemistry showed that BrdU labeling BM-MNCs were survived in the transplantation group.
Conclusion: Allogenic BM-MNCs transplantation in rat can reduce the left ventricular dilatation, enhance inhibition of left ventricular remodeling, and improve cardiac function after AMI.
Introduction
Myocardial infarction (MI) is a manifestation of coronary artery disease where coronary blood supply drastically reduced or interrupted. The corresponding myocardial tissue leads to serious and prolonged acute myocardial ischemia and induces acute necrosis. It is lethal and emergency cardiovascular disease which is one of leading causes of disability and death in clinical practice [1]. Despite the recent advances in percutaneous intervention, drug and device therapy, patients with acute myocardial infarction (AMI) and resulting left ventricular impairment have 13% mortality at 1 year [2]. Following the loss of over one billion cardiomyocytes in a functionally significant MI, the overloaded surviving cardiomyocytes undergo abnormal remodelling, eventually leading to heart failure. Ventricular remodeling after myocardial infarction refers to neuro-hormonal and genetic regulatory mechanisms activated by inflammatory cytokines causing changes in quality of cell morphology and functions of myocardial cells [3,4]. This condition, a leading cause of death and disability in the developed world, is associated with 5-year mortality rates of up to 70% in symptomatic patients [5]. Current conventional therapies do not correct underlying defects in cardiac muscle cell number [6].
Stem cells have the remarkable potential to develop into many different cell types in the body during early life and growth. In addition, in many tissues they serve as a sort of internal repair system, dividing essentially without limit to replenish other cells as long as the person or animal is still alive [1]. When a stem cell divides, each new cell has the potential either to remain a stem cell or become another type of cell with a more specialized function, such as a muscle cell, a red blood cell, or a brain cell. For example, adult stem cells isolated from liver tissue and reinjected into liver become hepatocytes, whereas the same cells injected into myocardium become myocytes [2].
Research on stem cells continues to advance knowledge about how an organism develops from a single cell and how healthy cells replace damaged cells in adult organisms. Stem cell research is one of the most fascinating areas of contemporary biology, but, as with many expanding fields of scientific inquiry, research on stem cells raises scientific questions as rapidly as it generates new discoveries.
Stem cells (unspecialized) are becoming the most important tool in “regenerative medicine” [3]. These cells have the potential to differentiate into cardiomyocytes. It would, therefore, be useful to find out if the differentiated cells can restore and improve cardiac function with efficacy and a good safety profile.
Katritsis et al. [4] have shown that intracoronary transplantation of mesenchymal stem cells and endothelial progenitors is feasible, safe, and may also contribute to regional regeneration of myocardial tissue early or late following MI. The demonstration of functional recovery of myocardium through cardiomyogenesis and neoangiogenesis in AMI in murine models by Orlic et al. [5-11] generated tremendous interest in the potential of bone marrow-derived stem cells. Since then, the cardiomyogenic ability of these cells has been challenged. However, studies continue to demonstrate improvement in cardiac function and reduction in infarct size.
Stem cell transplantation is emerging as a potential therapy to treat heart diseases. Promising results from early animal studies led to an explosion of small, non-controlled clinical trials that created even further excitement by showing that stem cell transplantation improved left ventricular systolic function and enhanced remodeling. However, the specific mechanisms by which these cells improve heart function remain largely unknown.
From September to November 2015, we performed allogeneic bone marrow mononuclear fine Cells (BM-MNCs) transplantation around the infarcted zone in rats with acute myocardial infarction (AMI) and observed its effect on ventricular remodeling and cardiac function.
Materials And Methods
Modeling and treatment
50 male, 250 ~ 300 g weight, Wistar rats were randomly divided into four groups, control 1, 2, 3 groups, each group with 12 rats, and transplant group with 14 rats. Anesthesia; intraperitoneal injection of 10% chloral hydrate (300 mg/kg), endotracheal intubation, positive pressure ventilator-assisted breathing, left lateral chest incision through the fourth intercostal thoracotomy to expose the heart. Control group 1, only thoracotomy without coronary artery ligation; control group 2, established AMI model, only left anterior descending coronary artery ligation; control group 3, immediately after AMI, using micro injection syringe (100 μl /points), a total of 4 points; DMEM-LG culture medium was injected in subepicardial region around the infarcted myocardium. Transplant group; immediately after AMI, BM-MNCs 107/ (100 μl/points), [a total of 4:points, the laboratory-made] implanted in epicardial region around the infarcted myocardium with a microsyringe, edema developed around injection site was a sign of successful model establishment. After observing 5 ~ 10 min for stable circulation, closed chest and continued feeding.
Observation of cardiac function
4 weeks after cell transplantation, heart function was checked, rats were anesthetized by 10% chloral hydrate Aldehyde intraperitoneal injection, synchronous ECG tracings. Echocardiographic measurements of left ventricular systolic diameter (LVEDs) and left ventricular enddiastolic diameter (LVEDd), averages five cardiac cycles were taken, left ventricular fractional shortening (LVFS) and left ventricular ejection fraction (LVEF) were calculated. After the end of echocardiography, catheter was introduced into the heart through the right common carotid artery which was connected to a pressure force transducer RM- 62000-type-4 conducting physiological recorder, measured heart rate (HR), left ventricular systolic pressure (LVSP), left ventricular enddiastolic pressure (LVEDP), maximal rate of left ventricular pressure (+ dp / dtmax) and the maximum rate of decrease of left ventricular pressure (dp / dtmax).
Histological examination
After the hemodynamic measurement, 3 ml of 10% potassium chloride was injection via the tail vein to establish cardiac arrest, quickly opened the chest and harvested the heart. Left atrium, right atrium and right ventricle were excised, the remaining part, left ventricle was washed with phosphate buffer, then fixed in 4% formaldehyde solution, paraffin-embedded sections, HE staining and immunohistochemical staining, ready for observation.
Statistical methods
Statistical analysis was done by using SPSS 10.0 software. Data presented in the form of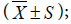 the groups were compared using analysis of variance. Test level α = 0.05.
the groups were compared using analysis of variance. Test level α = 0.05.
Result
MI model and treatment
Rats were healthy in each group. Postoperative death of 1 rat in Control group 1, 3 and transplant group, the cause of death was unknown; others were alive and healthy.
Echocardiography results
Compared with control group 1, LVEF (%) and LVFS (%) are decreased while, LVEDs (mm) and LVEDd (mm) are increased in treatment group. Compared with the control group 2 and 3, LVEF (%) and LVFS (%) are elevated, PTable 1).
| Group | n | LVEDs(mm) | LVEDd(mm) | LVEF(%) | LVFS(%) |
|---|---|---|---|---|---|
| Control 1 | 11 | 2.27±1.48 | 4.65±1.67 | 79.06±9.14 | 45.18±6.30 |
| Control 2 | 12 | 5.83±1.39# | 7.43±1.47# | 42.50±7.38# | 25.79±4.05# |
| Control 3 | 11 | 6.06±1.53# | 7.59±1.51# | 44.92±6.75# | 24.18±4.83# |
| Transplant | 13 | 3.81±1.26*▲ | 6.07±1.41#▲ | 54.89±7.92*Δ | 36.44±4.97*Δ |
Compared with control group 1,* P<0.05, #P< 0.01; Compared with control group 2,3 Δ P▲ P<0.01
Table 1: Echocardiography result of each group 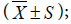
Cardiac catheterisation result
Compared with control group 1, (+ dp/dt max) and maximal decrease rate left ventricular pressure (-dp / dtmax) were significantly lower (P <0.05) in control group 2, 3 and transplantation group. LVEDP in tansplant group is higher than the control group 1 while lower than the control 2 and 3 groups (Table 2).
| Group | N | HR | LVEDP | LVSP | dp/dtmax | -dp/dtmax |
|---|---|---|---|---|---|---|
| (bpm) | (mmHg) | (mmHg) | (mmHg/s) | (mmHg/s) | ||
| Control 1 | 11 | 370±28 | 3.49±1.14 | 144.65±12.67 | 7228±385 | 5734±302 |
| Control 2 | 12 | 415±31 | 19.83±2.60▲ | 109.17±9.46* | 4729±316▲ | 3289±247▲ |
| Control 3 | 12 | 419±35 | 20.76±2.39▲ | 101.98±12.55* | 4534±403▲ | 3126±280▲ |
| transplant | 13 | 392±23 | 12.80±2.39▲Δ | 116.79±9.81* | 6104±375*Δ | 4153±294*Δ |
Note: Compared with control group 1, *P<0.05, ▲ P<0.01; Compared with control group 2,3 ΔP<0.05
Table 2: Cardiac catheterization results in each group 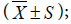
Histological examinations
Histomorphology in control group 1 was normal; control group 2, 3 showed a typical change in left ventricular anterior wall after AMI; transplant group performance related to immune rejection was not observed. Positive BrdU labeling BM-MNCs were visible in the focal coagulation necrosis of host myocardium, mainly in the local injection site. But also in the peri-infarct zone and in subendocardial region, a few focal BM-MNCs with disorderly growth and uneven distribution were seen. No obvious inflammatory cell groups around the cell mass were observed.
Discussions
Post AMI scar tissue repair of necrotic myocardial tissue, residual compensatory ventricular hypertrophy and ventricular remodeling eventually leading to congestive heart failure. Stem cells are a class of cells with potential of self-replication and differentiation. The replication and differentiation are affected by the surrounding microenvironment.
Our results showed, after AMI, a significant increase in LVEDP, LVEDs, LVEDd which describes left ventricular remodeling after AMI; while LVEF, LVFS, + dp / dtmax and -dp / dtmax were decreased significantly, indicating left ventricular dysfunction after AMI. Allogeneic BM-MNCs transplanted into the surrounding area of AMI after about four weeks, Immunohistochemical study found that a large number of positive Brdu markers of BM-MNCs were visible in the AMI regions which proved the viability of transplanted cells. In the transplant group, LVEDs, LVEDd, LEVDP were significantly reduced, while LVFS, LVEF, + dp / dtmax, -dp / dtmax were significantly increased showing allogeneic BM-MNCs transplantation can reduce left ventricular dilatation after myocardial infarction, can suppress left ventricular remodeling and can improve heart function. The mechanism may be:
1. Transplanted cells provide ventricular wall with a stent, thereby limiting myocardial fibrosis and cardiac dilatation after myocardial necrosis, also to maintain the structural integrity of the heart [12];
2. Transplanted BM-MNCs differentiate into cardiac tissue, add functional cell components in the infarct area, the formation of contractile proteins, repair of myocardial injury laid the foundation [12,13] to improve heart function;
3. Ability of transplanted BM-MNCs to differentiate into endothelial cells can promote capillary regeneration and improve local circulation in ischemic area [14];
4. Through the secretion and expression of transforming growth factor, the survival rate of transplanted cells can be improved and induces dormant acardiomyocytes to exert contractile function again [15,16];
5. Decreased inflammation reaction at infarction site, inhibition of ventriclar remodeling [14].
In a study of intracoronary BMMNCs or standard therapy 4.8 days after successful PCI following AMI, there was a significant improvement in global LVEF in the cell treatment group at 6 months without an effect on LV remodelling [17]. Intracoronary BMMNC transplantation 7 days post-AMI not only resulted in an early significant improvement in ejection fraction (EF) and infarct size, but there was also a significant reduction in mortality and improvement in exercise capacity compared to controls at 5 years [18]. LEUVEN-AMI study by Janssens et al. [19] showed that intracoronary re-infusion of BMMNCs within 24 hours of reperfusion was associated with a greater reduction in infarct size and improved regional systolic function, but no overall improvement in global left ventricular function compared to controls.
A recent comprehensive systematic review that included 13 trials with a total of 811 patients showed an improvement in LVEF by 2.99% in the BMMNC group compared to standard reperfusion therapy [20]. A previous meta-analysis by Lipinski et al. [21] that included 10 trials with AMI showed that intracoronary stem cell therapy (within the first 14 days after infarction) was associated with a small but significant (3.0%) improvement in LV systolic function compared to standard medical therapy.
Conclusion
Allogeneic BM-MNCs transplantation can reduce left ventricular dilatation after myocardial infarction, inhibit left ventricular remodeling and improve cardiac function. A variety of stem cells is currently used for transplantation in the treatment of AMI. In this study, we used BM-MNCs because there are a variety of stem cell components in bone marrow, source is abundant, simple and easy feasible, ability to induce both in- vivo and in-vitro differentiation into functional cardiomyocytes. It is suitable for clinical application. But complex composition of BM-MNCs and unclear of exact mechanism to suppress ventricular remodeling after acute AMI needs for further research to explore the mechanism is still required.
References
- Fox CS1, Coady S, Sorlie PD, D'Agostino RB Sr, Pencina MJ, et al. (2007) Increasing cardiovascular disease burden due to diabetes mellitus: the Framingham Heart Study. Circulation 115:1544-1550.
- Pfeffer M, John J, McMurray M, Velazquez E, Rouleau J, et al. (2003) Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 349: 1893-1906.
- Pfeffer MA, Braunwald E(1990) Ventricular remodeling after myocardial infarction: experimental observations and clinical implications. Circulation 81: 1161-1172.
- Rouleau JL, de Champlain J, Klein M, Bichet D, MoyéL,et al. (1993) Activation of neurohumoral systems in postinfarction left ventricular dysfunction. J Am CollCardiol22: 390-398.
- Braunwald E (1997) Cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med 337: 1360-1369.
- Mathur A, Martin JF (2004) Stem cells and repair of the heart. Lancet 364: 183-192.
- Graf T (2002) Differentiation plasticity of hematopoietic cells. Blood 99: 3089-3101.
- Donovan PJ, Gearhart J (2001) The end of the beginning for pluripotent stem cells.Nature 414: 92-97.
- Krishna KA, Rao KR (2010) India I K International Publishing Regenerative medicine: Stem cells and their applications. 1-208.
- Katritsis DG, Sotiropoulou PA, Karvouni E, Karabinos I, Korovesis S, et al. (2005)Transcoronary transplantation of autologous mesenchymal stem cells and endothelial progenitors into infarcted human myocardium. Catheter CardiovascInterv65: 321-329.
- Schächinger V, Assmus B, Britten MHonold J, Lehmann R, et al. (2004) Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: Final one year results of the TOPCARE-AMI trial. J Am CollCardiol44: 1690-1699.
- Fedak PW, Weisel RD, Verma S,Mickle DA, Li RK.et al. (2003) Restoration and regeneration of failing myocardium with cell transplantation and tissue engineering . SeminThoracCardiovascSurg 157: 277-286.
- Fedak PW, Altamentova SM, Weisel RD,Nili N, Ohno N, Â et al. (2003) Matrix remodeling in experimental and human heart failure: a possibleregulatory role for TIMP-3. Am J Physiol Heart CircPhysiol284 : 626-634.
- Kaikita K, Hayasaki T, Okuma T,Kuziel WA, Ogawa H, et al. (2004) Targeted deletion of chemokine receptor attenuates left ventricular remodeling after experimental myocardial infarction . Am J Pathol 165: 439-447.
- Schwartz Y, Kornowski R (2003) Autologous stem cells for functional myocardial repair . Heart Fail Rev 8: 237-245.
- Chiu RC (2003) Bone marrow stem cells as a source for cell therapy. Heart Fail Rev8: 247-251.
- Wollert K, Meyer G, Joachim L, Lichtenberg SR, Lippolt P, et al. (2004)Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet 364: 141-148.
- Yousef M, Schannwell CM, Kostering M, Zeus T, Brehm M, et al. (2009) The BALANCE Study: clinical benefit and long-term outcome after intracoronary autologous bone marrow cell transplantation in patients with acute myocardial infarction. J Am CollCardiol 53: 2262-2269.
- Janssens S, Dubois C, Bogaert J,Theunissen K, Deroose C, et al. (2006)Autologous bone marrow derived stem cell transfer in patients with ST-segment elevation myocardial infarction: double blind randomised controlled trial. Lancet 367: 113-121.
- Martin-Rendon E, Brunskill SJ, Hyde CJ, Stanworth SJ, Mathur A, et al. (2008) Autologous bone marrow stem cells to treat acute myocardial infarction: a systematic review. Eur Heart J 29: 1807-1818.
- Lipinski JM, Giuseppe GL,Zoccai B, Abbate A, Khianey R, et al. (2007)Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction. J Am CollCardiol 50: 1761-1767.
Citation: Firoj KM, Fa Xe, Yu Hb (2016) Influence of Bone Marrow Stem Cell Transplantation on Cardiac Function in Remodeled Left Ventricle of Rats after AMI. Cardiovasc Ther 1: 110.
Copyright: © 2016 Firoj KM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 11478
- [From(publication date): 7-2016 - Apr 05, 2025]
- Breakdown by view type
- HTML page views: 10600
- PDF downloads: 878
