Review Article Open Access
Inflammation, Free Radical Damage, Oxidative Stress and Cancer
Khanna RD1*, Karki K1, Pande D1, Negi R1 and Khanna RS21Department of Biophysics, Institute of Medical Sciences, Varanasi, India
2Department of Chemistry, Faculty of Science, Banaras Hindu University, Varanasi, India
- *Corresponding Author:
- Khanna RD
Professor Emeritus and Former Head
Department of Biophysics
Institute of Medical Sciences
Banaras Hindu University, Varanasi, India
Tel: 91-9450710446
Fax: 91-542-2367568
E-mail: hdkhanna@yahoo.co.in
Received date: August 29, 2014; Accepted date: July 24, 2014; Published date: July 26, 2014
Citation: Khanna RD, Karki K, Pande D, Negi R, Khanna RS (2014) Inflammation, Free Radical Damage, Oxidative Stress and Cancer. Microinflammation 1:109. doi: 10.4172/2381-8727.1000109
Copyright: © 2014 Khanna RD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Visit for more related articles at International Journal of Inflammation, Cancer and Integrative Therapy
Abstract
The health of our body is maintained by the equilibrium of Oxidation and Reduction. As long as a balance exists between oxidative stress and our antioxidant system, our body is maintained in a healthy state. However, excessive oxidative stress or inadequacy in a normal cell’s antioxidant defense system (or both) can cause the cell to experience oxidative stress. Tumor cells usually have an imbalanced redox status resulting in the damage to DNA, proteins and lipids. Higher levels of DNA damage and deficient DNA repair may predispose individuals to cancer. Reactive oxygen species (ROS) are involved in a variety of different cellular processes ranging from apoptosis and necrosis to cell proliferation and carcinogenesis. Molecular events, such as induction of cell proliferation, decreased apoptosis and oxidative DNA damage have been proposed to be critically involved in carcinogenesis. Carcinogenicity and aging are characterized by a set of complex end points, which appear as a series of molecular reactions. ROS can modify many intracellular signaling pathways including protein phosphatases, protein kinases and transcription factors suggesting that the majority of effect of ROS are through their actions on signaling pathways rather than via non- specific damage of macromolecules; however exact mechanism by which redox status induces cells to proliferate or to die, and how oxidative stress can lead to evoking tumor formation are still not clear. Our environment is oxidizing because of the prevalence of ambient Oxygen. The normal process of cellular metabolism, which requires oxygen from the air we breathe, leads to the production of free radicals- unstable, highly reactive molecules that lack an electron. Free radicals seek stability by stealing electrons from other stable molecules, creating a chain reaction of free radical formation that can cause damage to body cells, proteins and DNA. Aging and/or environmental stress may enhance this oxidative stress and may also lead to chronic inflammation, which can further exacerbate damage and increase cancer risk.
Keywords
Inflammation; Free radical damage; Oxidative stress; Cancer; Reactive oxygen species
Introduction
Inflammation, free radical damage and oxidative stress have become major health issues in recent years. Inflammation is body's natural reaction to invasion by an infectious agent, toxin or physical, chemical or traumatic damage. One purpose of inflammation is to protect the site of an injury. Most people are familiar with the kind of painful inflammation that occurs due to accidents and athletic injuries. Where infection is present, inflammation is not to be confused with the infection – rather it is body’s response to the cause of the infection [1]. Some of the inflammatory conditions can be recognized because their names end in “itis”–arthritis, appendicitis, gastritis, laryngitis, pancreatitis, dermatitis, meningitis (inflammation of the membranes that cover the central nervous system), peritonitis (inflammation of the membrane that covers all the abdominal organs and inside walls of the abdomen). Two inflammatory conditions that don’t end in “itis” are asthma (usually caused by an allergy to an ingested substance) and pneumonia, an inflammatory reaction to the invading micro-organisms in the lungs. Some of the above conditions are very serious and if not controlled, cause death.
Chronic systemic inflammation is not confined to a particular tissue, but involves the lining of blood vessels and many internal organs and systems. This inflammatory process is often associated with free radical damage and oxidative stress and may not cause pain, as some internal organs do not relay pain. Chronic inflammation is a pathological condition characterized by continued active inflammation response and tissue destruction. Many of the immune cells including macrophages, neutrophils and eosinophils are involved directly or by production of inflammatory cytokine production in pathology of chronic inflammation. From, literature it is appear that there is a general concept that chronic inflammation can be a major cause of cancers and express aging processes. Moreover, many studies suggest that chronic inflammation could have serious role in wide variety of age-related diseases including diabetes, cardiovascular and autoimmune diseases. Inflammatory process induces oxidative stress and reduces cellular antioxidant capacity. Overproduced free radicals react with cell membrane fatty acids and proteins impairing their function permanently. In addition, free radicals can lead to mutation and DNA damage that can be a predisposing factor for cancer and age-related disorders [2]. Khansari et al. described in their review free radicals in human diseases can help the investigators to consider the antioxidants as proper agents in preventive medicine, especially for cancer and aging processes [2].
Free Radicals and Oxidative Stress
Our bodies have powerful natural systems designed to protect our health. The risk of infectious diseases or cancers increases as our immune system gets weaker and weaker through the aging and other processes. Every second of our life, our cells are bombarded by particles called free radicals. Normally they protect us from bacteria viruses and other foreign substances. When our antioxidant defenses are adequate, damage caused by those free radicals is repaired without many consequences. However when excessive amount of free radicals generates it can damage proteins, lipids, enzymes and DNA that can alter downstream cell signaling and a cause a variety of diseases.
Reactive oxygen species (ROS) including the hydroxyl radical, superoxide anion, singlet oxygen, and peroxynitrate figures prominently in the etiology and progression of numerous cancers. During endogenous metabolic reactions, aerobic cells produce ROS such as superoxide anion (O2•-), hydrogen peroxide (H2O2), hydroxyl radical (OH•), and organic peroxides as normal products of the biological reduction of molecular oxygen. Oxygen radicals are not only generated in the mitochondria. Neutrophils and macrophages produce ROS via a plasma membrane bound nicotinamide adenine dinucleotide phosphate, reduced form (NADPH)-oxidase. The radicals are generated for cell killing and bactericidal activities. The NADPH-oxidase is not exclusive to these cells, however. Panels of human tumour cell lines were shown to produce large quantities of hydrogen peroxide in vitro [3]. Most ROS are generated in cells by the mitochondrial respiratory chain. Under normal metabolic conditions, these ROS are eliminated rapidly in normal cells by a wide variety of enzymatic and non-enzymatic antioxidant defences. The imbalance between production of reactive oxygen species (ROS) and their elimination by antioxidant defence system results in oxidative stress. Exposure to endogenous and environmental carcinogens causes DNA damage, protein and lipid oxidation indicative of oxidative stress, with consequences for cytotoxic and mutagenic activity as well as aberrant changes to cell cycle progression and replication which potentially impact on the whole organism. Oxygen free radicals are powerful DNA damaging agents that can cause base substitution, deletion, and strand fragmentation which may inactivate tumour suppressor genes with in tumour cells, or increase expression of proto-oncogenes that are critical initial events in carcinogenesis. Moreover, oxidation of cellular lipids and proteins can adversely affect several steps of the carcinogenic process through changes in a variety of cell regulatory functions, including signal transduction and gene expression [4].
Trace elements are major components of antioxidant enzymes. A growing body of evidence has indicated that many trace elements play an important role in a number of biological processes by activating or inhibiting enzymes, by competing with other elements and metalloproteins for binding sites or by affecting the permeability of cell membranes. Thus trace elements may exert action, directly or indirectly, on the carcinogenic process [5].
Reactive oxygen species (ROS) are involved in a variety of different cellular processes ranging from apoptosis and necrosis to cell proliferation and carcinogenesis. ROS play vital role in the stimulation of signaling pathways in cells in response to changes in intra- and extracellular environmental conditions. ROS can modify many intracellular signaling pathways including protein phosphatases, protein kinases, and transcription factors, suggesting that the majority of the effects of ROS are through their actions on signaling pathways rather than via non-specific damage of macromolecules.
Cancer is a multistage process defined by at least three stages: initiation, promotion, and progression. Oxidative stress interacts with all three stages of this process. During the initiation stage, ROS may produce DNA damage by introducing gene mutations and structural alterations of the DNA. In the promotion stage, ROS can contribute to abnormal gene expression, blockage of cell- to cell communication, and modification of second messenger systems, thus resulting in an increase of cell proliferation or a decrease in apoptosis of the initiated cell population. Finally, oxidative stress may also participate in the progression stage of the cancer process by adding further DNA alterations, lipid peroxidation and protein oxidation to the initiated cell population.
Consequences of oxidative stress are: (1) it can increase mutation rate and accelerate tumour progression by inactivating tumour suppressor genes within tumour cells, or by increasing expression of proto-oncogenes, (2) it can activate the growth-promoting signaling pathways, (3) Adaptation to oxidative stress, results in increased resistance to therapy. Severe oxidative stress leads to apoptosis. Conversely, persistent oxidative stress at sublethal levels may cause resistance to apoptosis, (4) it can increase blood supply to tumour cells. Oxygen radicals increase tumour cell production of the angiogenic factors IL-8 and vascular endothelial growth factor (VEGF).
Extensive investigations over the past decade have uncovered that chronic inflammation can promote all stages of tumorigenesis, including DNA damage, replication, apoptosis, angiogenesis, growth signaling, tissue invasion or metastasis. Chronic inflammation is triggered by environmental (extrinsic) factors (eg, infection, tobacco, asbestos) and host mutations (intrinsic) factors (eg, Ras, Myc, p53). However, the precise molecular mechanisms involved and the interconnecting crosstalk between pathways remain incompletely understood. Damage associated molecular patterns (DAMPs) are endogenous molecules released from dying host cells upon oxidative stress or tissue damage can trigger TLR family of receptors involved in alerting the innate immune system of danger [6]. Elevated expression of some TLRs has been reported in many tumor cells, tissues or tumor cell lines which may play a crucial role promoting angiogenesis and metastasis [7]. Toll-like receptor 9 (TLR9) is a cellular DNA-receptor whose activation with cognate ligands triggers an immune reaction, with increased production of inflammatory cytokines, chemokines. Recent publications revealed the existence of a soluble form of TLR9 in human plasma, serum and pleural effusions [8].
Biomarkers
Biomarkers are defined as characteristics that can be objectively measured and evaluated as indicators of normal biological processes, pathogenic processes, or pharmacological responses to a therapeutic intervention. Several in vitro markers of oxidative stress are available, including free radicals themselves, but most are of limited value in vivo because they lack sensitivity and specificity. Although some free radicals have been directly detected in vitro by electron spin resonance with or without spin-trapping reagents, or by chemiluminescence, these direct detection methods are not yet applicable for clinical research because of the instability of many free radicals and the need for expensive equipment. Furthermore, free radicals are generally too reactive and have a half-life too short to allow direct measurement in cells, tissues, or body fluids. Because molecular products formed from the reaction of free radicals with biomolecules are generally considered more stable than free radicals themselves, most commonly, free radicals have been tracked by measuring stable metabolite concentrations of their oxidation target products. such as Malondialdehyde (MDA), 8-hydroxy-2 deoxyguanosine (8-OHdG), protein carbonyl (PC) [3,9].
Therefore free radicals have been tracked by measuring stable metabolite concentrations of their oxidation target products eg. Malondialdehyde (MDA), 8-hydroxy-2 deoxyguanosine (8-OHdG), protein carbonyl (PC) [3,9].
In recent years the emerging science of genomics, proteomics and biophysics has generated a plethora of candidate cancer biomarkers. Unfortunately few of these markers immediately stand out as superior prognostic or diagnostic marker, and even fewer have been validated and approved. Therefore it is very important to validate pre-existing biomarkers and create new biomarkers with high specificity and sensitivity because without measuring parameters relevant to the status of antioxidant defenses and oxidative stress, it is not possible to determine whether the selection, dose, and duration of an antioxidant intervention achieves its intended biochemical or physiological endpoint or whether the enrolled subjects even present with oxidative stress.
Redox Homeostasis
The health of our body is maintained by the equilibrium of redox control. Excessive external factors can disturb this equilibrium triggering disease and illnesses. Most of the diseases are caused by oxidative stress due to active oxygen, other free radicals and lipid peroxides. Active oxygen, though vital to our body, causes considerable damage to the essential components of human body and disturbs the physiologically important functions of proteins, lipids, enzymes and DNA bearing the genetic code. DNA damage caused by oxidative stress is particularly dangerous and has the potential to lead to serious disorders.
Redox control means maintaining the balance between active oxygen species and antioxidant enzymes. A biomarker indicates if the redox system is functioning properly in our body. Active oxygen species are vital for life, however once this active oxygen exceeds a certain level; it causes considerable damage to our cells and threatens our health. Normally about 2% of oxygen inhaled by our respiratory system turns into active oxygen in our body and is used to protect us against external attack from bacteria, germs, viruses and other foreign substances. However, when levels of active oxygen species are increased by tobacco, alcohol, ultra-violet rays, air-pollution, food additives, emotional stress, etc., excessive active oxygen starts attacking not only dangerous substances but also normal functioning cells, damaging proteins, lipids, enzymes and DNA. This state is called oxidative stress. As long as a balance exists between oxidative stress and our antioxidant system, our body is maintained in a healthy state. However, excessive oxidative stress or inadequacy in a normal cell’s antioxidant defense system (or both) can cause the cell to experience oxidative stress. Tumor cells usually have an imbalanced redox status resulting in the damage to DNA, proteins and lipids. Higher levels of DNA damage and deficient DNA repair may predispose individuals to cancer, ageing and other neurodegenerative diseases.
Intracellular redox environment plays an important role in the maintenance of proper cellular homeostasis and functions. Disturbances in redox equilibrium of cells lead to pro-inflammatory conditions and these inflammatory conditions can induce carcinogenesis or increase the malignant potential of the tumour. Oxidative stress or tissue damage can trigger TLR family of receptors involved in altering innate immune system.
Inflammatory Mediators, Oxidative Stress and Cancer
Chronic inflammation and infection are major causes of cancer. Key mediators of inflammation-induced cancer include NF-κB, reactive oxygen and nitrogen species, inflammatory cytokines, prostaglandins and specific micro RNAs. The collective activity of these mediators is largely responsible for either a pro-tumorigenic or anti-tumorigenic inflammatory response through changes in cell proliferation, cell death, cellular senescence, DNA mutation rates, DNA methylation and angiogenesis [10]. Inflammatory mediators provide opportunities to develop novel diagnostic and therapeutic strategies. Schetter et al. described a general overview of the connection between inflammation, micro RNAs and cancer and highlight how the improved understanding of these connections may provide novel preventive, diagnostic and therapeutic strategies to reduce the health burden of cancer [10].
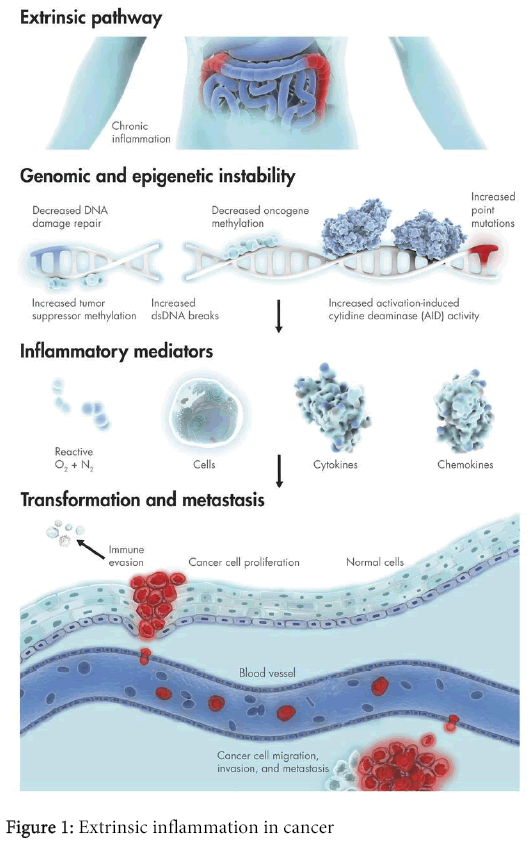
While much effort has been made on understanding the effects of ROS on DNA damage and induction of mutations, the role of ROS on epigenetic effects has also been studied [11]. Carcinogenesis is associated with various epigenetic mechanisms, which can alter intra and inter-cellular communications and gene expression, affecting cell proliferation, differentiation, and apoptosis (Figure 1). When oxidative stress is generated, causing selective DNA damage, it can be one of the most important factors on carcinogenesis. Additionally, protein structure and function can also be altered by ROS produced either by cell metabolism or by external oxidants. Among these and other factors, an oxidative/signaling network will induce cells to proliferate or to commit suicide. These events lead to the transformation of normal cells to carcinoma cells. However, the role of ROS may not be limited to early mutagenic events and cell transformation.
Nuclear factor kappa B (NF-κB) is a family of transcription factors, the functions of which are very well known in apoptosis and inflammation [12,13]. NF-κB is recognized as a redox-sensitive transcription factor and has been implicated in cellular response to oxidative stress. In normal cells NF-κB activation is regulated tightly. Normally, NF-κB can be activated by an appropriate stimulus and returns to the inactive status after the transcription in the target genes have been completed. In tumour cells, different types of molecular alterations may result in damaged regulation of NF-κB activation. ROS also activate NF-κB signal transduction pathways, which in turn lead to the transcription of genes involved in cell growth regulatory pathways. NF-κB pathway play important role in the control of cell proliferation, differentiation, apoptosis, stress response, cell signaling transduction, and other physiological processes.
A number of somatic mutations have been identified in cancer these include transversion mutations of p53. These key genes have been linked to cancer progression and the mutations found in them can be produced by ROS. Although the direct link between ROS modification of DNA and mutation of these genes remains to be established, they should be considered important candidates for the induced carcinogenesis because mutations in these genes could be responsible for tumour initiation as well as progression. Finally, ROS has been shown to increase production of the angiogenic factor such as vascular endothelial growth factor (VEGF) by tumour cells resulting in carcinogenesis.
Infections caused by different pathogens are often associated with systemic symptoms and may compromise the functional integrity. In the mediation of the systemic effect of pathogens Toll-like receptors (TLRs) play a significant role. TLRs are a type of pattern recognition receptor and recognize molecules that are broadly shared by pathogens but distinguishable from host molecules. TLRs are broadly distributed on cells of the immune system and function as primary sensors of invading pathogens. There is also growing experimental evidence indicating that Toll-like receptors are expressed on different non-immune cell types as well, like epithelial or endothelial cells. Oxidative stress significantly up-regulate the expression of these receptors whereas TNF-alpha up-regulates the expression of TLR2 and TLR3. Furthermore the activation of TLR2/6 leads to an increased permeability which is accompanied by a down regulation of occludin and claudin-5 expression and disappearance of these tight junction proteins from the cell membrane. Cell biology of TLRs provides new opportunities for drug development for drug intervention to manipulate immune responses. TLRs are mostly associated with initiation of the innate response and inflammation, and inhibition of TLR activity- which may help combat an over active innate response characteristic of numerous inflammatory disorders.
Conclusion
The concept of “Redox Regulation” in the field of cancer prevention is emerging to understand the mechanisms behind the pathogenesis of several disorders including tumor initiation and malignant transformation. Redox sensitive transcription factors are the key players in regulating several pathways that leads to carcinogenesis. Transcription factors/activators are a group of proteins that bind to specific consensus sequences (cis elements) in the promoter regions of downstream target/effector genes and either trans activate or repress effector gene expression. The change in the expression of the effector genes eventually leads to several biological modifications such as proliferation, growth suppression, differentiation or senescence. Furthermore, redox induced biochemical alterations sometimes lead to change in the biological functions of these proteins. Therefore, differential regulation of these transcriptional activators, which in turn, regulate many target/effector genes, may provide an additional mechanism by which small antioxidant molecules play protective roles in anti-cancer processes and thus have an important impact on drug discovery and therapy for the inhibition of cancer. Inflammation of external origin is responsible for increased cancer risk via induction of genetic and epigenetic aberrations in affected cells [14].
References
- Beverly Nadler (2007) Inflammation, Free Radicals, Oxidative Stress and Antioxidants. Unlimited Vision –Nutrition.
- Nemat K, Yadollah S, Mahdi M (2009) Chronic Inflammation and Oxidative Stress as a Major Cause of Age- Related Diseases and Cancer. Recent Patents on Inflammation & Allergy Drug Discovery 3: 73 -80.
- Halliwell B, Cross CE (1994) Oxygen-derived species: their relation to human disease and environmental stress. Environ Health Perspect 102 Suppl 10: 5-12.
- McKeown N (1999) Antioxidants and breast cancer. Nutr Rev 57: 321-324.
- Huang YL, Sheu JY, Lin TH (1999) Association between oxidative stress and changes of trace elements in patients with breast cancer. ClinBiochem 32: 131-136.
- Gill R, Tsung A, Billiar T (2010) Linking oxidative stress to inflammation: Toll-like receptors. Free RadicBiol Med 48: 1121-1132.
- Harmey JH, Bucana CD, Lu W (2002) Lipopolysaccharide-induced metastatic growth is associated with increased angiogenesis, vascular permeability and tumor cell invasion. International journal of cancer 101: 415-422.
- LeBouder E, Rey-Nores JE, Rushmere NK, Grigorov M, Lawn SD, et al. (2003) Soluble forms of Toll-like receptor (TLR)2 capable of modulating TLR2 signaling are present in human plasma and breast milk. J Immunol 171: 6680-6689.
- Nagini S, Saroja M (2001) Circulating lipid peroxides and antioxidants as biomarkers of tumor burden in patients with oral squamous cell carcinoma. J BiochemMolBiolBiophys 5: 55–59.
- Schetter AJ, Heegaard NH, Harris CC (2010) Inflammation and cancer: interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis 31: 37-49.
- Evans MD, Dizdaroglu M, Cooke MS (2004) Oxidative DNA damage and disease: induction, repair and significance. Mutat Res 567: 1-61.
- Gilmore TD (2006) Introduction to NF-kappaB: players, pathways, perspectives. Oncogene 25: 6680-6684.
- Bonizzi G, Karin M (2004) The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol 25: 280-288.
- Liu C, Ehrlich E (2011) The Role of Inflammation in Cancer.
Relevant Topics
Recommended Journals
- Journal of Lung Cancer Diagnosis & Treatment
- Advances in Cancer Prevention
- Breast Cancer: Current Research
- Cancer Surgery
- Immunology: Current Research
- Current Trend in Gynecologic Oncology
- Journal of Cancer Diagnosis
- Journal of Gastrointestinal Cancer and Stromal Tumors
- Cervical Cancer: Open Access
- Journal of Mucosal Immunology Research
- Journal of Oncology Research and Treatment
- Journal of Orthopedic Oncology
- Journal of Prostate Cancer
- Research and Reviews on Pathogens
Article Tools
Article Usage
- Total views: 29794
- [From(publication date):
September-2014 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 24643
- PDF downloads : 5151