Research Article Open Access
Influence of an Oral Supplementation Based on Orthosilicic Acid Choline-Stabilized on Skin, Hair and Nails: A Clinical Study with Objective Approach
Gabriela Favaretto1* and Patricia M B G Maia Campos1
Faculty of Pharmaceutical Sciences of Ribeirao Preto, University of Sao Paulo, Ribeirão Preto, SP, Brazil
- *Corresponding Author:
- Gabriela Favaretto
Faculty of Pharmaceutical Sciences of Ribeirao Preto
University of Sao Paulo, Av. do Café S/N
Monte Alegre 14040-903, Ribeirão Preto, SP, Brazil
Tel: 55 (016) 3315 4197
Fax: 55 (016) 3625 7202
E-mail: gabi_fava@hotmail.com
Received date: August 05, 2016; Accepted date: August 26, 2016; Published date: August 30, 2016
Citation: Favaretto G, Campos PMBGM (2016) Influence of an Oral Supplementation Based on Orthosilicic Acid Choline-Stabilized on Skin, Hair and Nails: A Clinical Study with Objective Approach. Clin Pharmacol Biopharm 5:160. doi:10.4172/2167-065X.1000160
Copyright: © 2016 Favaretto G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
In recent years, various dietary supplements have been released in the market with the promise of health benefits and functional properties. New trends involving nutrition have been highlighted due to the potential effects of certain food ingredients in the cutaneous aging process, the healthy appearance of skin, hair and nails. Looking at the growing demand for improvement in the appearance of hair, skin and nails and the increased interest of the population by use of nutricosmetics, the aim of this study was to evaluate an oral supplementation with orthossilicic acid choline-stabilized. For this, it was done a randomized, placebo-controlled clinical test. After approval of the ethics committee, were selected 60 women, aged 40 and 65 and they were divided into two groups (treatment and placebo). The daily dose of the supplement for evaluation was 400 mg of orthossilicic acid choline-stabilized for a period of 3 months. Analyzes were done before the treatment (basal-T0) and after 30, 60 and 90 days of treatment and it were evaluated the structural characteristics of the dermis, the mechanical properties of hair and the perception of effectiveness by the volunteers. According to the results, it was observed for the treatment group an increase in echogenicity of the dermis after 90 days of treatment. Thus, it proved that the treatment increased the density of the skin. In addition, the high resolution images showed improvement in skin micro relief and an improvement in skin roughness. There was also increased resistance of the hair and volunteers of treatment group reported improvement in skin, hair and nails. Finally, the oral supplementation of the nutricosmetic with orthossilicic acid choline-stabilized can be suggested as an effective product to increase skin density and to improve hair and nails conditions, complementary to topical treatments.
Keywords
Silicon; Orthosilicic acid; Oral supplementation; Collagen; Osteoporosis
Introduction
Silicon is a trace element that plays an important structural role by continuous deposition in bones and connective tissue proteins such as elastin, collagen and proteoglycan [1,2]. It seems to have some role in modulating immune and inflammatory response and be associated with mental health by reducing the deposition of heavy metals [3].
Several chemical forms of silicon are found in nature, since it is the second most prevalent chemical element, after oxygen. The knowledge of their chemical structure is critical to differentiate the silicon that contributes to the health of that organic xenobiotics and which acts as a potent toxin [4].
Silicon is usually found in the human diet, but do not always have good bioavailability due to enzymatic action that results in silica or silicate [5]. It has been demonstrated that the bioavailability improves when the silicon is in the form of orthossilicic acid stabilized with choline [1] or collagen, water soluble chemical forms which can be found in food, drinks or dietary supplements [6].
In the organic structures, silicon is deposited on macromolecules such as lipids, carbohydrates and proteins and can be found in the form of orthosilicic acid in bones, tendons, aorta, liver and kidneys [2]. Silicon deficiency may be associated with deterioration of cartilage and collagen, and the imbalance between the minerals contributes to osteoporosis [4].
Silicon has long been considered an agent capable of improving the quality of hair, skin and nails. It has been reported topical use on the hair for cosmeceuticals, together with other ingredients, such as: Fat, hydrolyzed proteins, cationic polymers or cationic quaternized derivatives. These products are designed to give softness, shine, strengthen the wires and detangle easily [7].
In the mechanism of intrinsic and extrinsic skin aging occurs by reduction of dermal fibroblast collagen synthesis and increased degradation by metalloproteinases leading to reduction of skin elasticity, sagging and wrinkles appear to alter the micro relief [8].
Silicon appears to play some influence on the dermis structure by mechanisms: 1) Modulates the action of the enzyme responsible for hydroxylation required for cross-linking of collagen fibers [9]; 2) Participation in the activity of the enzyme proline hydroxylase responsible for the synthesis of proline [10]; 3) Activation of ornithine aminotransferase enzyme that participates in collagen synthesis, action demonstrated in private silicon animals that had a decrease of this enzyme in the liver, reducing the hydroxyproline concentration in the tibia [11]; 4) Connection to the hydroxyl group of polyols, interfering with the binding of glycosaminoglycans to water, mucopolysaccharides and collagen production [12]; 5) The neutralization of free radicals and decrease in collagen glycation reactions. A study has demonstrated that silicon plus vitamin C stimulated the synthesis of hyaluronic acid and proteoglycans, reducing the disruption of the dermal matrix [13]; 6) Anti-inflammatory action, demonstrated in vitro by a reduction in interleukin production and in vivo by the reduction of erythema and edema [14]; 7) Decreased inhibitory activity of cyclooxygenase I (COX1) [15].
In recent years, various dietary supplements have been launched in the market with the promise of health benefits and functional properties. New trends involving nutrition, skin, hair and nails have been highlighted due to the potential beneficial effects of certain ingredients in the cutaneous aging process, the healthy appearance of skin, hair and nails. Clinical studies have demonstrated positive effects of the ingredients in the biomechanical properties of the skin as well as the barrier functions [1,4,5,12,14]. Parallel to this, in vitro and in animal experiments reveal the mechanism of action of food ingredients in cellular and molecular biology of skin cells. Healthy skin is a manifestation of general health and, as such, may be influenced by the consumption of food ingredients, including vitamins, minerals, antioxidants and bioactive peptides.
Looking at the growing demand for improvement in the appearance of hair, skin and nails and the increased interest of the population by use of nutricosmetics, this study aims to promote clinical efficacy test (using appropriate equipments) to analyze the use of oral supplementation of orthossilicic acid stabilized with choline, product already available in the Brazilian market by new clinical study that shows more objective tests (ultrasound skin, hair resistance test, for example) and high resolution images to evaluate changes in the hair, skin and nails and make more visible and measurable results for consumers.
Materials and Methods
The study was placebo-controlled, randomized, double-blind, 60 women aged between 40 and 65 years were voluntary, with the primary objective of evaluating the effect of nutricosmetic intake containing orthossilicic acid stabilized with choline in the skin tissue in the hair shaft and the nail. The study was approved by the Ethics Committee of the Faculty of Pharmaceutical Sciences of Ribeirão Preto/SP (CEP/ FCFRP 339) and followed current Good Clinical Practice regulations [16]. The study duration was 90 days, with 4 reviews at the periods 0, 30, 60 and 90 days. Participants signed the Informed Consent before accepting participation in the study.
After evaluating the terms of inclusion and exclusion, acceptance to participate in the study and signing the consent form, participants were divided into two groups: Group 1 which was the test group, which made the intake of 400 mg/day of orthossilicic acid stabilized with choline and group 2 which was the placebo group that did intake of 400 mg/day placebo with maltodextrin. The duration was 3 months for each participant, with a total of 4 visits: Visit 1 (week 0 and D0), visit 2 (week 4 or D30 ± 3), visit 3 (week 8 or D60 ± 3), visit 4 (week 12 or D90 ± 3).
Inclusion criteria
The inclusion criteria were as follows: Healthy females ranging in age from 40 to 65 years (homogeneous distribution between treatment groups); general good health and mental condition; personal informed consent to participate in the study; personal presence on the predefined days at the institute, and willingness and capability to follow the study rules and a fixed schedule, know that the data could be used to share the project. It was also instructed to the volunteers to not use other oral supplements or cosmetic products (except sunscreen) and to not change their alimentary habits during the study period [16]. All inclusion criteria were evaluated at every visit by means of a questionnaire that assessed whether there was no criterion that was unrequited.
Exclusion criteria
The exclusion criteria was as follows: treatment with topical retinoids, alpha hydroxy acids, poly hydroxy acids, β hydroxy acids and ascorbic acid less than three months; Topical treatment of the facial skin with anti-aging products; Pre-treatment with oral retinoids for less than 6 months; Pre-treatment with nutraceuticals for less than 3 months; Skin Treatment with superficial chemical peels, microdermabrasion and/or laser ablative not less than three months; Treatment with oral products and/or threads to changes of hair shaft for less than 3 months; Stomach diseases such as gastritis and ulcers; Use of antacids and medicines such as omeprazole; Hypothyroidism; Current smoking; Chronic use of corticosteroids (systemic or topical); Chronic Kidney Diseases; Chronic Liver Diseases; Diabetes Mellitus; transplanted patients; photodermatoses Presence; Presence of inflammatory or infectious skin disease on the face; Chemotherapy for less than 3 months; Clinical evidence of immunosuppression; Women on hormone replacement therapy [16].
Randomization
A simple randomization was performed based on the table of random numbers was used to scramble the participants in the treatment group (n = 30) and placebo (n = 30). It was taken into account only the age and color of the skin of volunteers and it was not taken into account the menstrual status (pre/post-menopause) nor recorded the weight of volunteers (Table 1).
| Main Characteristics | Treatment | Placebo | p |
|---|---|---|---|
| Sex F | 30 | 30 | - |
| Age (Years) (Mean) | 50,6 | 50,0 | 0.652 |
| Skin Color | |||
| White | 26 | 25 | 0.325 |
| Nonwhite | 4 | 5 | - |
Table 1: Comparison of randomized groups. P-value calculated using student t-test.
Study limitations
The study depended on voluntary, since it is prohibited by the ethics committee any payment or bonus to participants in clinical studies. Thus, the visits were scheduled according to the availability of participants and their discontinuation in the project was free.
Another limitation of the study was that the criteria for inclusion and exclusion were conducted through questionnaires, which was fully dependent on voluntary responses to our acceptance.
Assessments
Test areas
The test areas were the frontal, periorbital and nasolabial regions of the face, being the periobital and nasolabial sides randomly chosen. On every measurement day, the subjects had to expose their uncovered test areas to the indoor climate conditions (21.5 ± 1ºC and 50 ± 5% relative humidity) for 20 min.
Biophysics techniques and skin image analysis
Evaluation of dermal characteristics: For the evaluation of thickness and dermis echogenicity was used a 20 MHz ultrasound equipment (Dermascan® C, Cortex Technology). The thickness of the skin was determined by an image analyzer. The echogenicity was analyzed per unit area, in pixels determined with the aid of software, which is connected with the water retention between the collagen fibers and with aging and photoaging [17].
Evaluation by high resolution image of the skin: For this study we used the Visioface® equipment which analyzes the visible spots and only visible with UV light by means of image analysis of skin illuminated by white light-emitting diodes (white LEDs) and light emitting diodes similar to UV (UV-like LED), respectively [18]. The equipment has software which can compare images of the same face region and quantify the wrinkles and pores.
Hair analysis techniques
Evaluation of the mechanical properties: The mechanical properties (tensile strength) were tested with the Texturômetro® TA.XT Plus device, operating according to the method described for tensile strength. The hairs used were provided by the volunteers of the study and the measurements were made in triplicate only at 0 and 90 days. The strands were removed intact and the part used was the 5 cm plus near to hair root. The tensile strength was calculated using the maximum force in breaking the wires (measured in Newton).
Subjective evaluation of skin, hair and nails: The volunteers were submitted to sensory analysis to such a questionnaire was applied before the use of nutraceutical and after the end of use, on days 0 and 90 of treatment. The volunteers were asked about the condition of their skin, hair and nails before treatment, as well as the views change after using the nutraceutical.
Statistical evaluation: The experimental data obtained in clinical trials efficacy assessment were submitted to statistical analysis for their interpretation, when the distribution was normal, the indicated test was the analysis of variance, and when the distribution was not normal, the statistics were nonparametric, applying the Kruskal-Wallis test for data not linked [16]. The age of the volunteers were not used as co-variate in this study. The results were presented in form of graphs, tables and figures and discussed across the data provided by the literature. Differences were accepted as statistically significant at p < 0.05.
Results and Discussion
Evaluation of dermal characteristics
The high-frequency ultrasound provide measures of parameters related to the skin histology, analyzing the skin aging process and the echogenicity of the dermis-which is important as it varies with the chronological aging (intrinsic) and photoaging (extrinsic). This equipment also quantifies and qualifies the collagen and elastin fibers in the skin, being this way, an important contribution to clinical efficacy studies of dermocosmetic formulations [19].
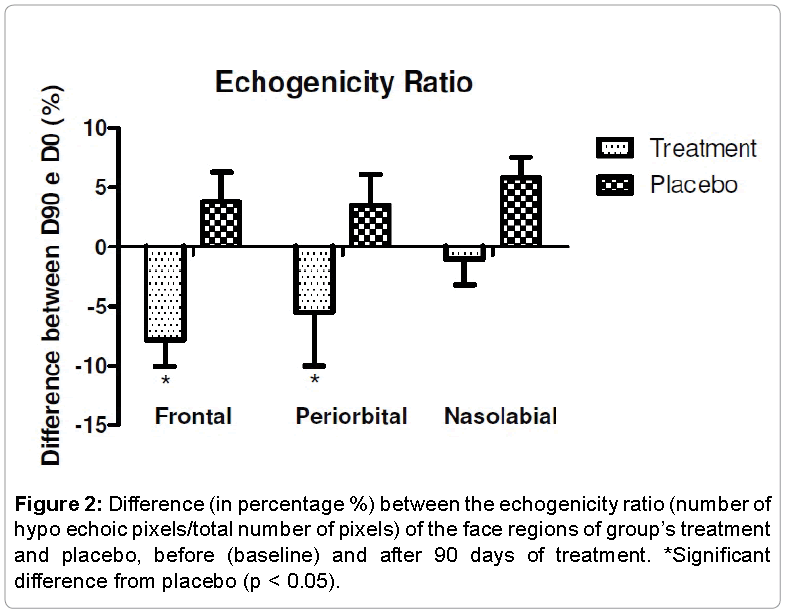
This study showed the increase in the face of the skin echogenicity in the treatment group compared to baseline values (initial time), showing an improvement in skin condition (Figure 1). In the placebo group, there was no increase in echogenicity and some areas had decreased echogenicity as compared with the initial measurement (baseline). This way, the product acted on the dermis, increasing of fibroblast density, enhancing the formation of collagen fibrils, and acting as a repairman of the present damages, slowing the chronological aging and photoaging process, increasing the echogenicity of the dermis and improving the skin density [20-22].
A high dermis echogenicity is related to a high content of collagen fibers, so the lower this ratio is, the more echogenic the skin. Thus, the treatment with orthossilicic acid stabilized with choline has improved the dermis echogenicity when compared to placebo treatment in all regions. The Figure 2 shows the Echogenicity Ratio of all study regions. This parameter reflects the number of hypo echoic pixels/total number of pixels that increases during the aging process.
Evaluation by high resolution image of the skin
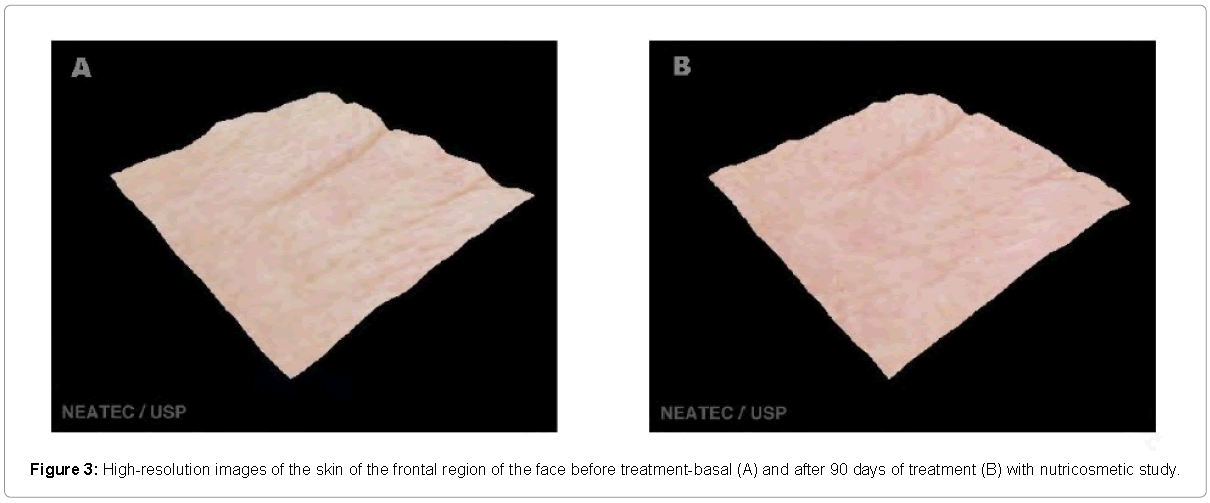
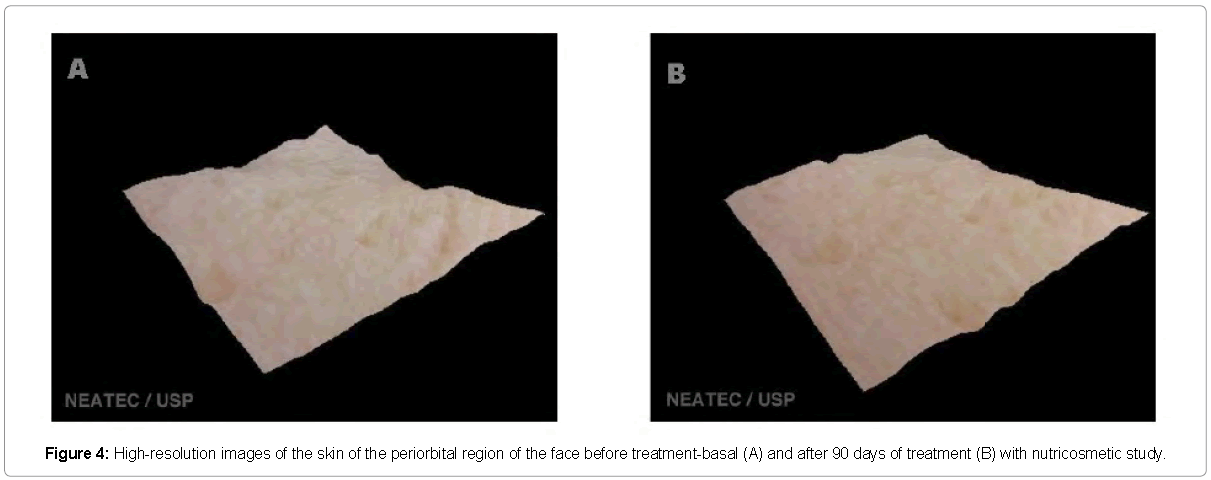
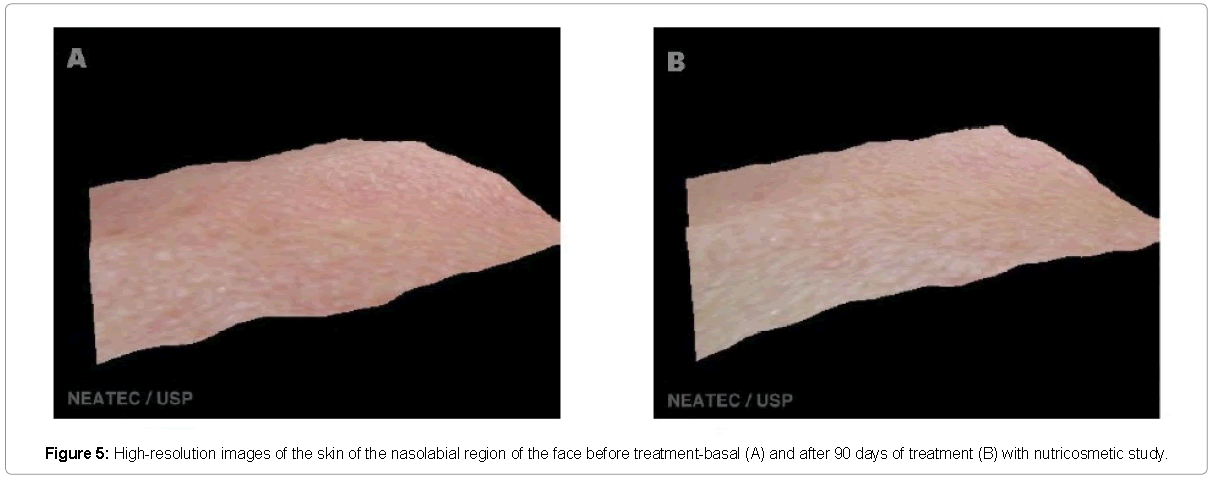
The evaluation by high resolution image (Visioface®) also showed improvement in the skin of the treatment group, but each volunteer had different improvement in each of the regions studied. Figures 3-5 shows a three-dimensional image shown where an evident reduction of wrinkles on the forehead to treatment with the product is visible. Alterations of collagen and elastin can directly the affect wrinkle [23]. Similar results in the reduction of wrinkles after the treatment with oral supplementation and analyzed with other techniques was related in the literature, demonstrating this way, the effectiveness of this type of products in the improvement skin appearance [24].
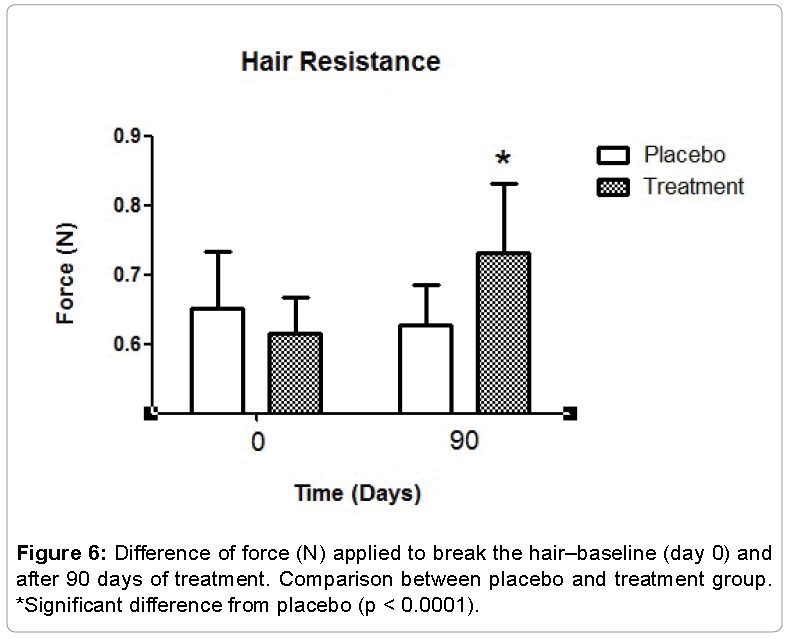
Evaluation of the mechanical properties of hair
The hair resistance test made by Texturômetro® TA.XT plus showed that the hair treatment group necessitated increased strength (force in Newton) to the break, whereas in the placebo group was not seen any significant alteration in the breaking strength (Figure 6). Thus, it was found that oral supplementation with orthosilicic acid choline stabilized promoted greater resistance to the hair, improving hair fiber quality in general.
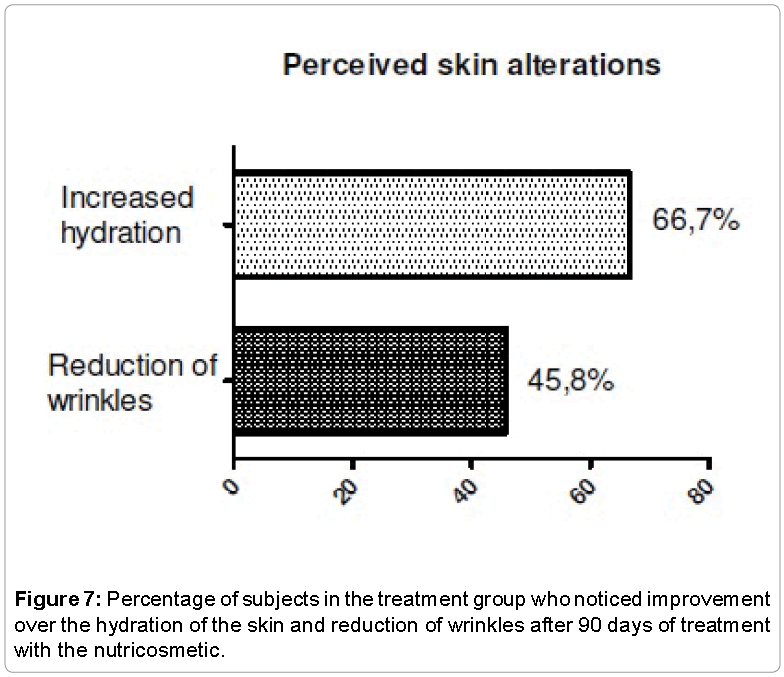
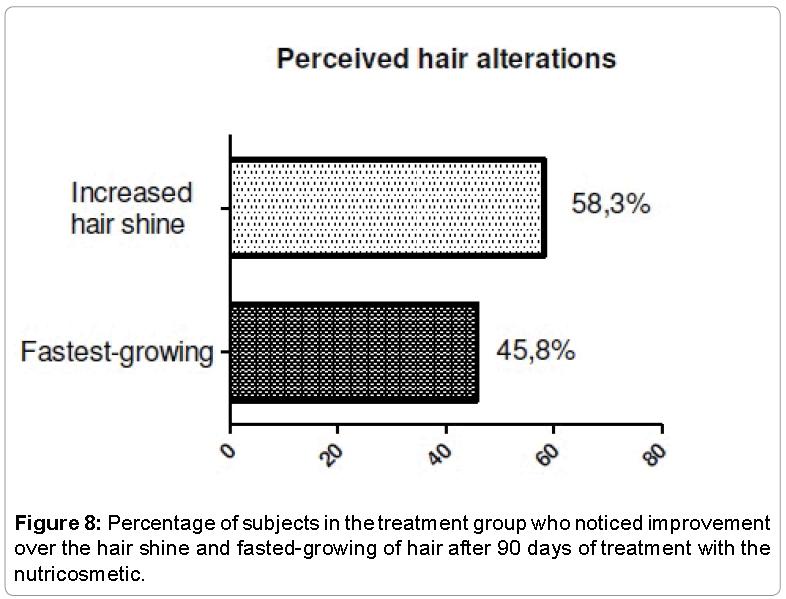
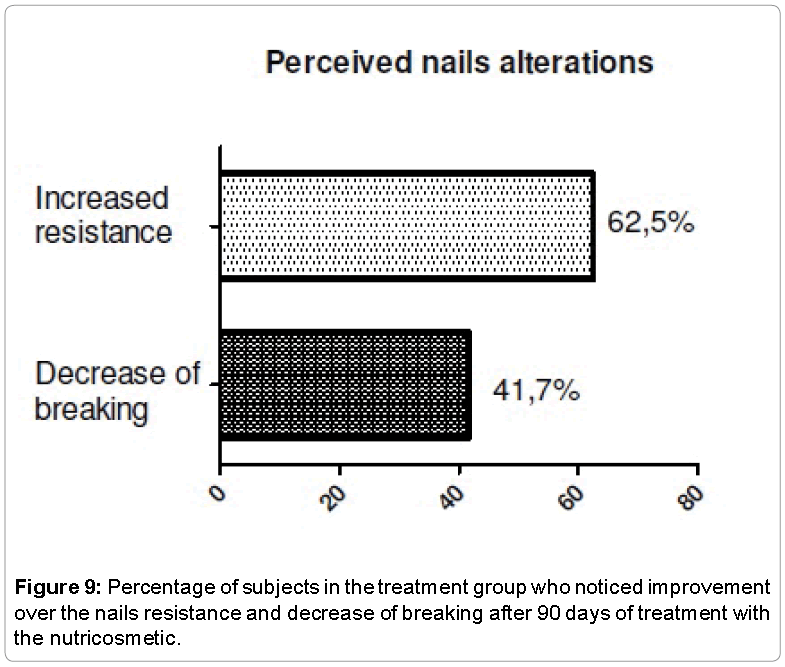
Subjective evaluation of skin, hair and nails
The evaluation of the perception of efficacy was performed to identify what were the changes noted by the volunteers, and check the effect of the use of placebo capsules in this type of treatment. The two groups noted changes related to skin, hair and nails. Thus, it was possible to realize the importance of making a comparison between the treatment group and the placebo group. Among the responses, we observed that voluntary treatment group saw greater improvement in skin, hair and nails appearance (Figures 7-9).
Conclusion
From the results obtained in this study, it was possible to assess the actual clinical efficacy of orthosilicic acid choline-stabilized as a nutricosmetic. Oral supplementation of this compound had satisfactory results regarding the improvement of the skin, hair and nails of the volunteers. Techniques skin image analysis showed positive results in increased echogenicity of the dermis and the micro relief of the improvement of the studied regions. It was also concluded that the supplementation improved the quality of the hair, since the hair after treatment were more resistant. In assessing the perception of the effectiveness of the volunteers, the results were better in the treatment group compared to the placebo group, showing results that coincided with the objective tests performed. In summary, the use of orthosilicic acid choline-stabilized showed significant effects in improving the general conditions of the skin, hair, nails, and can therefore be suggested as an effective nutricosmetic in the treatment of changes resulting from aging, complementary to the use of cosmetic products.
Acknowledgments
The authors thank the company Galena Química e Farmacêutica for the supply of Orthosilicic Acid Choline-Stabilized for the accomplishment of the study.
References
- Barel A, Calomme M, Timchenko A, De Paepe K, Demeester N, et al. (2005) Effect of oral intake of choline-stabilized orthosilicic acid on skin, nails and hair in women with photodamaged skin. Arch Dermatol Res 297:147-153.
- Bissé E, Epting T, Beil A, Lindinger G, Lang H, et al. (2005) Reference values for serum silicon in adults. Anal Biochem 337:130-135.
- Nielsen FH, Poellot R (2004) Dietary silicon affects bone turnover differently in ovariectomised and sham-operated growing rats. J Trace Elem Exp Med 17: 137-149.
- Martin KR (2007) The chemistry of silica and its potential health benefits. J Nutr Health Aging 11:94-97.
- Calomme MR, VandenBerghe DA (1997) Supplementation of calves with stabilized orthosilicic acid. Effect on the Si, Ca, Mg, and P concentrations in serum and the collagen concentration in skin and cartilage. Biol Trace Elem Res 56:153-165.
- Jugdaohsingh R, Anderson SH, Tucker KL, Elliott H, Kiel DP, et al.(2002) Dietary silicon intake and absorption Am J ClinNutri 75: 887-893.
- Trüeb RM (2001) The value of hair cosmetics and pharmaceuticals. Dermatology 202: 275-282
- Rabe JH, Mamelak AJ, McElgunn PJ, Morison WL, Sauder DN (2006)Photoaging: Mechanisms and repair.J Am AcadDermatol55:1-19.
- Keeting PE, Oursler MJ, Wiegand KE, Bonde SK, Spelsberg TC, et al (1992). Zeolite A increases proliferation, differentiation, and transforming growth factor beta production in normal adult human osteoblast-like cells in vitro. J Bone Miner Res 7:1281-1289.
- Carlisle EM(1981) Silicon: A requirement in bone formation independent of vitamin D1. Calcified Tissue International 33: 27-34.
- Seaborn CD, Nielsen FH (2002) Silicon deprivation decreases collagen formation in wounds and bone, and ornithine transaminase enzyme activity in liver. Biol Trace Elem Res 89:251-261.
- Nielsen FH (2014) Update on the possible nutritional importance of silicon. J Trace Elem Med Biol28:379-382.
- Schwarz K (1973) A Bound Form of Silicon in Glycosaminoglycans and Polyuronides (polysaccharide matrix/connective tissue). Proc Nat AcadSci 70: 1608-1612.
- Lansdown ABG (2007) A prospective analysis of the role of silicon in wound care. J Wound Care 16: 404-407.
- Englebienne P (2005) Effects of introducing silicon isosteres in COX-2 inhibitors: a preliminary in silico evaluation. Med Chem1: 215-226.
- Maia Campos P. Melo MO, Calixto LS, Fossa MM (2015) An oral supplementation based on hidrolyzed collagen and vitamins improves skin elasticity and dermis achogenicity: A clinical placebo-controlled study. ClinPharmacolBiopharm 4: 3.
- Gniadecka M, Jemec GB (1998) Quantitative evaluation of chronological ageing and photoageing in vivo: studies on skin echogenicity and thickness. Br J Dermatol 139: 815-821.
- Levy JL, Trelles M, Servant JJ, Agopian L (2004) Non-ablative skin remodeling: an 8-month clinical and 3D in vivo profilometric study with an 810 nm diode laser. J Cosmet Laser Ther 6: 11-15.
- Uhoda E, Piérard-Franchimont C, Petit L, Piérard GE (2005) The conundrum of skin pores in dermocosmetology. Dermatology 210: 3-7.
- Kang S, Krueger GG, Tanghetti EA, Lew-kaya D, Sefton J, et al. (2005) A multicenter, randomized, double-blind trial of tazarotene 0.1% cream in the treatment of photodamage. J Am AcadDermatol 52: 268-274.
- Whang SW, Lee KH, Lee JB, Chung KY (2007) Chemical reconstruction of skin scars (CROSS) method using a syringe technique. DermatolSurg 33: 1539-1540.
- Gniadecka M (2001) Effects of ageing on dermal echogenicity. Skin Res Technol 7: 204-207.
- Pugliese PT (1998) The skin’s antioxidant systems. DermatolNurs 10: 401-416
- Proksch E, Segger D, Degwert J, Schunck M, Zague V, et al. (2014) Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: a double-blind, placebo-controlled study. Skin PharmacolPhysiol 27: 47-55.
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 13363
- [From(publication date):
August-2016 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 12474
- PDF downloads : 889