Research Article Open Access
Influence of Bile Salts as Excipients in Ranitidine, Aminophylline and Phenobarbital Tablets on Dissolution Rate
Marta Pocuca1*, Jelena Cvejic2, Sasa Vukmirovic1, Nebojsa Stilinovic1, Ksenija Kuhajda3, Slavko Kevresan4 and Momir Mikov1
1Department of Pharmacology, Toxicology and Clinical Pharmacology, University of Novi Sad, 21000 Novi Sad, Republic of Serbia
2Department of Pharmacy, University of Novi Sad, 21000 Novi Sad, Republic of Serbia
3Department of Chemistry, University of Novi Sad, 21000 Novi Sad, University of Novi Sad, Republic of Serbia
4Faculty of Agriculture, University of Novi Sad, 21000 Novi Sad, Republic of Serbia
- *Corresponding Author:
- Marta Pocuca
Medical Faculty Novi Sad
Department of Pharmacology
Toxicology and Clinical Pharmacology
University of Novi Sad
Hajduk Veljkova 3, 21000 Novi Sad, Republic of Serbia
Tel: +38163/594220
E-mail: marta.pocuca@gmail.com
Received Date: May 22, 2017; Accepted Date: May 30, 2017; Published Date: May 31, 2017
Citation: Pocuca M, Cvejic J, Vukmirovic S, Stilinovic N, Kuhajda K, et al. (2017) Influence of Bile Salts as Excipients in Ranitidine, Aminophylline and Phenobarbital Tablets on Dissolution Rate. Clin Pharmacol Biopharm 6:171. doi: 10.4172/2167-065X.1000171
Copyright: © 2017 Pocuca M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
Aim: The aim of this study is to investigate the influence of bile salts, sodium cholate, sodium 12-ketocholate and sodium dehydrocholate, as excipients in ranitidine, aminophylline and phenobarbital tablets on dissolution rate.
Methods: Four groups of tablets (control without bile salts and three investigational groups containing different bile salts) were prepared for three different drug substances: ranitidine, aminophylline and phenobarbital. Dissolution rate was measured.
Results: Dissolution rate is increased significantly in all investigational groups comparing to the control group in all three drug substances.
Discussion: Presented results are very favourable and encouraging in case of dissolution enhancing and should be further investigated, especially in drug substances that are classified in class II and IV as per Biopharmaceutical Classification System (BCS) classification.
Conclusion: Bile acid salts are very promising excipients, proven to act as surfactants and as lubricants. Running title: Bile salts as excipients in ranitidine, aminophylline and phenobarbital tablets.
Keywords
Bile salts; Excipient; Dissolution; Ranitidine; Aminophylline; Phenobarbital
Introduction
Bile acids are amphyphilic molecules with two functionally different molecular surfaces, convex of steroid core that is hydrophobic and concave that is hydrophilic. This coexistence of non-polar and polar surfaces influences their physico-chemical properties [1-4] such as formation of self-association micelles that have significant role in the physiology of the metabolism of fats, absorption of liposoluble vitamins and hydrophobic drugs. Beside their physiological role, micellar solutions of bile acids can solubilizate poorly soluble organic substances [5]. Furthermore, bile acids have a promoting effect in the transport of some polar drugs [6]. It has also been shown that the glycosylated derivates of bile acids facilitate transport of insulin and calcitonin through cell membrane [7]. On the other hand, it has been observed that hydrophobic cationic drugs enter the interaction with bile acid salts [8]. Cholic acid keto derivates prolonged the analgesic effect of lidocaine [9]. Pharmacological studies on promotory action of keto derivates of cholic acids, first of all of 12-monoketocholic acid indicates that keto derivatives of bile acids are of growing interest from a biomedical point of view [10-13]. Bile acids have also been studied as permeability enhancers of other biological membrane such as skin, cornea and the blood-brain barrier [14]. In pharmacological studies bile acids keto derivates are of special interest as introduction of keto group in bile acid molecule leads to Critical Micellar Concentration (CMC) increase which results in decrease in membrane-toxic effect associated with bile acids [15].
Ranitidine hydrochloride is highly soluble in water (24.7 mg/mL) pH of water solution is 4.5-6 [16,17]. It is an acidic salt of a weak base ranitidine and has two pKa values, 2.7 (side chain) and 8.2 (dimethylamine). Per oral absorption is variable, biological availability after per oral dose of 150 mg is 50% (in the range from 40 to 88%), volume of distribution is 1.4 L/kg and elimination half time is 2.5 to 3 hrs. Ranitidine has an unpredictable dose-response ratio i.e., doses of 75, 100 and 150 mg lead to the inhibition of hydrochloride acid overnight in the following percentage: 95, 96 and 92% [16,18]. According to Biopharmaceutical Classification System (BCS) ranitidine belongs to a class III i.e., high solubility-low permeability drugs [19,20].
Aminophylline, theophylline ethylenediamine, is highly soluble in water (one g dissolves in 25 mL of water to give a clear solution), pH of water solution is alkaline. It is a weak base, pKa value is 5.0 [21,22]. Absorption from gastrointestinal tract after per oral or rectal administration is incomplete, slow and variable. Approximately 79% is converted into theophylline. Upon per oral administration of non-coated tablets peak concentration in plasma is reached in 2 hrs. Mean volume of distribution is 0.5 L/kg. Elimination kinetics is highly variable, half-life of elimination is 7-9 hrs in adults, non-smokers, 4-5 hrs in adults, smokers, 3-5 hrs in children and 20-30 hrs in premature babies [23]. According to BCS theophylline belong to a class I i.e., high solubility-high permeability drugs [24].
Phenobarbital is hardly soluble in water (1.11 mg/mL), pH of saturated water solution is 5 [25]. It is a weak acid, pKa is 7.6 [26]. Approximately 80% of per oral dose is absorbed and is distributed in all tissues and central nervous system, peak concentration is reached after 16-18 hrs i.e., phenobarbital has a slow onset of therapeutic effect. Volume of distribution is 0.7 to 1 L/kg. Plasma life halftime is 50-120 hrs in adults and in children 40-70 hrs [26-28]. According to BCS phenobarbital also belongs to a class I [24].
Bile acids and their salts are a special group of biological surfactants and were therefore investigated in this study as excipients in tablets. The aim of this study was to investigate effectiveness of bile salts as excipients used instead of an equimolar concentration of a commonly used excipient magnesium stearate. Due to their physico-chemical properties, it was expected that they would act as surfactants (ionic) and as lubricants.
Materials and Methods
Materials
Tablets
Cholic acid (Sigma, New Zealand, 98%) was used as the starting compound for the synthesis of its keto derivatives. 3α,7α- dihydroxy-12-keto-5β-cholanoic acid (12-monoketocholic acid) and 3,7,12-triketo-5β-cholanoic acid (3,7,12-triketo-cholic acid or dehydrocholic acid)were prepared according to the procedures published earlier [29,30]. Investigated bile acids were transformed to sodium salts by the known procedure [31].
Material for a preparation of ranitidine hydrochloride, aminophylline and phenobarbital tablets is presented in Tables 1-3 respectively. Formulations of control groups (Ranitidine control (RC), Aminophylline Control (AC) and phenobarbital control (PhC)) were developed according to commercial formulations [32-34], while in investigational groups magnesium stearate was replaced with an equimolar concentration of sodium cholate in the investigational group 1 (ranitidine (RIG1), aminophylline (AIG1) and phenobarbital (PhIG1); sodium 12-monoketocholate in the investigational group 2 (ranitidine (RIG2), Aminophylline (AIG2) and phenobarbital (PhIG2)) and sodium dehydrocholate in the investigational group 3 (ranitidine (RIG3), aminophylline (AIG3) and phenobarbital (PhIG3)). Adjustment of lactose or starch quantity was made in order to keep the same tablet mass for all formulations (Tables 1-3).
| Substance | mg/tablet |
|---|---|
| Ranitidine hydrochloride | 167.4 |
| Lactose monohydrate | 57.00-57.68 |
| Corn starch | 24 |
| Povidone k30 | 6.2 |
| Microcrystalline cellulose | 30 |
| Talc | 12 |
| Silicon dioxide colloidal anhydrous | 1 |
| RC-Magnesium stearate* | 2.4 |
| RIG1-Sodium cholate* | 1.8 |
| RIG2-Sodium 12-ketocholate* | 1.74 |
| RIG3-Sodium dehydrocholate* | 1.72 |
| In total | 300 mg |
Table 1: Substances used for preparation of tablets containing ranitidine hydrochloride as active compound (expressed in mg/tablet). *One of the four substances in each of the four groups.
| Substance | mg/tablet |
|---|---|
| Aminophylline | 100 |
| Microcrystalline cellulose | 102.4875 |
| Starch pregelatinised | 25.00-26.43 |
| Sodium starch glycolate | 7.5 |
| Povidone | 7.5 |
| Talc | 2.5 |
| Quinoline yellow | 0.0125 |
| AC-Magnesium stearate* | 5 |
| AIG1-Sodium cholate* | 3.62 |
| AIG2-Sodium 12-ketocholate* | 3.6 |
| AIG3-Sodium dehydrocholate* | 3.57 |
| In total | 250 mg |
Table 2: Substances used for preparation of tablets containing aminophylline as active compound (expressed in mg/tablet). *One of the four substances in each of the four groups.
| Substance | mg/tablet |
|---|---|
| Phenobarbital | 65 |
| Starch | 20-20.0878 |
| Talc | 20 |
| Lactose | 26 |
| PhC-Magnesium stearate | 0.3 |
| PhIG1-Sodium cholate | 0.2152 |
| PhIG2-Sodium 12-ketocholate | 0.2142 |
| PhIG3-Sodium dehydrocholate | 0.2122 |
| In total | 131.3 mg |
Table 3: Substances used for preparation of tablets containing phenobarbital as active compound (expressed in mg/tablet). *One of the four substances in each of the four groups.
Solutions
All chemicals used for a preparation of solutions were pro analysi (p.a.) degree of purity.
For all dissolution testing that were performed, solution of potassium dihydrogen phosphate (0.05 M, pH 4.5) was used. Phosphoric acid (85%) and sodium hydroxide (6 M) were used for pH adjustment.
For a preparation of mobile phase for HPLC analysis, methanol, 0.1 M ammonium chloride and deionised water were used.
Methods
Tableting
All ranitidine, aminophylline and phenobarbital tablet formulations were produced manually i.e., matrix for tableting was filled in manually, at Faculty of Technology, Novi Sad using the ERWEKA EK O tablet machine with AR 400 electromotor. Ranitidine and aminophylline tablets were prepared by wet granulation method, phenobarbital by dry granulation method [32-34].
Dissolution test
For dissolution testing of all formulations, ERWEKA DT 800 dissolution paddle apparatus (United States Pharmacopeia-USP 2), with fractional collector, was used. The following conditions were applied: 37°C, 50 rotation per minute (rpm), run schedule 10, 20, 30, 40, 50, 60 min, volume of dissolution media 500 ml. Dissolution media pH was changed from 4.5 to 6.5 at 30 min and to 7.0 at 60 min. Sodium hydroxide solution (6 M) was used for pH adjustment. Obtained samples were analysed by High Performance Liquid Chromatography (HPLC) [35].
HPLC analysis
All samples were analysed using a HPLC system consisting of an Agilent Technology Series 1100 auto injector, a C18 guard column (4.6 × 15 mm, 5 μm, Zorbax), a C18 analytical column (4.6 × 150 mm, 5 μm, Zorbax) and Agilent series 1100 with DAD detector.
Ranitidine hydrochloride samples obtained from dissolution were analysed for ranitidine hydrochloride concentrations at 314 nm. The mobile phase was a mixture of ammonium chloride (0.1 M) and methanol (15:85 v/v), injection volume 10 μl. The flow rate was 1.5 ml/ min. Under these conditions, the retention time for ranitidine was 1.18 min. For calibration curve concentration range was 0.01-0.1 mg/ml, R=0.9980 [36].
Aminophylline samples obtained from dissolution were analysed for aminophylline concentrations at 273 nm. The mobile phase was a mixture of methanol and water (45:55 v/v), injection volume 10 μl. The flow rate was 2 ml/min. Under these conditions, the retention time for aminophylline was 1.24 min. For calibration curve concentration range was 0.01-0.2 mg/ml, R=0.9946 [37].
Phenobarbital samples obtained from dissolution were analysed for phenobarbital concentrations at 235 nm. The mobile phase was a mixture of methanol and water (50:50 v/v), injection volume 10 μl. The flow rate was 1.2 ml/min. Under these conditions, the retention time for phenobarbital was 3.80 min. For calibration curve concentration range was 0.005-0.1 mg/ml, R=0.9999 [38].
Statistical analysis
All statistical calculations were performed using statistical method ANOVA in Origin 5.0.
Results
Pharmaceutical-technological assessment of tablets was done for all groups of prepared tablets and all tablets fulfil the pharmaceutical-technological requirements in terms of disintegration, dissolution, mass variation and drug content uniformity (results are not presented in this article apart from results for dissolution).
Dissolution
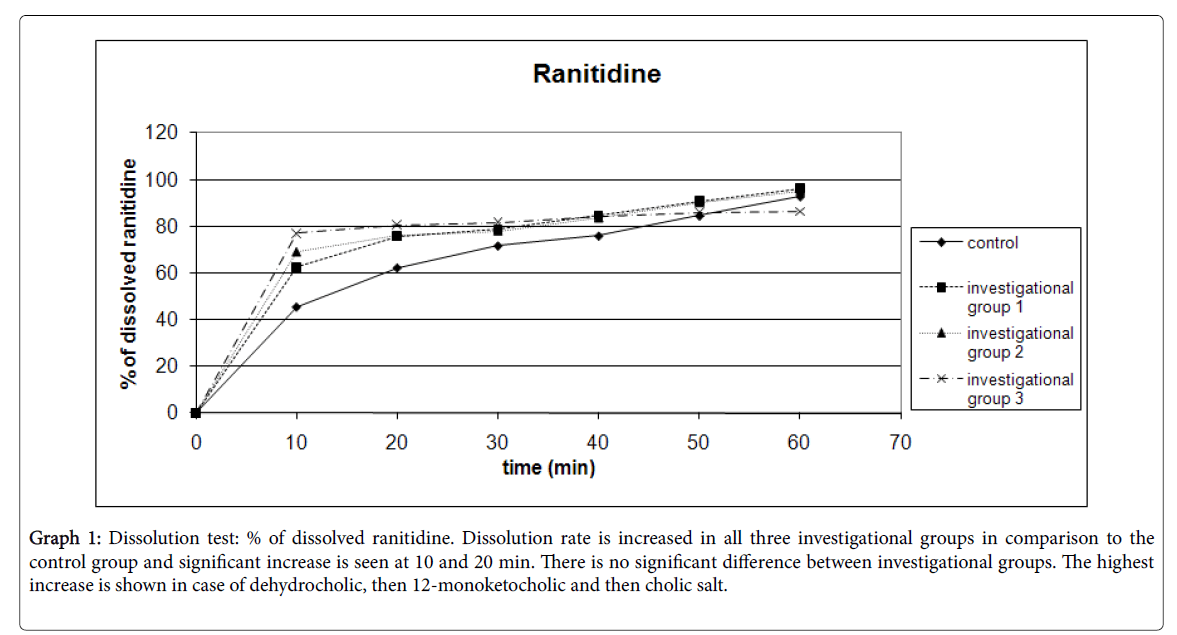
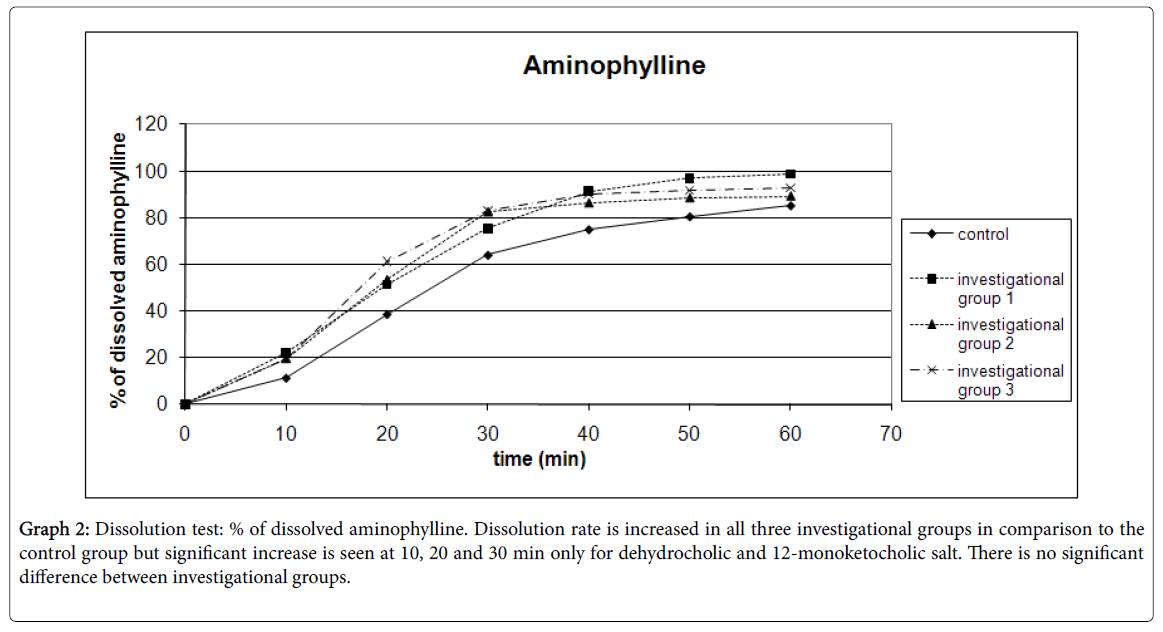
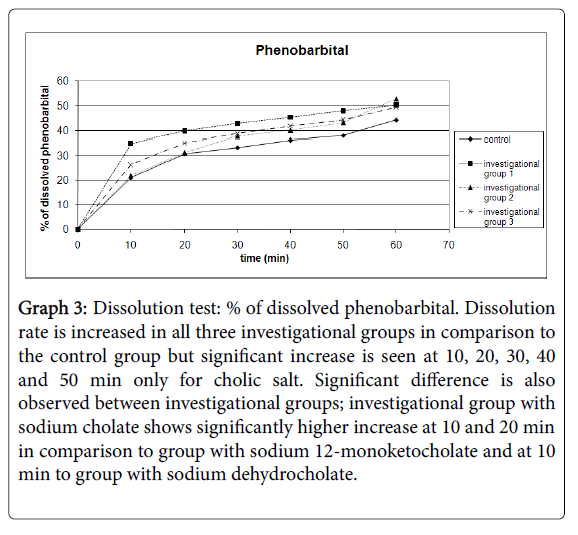
Results for dissolution testing are presented for ranitidine in Table 4 and Graph 1; aminophylline in Table 5 and Graph 2 and for phenobarbital in Table 6 and Graph 3.
Graph 1: Dissolution test: % of dissolved ranitidine. Dissolution rate is increased in all three investigational groups in comparison to the control group and significant increase is seen at 10 and 20 min. There is no significant difference between investigational groups. The highest increase is shown in case of dehydrocholic, then 12-monoketocholic and then cholic salt.
Graph 2: Dissolution test: % of dissolved aminophylline. Dissolution rate is increased in all three investigational groups in comparison to the control group but significant increase is seen at 10, 20 and 30 min only for dehydrocholic and 12-monoketocholic salt. There is no significant difference between investigational groups.
Graph 3: Dissolution test: % of dissolved phenobarbital. Dissolution rate is increased in all three investigational groups in comparison to the control group but significant increase is seen at 10, 20, 30, 40 and 50 min only for cholic salt. Significant difference is also observed between investigational groups; investigational group with sodium cholate shows significantly higher increase at 10 and 20 min in comparison to group with sodium 12-monoketocholate and at 10 min to group with sodium dehydrocholate.
| Time, min | % of dissolved ranitidine in | N | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ranitidine control | Ranitidine investigational group 1 | Ranitidine investigational group 2 | Ranitidine investigational group 3 | ||||||
| Mean, % | SD | Mean, % | SD | Mean, % | SD | Mean, % | SD | ||
| 0 | 0 | - | 0 | - | 0 | - | 0 | - | 6 |
| 10 | 45.52 | 7.33 | 62.47** | 9.21 | 68.95** | 12.38 | 77.07** | 17.45 | 6 |
| 20 | 62.28 | 6.57 | 75.72** | 7.45 | 75.89* | 12.34 | 80.56* | 15.08 | 6 |
| 30 | 71.68 | 10.39 | 78.69 | 9.71 | 77.99 | 11.27 | 81.73 | 13.86 | 6 |
| 40 | 75.91 | 9.52 | 84.68 | 4.09 | 83.48 | 8.62 | 84.34 | 12.19 | 6 |
| 50 | 84.5 | 12.41 | 90.85 | 5.06 | 89.98 | 6.08 | 85.99 | 10.53 | 6 |
| 60 | 92.75 | 8.6 | 96.2 | 3.97 | 95.07 | 3.95 | 86.41 | 10.41 | 6 |
Table 4: Dissolution test: % of dissolved ranitidine. Standard error is within 1.61 to 7.13. *Result is statistically significantly increased in comparison to the control group at P<0.05. **Result is statistically significantly increased in comparison to the control group at P<0.01.
| Time, min | % of dissolved aminophylline in | N | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Aminophylline control | Aminophylline investigational group 1 | Aminophylline investigational group 2 | Aminophylline investigational group 3 | ||||||
| Mean, % | SD | Mean, % | SD | Mean, % | SD | Mean, % | SD | ||
| 0 | 0 | - | 0 | - | 0 | - | 0 | - | 6 |
| 10 | 11.36 | 3.31 | 22.16* | 9.41 | 19.83** | 4.55 | 19.72* | 6.18 | 6 |
| 20 | 38.72 | 5.08 | 51.32 | 14.87 | 53.41* | 11.59 | 61.19* | 15.13 | 6 |
| 30 | 64.15 | 12.12 | 75.44 | 9.8 | 82.41* | 10.77 | 83.15* | 6.67 | 6 |
| 40 | 75.08 | 12.85 | 91.31 | 14.4 | 86.22 | 10.26 | 90.09 | 9.87 | 6 |
| 50 | 80.62 | 10.51 | 96.9 | 19.09 | 88.41 | 9.73 | 91.79 | 9.66 | 6 |
| 60 | 85.33 | 8.23 | 98.73 | 17.02 | 89.18 | 10.16 | 92.8 | 7.38 | 6 |
Table 5: Dissolution test: % of dissolved aminophylline. Standard error is within 1.48 to 8.54. *Result is statistically significantly increased in comparison to the control group at P<0.05. **Result is statistically significantly increased in comparison to the control group at P<0.01.
| Time, min | % of dissolved phenobarbital in | N | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Phenobarbital control | Phenobarbital investigational group 1 | Phenobarbital investigational group 2 | Phenobarbital investigational group 3 | ||||||
| Mean, % | SD | Mean, % | SD | Mean, % | SD | Mean, % | SD | ||
| 0 | 0 | - | 0 | - | 0 | - | 0 | - | 8 |
| 10 | 21.06 | 7.66 | 34.90** | 5.22 | 21.95 | 6.2 | 26.43 | 5.83 | 8 |
| 20 | 30.85 | 8.42 | 39.84* | 5.83 | 31.3 | 8.4 | 35.11 | 5.81 | 8 |
| 30 | 33.3 | 7.72 | 42.79* | 5.51 | 37.7 | 9.8 | 38.79 | 5.79 | 8 |
| 40 | 36.22 | 7.65 | 45.33* | 5.54 | 40.11 | 10.44 | 41.84 | 5.73 | 8 |
| 50 | 37.99 | 7.73 | 47.91** | 4.65 | 43.15 | 10.49 | 44.31 | 6.31 | 8 |
| 60 | 44.24 | 16.85 | 50.25 | 4.01 | 52.78 | 11.47 | 49.4 | 13 | 8*** |
Table 6: Dissolution test: % of dissolved phenobarbital. Standard error is within 1.51 to 5.96. *Result is statistically significantly increased in comparison to the control group (and only at 20 min investigational group 2) at P<0.05. **Result is statistically significantly increased in comparison to the control group (and only at 10 min investigational groups 2 and 3) at P<0.01. ***For PhIG1 N are 7 at 60 min.
Discussion
The results are very favourable and encouraging in case of dissolution enhancing. It has been demonstrated that dissolution rate is increased significantly for all three substances even though they differ in their physico-chemical properties: ranitidine hydrochloride is an acid salt, aminophylline is a weak base and phenobarbital is a weak acid. All of three investigational cholic salts have demonstrated positive effect on dissolution rate increase.
Ranitidine and aminophylline are both highly water-soluble, so the final dissolved concentration is approximately equal for all four groups and significant increase in dissolution rate is observed in the first 30 min only. In contrast, for phenobarbital that is poorly water-soluble, final dissolved concentration in all three investigational groups is higher than in the control group. Important observation is that bile salts have increased dissolution rate at low pH similar to gastric environment. BCS is an experimental model, centrally embracing permeability and solubility, with qualifications related to pH and dissolution. The objective of the BCS is to predict in vivo pharmacokinetic performance of drug products from measurements of permeability and solubility. A drug substance is considered highly soluble when the highest dose strength is soluble in 250 ml or less of aqueous media over a pH range of 1-7.5 at 37°C. A drug substance is considered to be highly permeable when the extent of absorption in humans is determined to be ≥90% of an administered dose based on a mass balance determination or in comparison to an intravenous reference dose [39]. According to BCS, substances that are classified in class II (low solubility-high permeability) and IV (low solubility-low permeability) have low solubility. Drugs whose rate of absorption is much faster than the dissolution rate are said to be dissolution limited because the rate of entry of the drug into the blood is limited by the dissolution process rather than the absorption process. Such drugs are of great interest in the pharmaceutical industry because the rate of dissolution is amenable to control by means of the formulation. In the case of dissolution-limited drugs there may be a relationship between the absorption in human subjects (in vivo) and the dissolution under laboratory conditions (in vitro). Therefore, observed property of bile acid salts to increase dissolution rate should be further investigated especially in dissolution limited drugs.
Conclusion
Bile acid salts are very promising excipients. This study confirmed their role as surfactants and lubricants.
Acknowledgement
This research was supported by Ministry of Education and Science, Republic of Serbia project no. III 41012.
References
- Posa M, Kevresan S, Mikov M, Cirin-Novta V, Sarbu C, et al. (2007) Determination of critical micellar concentrations of cholic acid and its keto derivatives. Colloids Surf B: Biointerf 59: 179-183.
- Posa M, Kevresan S, Mikov M, Cirin-Novta V, Kuhajda K (2008) Critical micellar concentrations of keto derivatives of selected bile acids: thermodynamic functions of micelle formation. Colloids Surf B: Biointerf 64: 151-161.
- Sarbu C, Onis C¸ Posa M, Kevresan S, Kuhajda K (2008) Modeling and prediction (correction) of partition coefficients of bile acids and their derivatives by multivariate regression methods. Talanta 75: 651-657.
- Staels B, Fonseca VA (2009) Bile acids and metabolic regulation: mechanisms and clinical responses to bile acid sequestration. Diabetes Care 32: S237-S245.
- Atanackovi─? M, Posa M, Heinle H, Gojkovi─?-Bukarica L, Cveji─? J (2009) Solubilization of resveratrol in micellar solutions of different bile acids. Colloids Surf B Biointerfaces 72: 148-154.
- Gordon GS, Moses AC, Silver RD, Flier JS, Carey MC (1985) Nasal absorption of insulin: enhancement by hydrophobic bile salts. Proc Natl Acad Sci USA 82: 7419-7423.
- Bowe CL, Mokhtarzadeh L, Venkatesen P, Babu S, Axelrod RH, et al. (1997) Design of compounds that increase the absorption of polar molecules. Proc Natl Acad Sci USA 94: 12218-12223.
- Schwartz MA, Neubert RH, Dongowski G (1996) Characterization of interactions between bile salts and drugs by micellar electrokinetic capillary chromatography, Part I. Pharm Res 13: 1174-1180.
- Posa M, Kevresan S, Mikov M, Cirin-Novta V, Kuhajda K (2007) Effect of cholic acid and its keto derivatives on the analgesic action of lidocaine and associated biochemical parameters in rats. Eur J Drug Metab Pharmacokin 32: 109-117.
- Mikov M, Fawcett JP (2007) (eds.) Bile Acids Medishet Publisher, Geneva, 2007.
- Jakovljevic V, Vasovic V, Vukmirovic S, Posa M, Raskovic A (2006) The influence of bile acids on the pharmacological effects of cardioactive drugs. Acta Pharmacol Sin 27: 172.
- Vasovic V, Vukmirovic S, Posa M, Mikov M, Raskovic A, et al. (2006) Effect of rat pretreatment with aqueous solutions of stevioside and bile acids on the action of certain cardioactive drugs. Eur J Drug Metab Pharmacokinet 31: 311-314.
- Raskovic A, Mikov M, Skrbic R, Jakovljevic V, Vasovic V, et al. (2008) Effect of stevioside and sodium salt of monoketocholic acid on glycemia in normoglycemic and diabetic rats. Eur J Drug Metab Pharmacokinet 33: 17-22.
- Yang L, Zhang H, Mikov M, Tucker I (2009) Physicochemical and biological characterization of monoketocholic acid, a novel permeability enhancer. Mol Pharm 6: 448-456.
- Posa M (2011) QSPR study of the effect of steroidal hydroxy and oxo substituents on the critical micellar concentration of bile acids. Steroids 76: 85-93.
- Riley TN, DeRuiter J (1998) Histamine and Antihistaminic Agents. 680 In Wilson and Gisvoid’s textbook of organic medicinal and pharmaceutical chemistry. In: Delgado JN, Remers WA (eds.) Lippincott Williams & Wilkins, USA.
- Ranitidin-hidrohloride (2000) In Jugoslovenska farmakopeja, Savremena administracija, Beograd.
- Toiman KG (2000) Gastrointestinal and Liver Drugs: In Remington: The Science and Practice of Pharmacy. Gennaro AF, Lippincott Williams & Wilkins, USA.
- Amidon GL, Lennernas H, Shah VP, Crison JR (1995) A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res 12: 413-420.
- Kortejarvi H, Yliperttula M, Dressman JB, Junginger HE, Midha KK, et al. (2005) Biowaiver monographs for immediate release solid oral dosage forms: ranitidine hydrochloride. J Pharm Sci 94: 1617-1625.
- Teofilin-etilendiamin (2000) In Jugoslovenska farmakopeja, Savremena administracija, Beograd.
- http://www.pharminfotech.co.nz/manual/Formulation/mixtures/aminophylline.html accessed on 16.04.2011
- Nichols WK (2000) Respiratory Drugs, In Remington: The Science and Practice of Pharmacy. Gennaro AF, Lippincott Williams & Wilkins, USA.
- Wu CY, Benet LZ (2005) Predicting drug disposition via application of BCS: transport/absorption/ elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm Res 22: 11-23.
- Fenobarbiton (2000) Jugoslovenska farmakopeja, Savremena administracija, Beograd.
- White HS (2000) Sedative and Hypnotic Drugs, In Remington: The Science and Practice of Pharmacy. Gennaro AF, Lippincott Williams & Wilkins, USA.
- Fitzpatrick RW (1999) Practical pharmacokinetics. 1999;10-5 In Clinical Pharmacy and Therapeutics. In: Walker R, Edwards C (eds.) Churchill Livingstone, UK.
- Psihofarmakologija (2003) In Farmakologija, Klini─Źka farmakologija. Ka┼żi─? T, Integra, Beograd pp: 119-120.
- Miljkovic D, Kuhajda K, Hranisavljevic J (1996) Selective C-12 oxidation of cholic acid. J Chem Res pp: 106-107.
- Fieser LF, Rajagopalan S (1950) Oxidation of Steroids. III. Selective Oxidations and Acylations in the Bile Acid Series. J Am Chem Soc 72: 5530-5536.
- Roda A, Hofmann AF, Mysels KJ (1983) The influence of bile salt structure on self-association in aqueous solutions. J Biol Chem 258: 6362-6370.
- Mannur VS, Karki SS, Ramani KB (2010) Formulation and characterization of ranitidine hydrochloride fast disintegrating tablets. Int J ChemTech Res 2: 1163-1169.
- http://www.pharma-ingredients.basf.com/Statements/Technical%20Informations/EN/Pharma%20Solutions/03_040101e_Aminophylline%20hydrous
- Rudnic EM, Schwartz JD (2000) Oral solid dosage forms: In Remington: The Science and Practice of Pharmacy. Gennaro AF, Lippincott Williams & Wilkins, USA.
- Likar MD, Mansour HL, Harwood JW (2005) Development and validation of a dissolution test for a once-a-day combination tablet of immediate-release cetirizine dihydrochloride and extended-release pseudoephedrine hydrochloride. J Pharm Biomed Anal 39: 543-551.
- Xu QA, Trissel LA (2003) Ranitidine: In Stability-indicating HPLC methods for drug analysis. Pharmaceutical Press, London.
- Xu QA, Trissel LA (2003) Aminophylline: In Stability-indicating HPLC methods for drug analysis. Pharmaceutical Press, London
- Xu QA, Trissel LA (2003) Phenobarbitol: In Stability-indicating HPLC methods for drug analysis. Pharmaceutical Press, London.
- Wu CY, Benet LZ (2005) Predicting drug disposition via application of BCS: transport/absorption/ elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm Res 22: 11-23.
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 4431
- [From(publication date):
May-2017 - Jul 09, 2025] - Breakdown by view type
- HTML page views : 3502
- PDF downloads : 929