Research Article Open Access
Inflammasome Activation P2X7-Dependent in Crohns Disease
Zelante Angelo1*, Borgoni Riccardo4, Falzoni Simonetta2, D’Incà Renata3, Sturniolo Giacomo Carlo3, Cifalà Viviana1and Di Virgilio Francesco21Gastroenterology Unit, Department of Medicine, University Hospital "Sant'Anna", Ferrara, Italy
2Department of Experimental and Diagnostic Medicine, University of Ferrara, Italy
3Department of Surgical, Oncological and Gastroenterological Sciences, Padua, Italy
4Department of Statistics, University of Milano-Bicocca, Milan, Italy
- *Corresponding Author:
- Zelante Angelo
Gastroenterology Unit
Department of Medicine
University Hospital "Sant'Anna"
Ferrara, Italy
Tel: +0393470125037
E-mail: zelans@libero.it
Received date: June 30, 2015 Accepted date: July 16, 2015 Published: July 25, 2015
Citation:Angelo Z, Riccardo B, Simonetta F, Incà Renata D, Carlo SG, et al. (2015) Inflammasome Activation P2X7-Dependent in Crohn’s Disease. J Gastrointest Dig Syst 5: 316. doi:10.4172/2161-069X.1000316
Copyright: ©2015 Angelo Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License; which permits unrestricted use; distribution; and reproduction in any medium; provided the original author and source are credited
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
The Inflammasome represents an intracellular multiprotein complex belonging to the innate immune system that identifies molecular damage. The activation of the Inflammasome triggers the maturation and secretion of cytokines IL-1β, IL-18, IL-33 which activate on his part inflammatory processes. The most important Inflammasome is NALP3 (NOD-like family) with his components P2X7, NALP3, the adapter ASC and caspase-1. NALP3 and NOD2 polymorphisms are associated with development of Crohn's disease (CD). This study analyzed the expression and function of the NALP3-inflammasome by stimulating in vitro PBMCs of CD patients. We enrolled 62 CD patients: 36 female (58.1%) and 26 male (41.9%) with a mean age of 53.7 years; the control group included 58 subjects (38 female; 20 male; mean age 46 years). The patients have been analyzed with regard to duration, location and disease activity, smoking habits, comorbidities, type of therapy, previous surgery, familiarity for IBD and CRP values. We isolated PBMCs to extract RNA for rt-PCR and proteins for Western Blotting.
Abstract
The Inflammasome represents an intracellular multiprotein complex belonging to the innate immune system that identifies molecular damage. The activation of the Inflammasome triggers the maturation and secretion of cytokines IL-1β, IL-18, IL-33 which activate on his part inflammatory processes. The most important Inflammasome is NALP3 (NOD-like family) with his components P2X7, NALP3, the adapter ASC and caspase-1. NALP3 and NOD2 polymorphisms are associated with development of Crohn's disease (CD). This study analyzed the expression and function of the NALP3-inflammasome by stimulating in vitro PBMCs of CD patients.
We enrolled 62 CD patients: 36 female (58.1%) and 26 male (41.9%) with a mean age of 53.7 years; the control group included 58 subjects (38 female; 20 male; mean age 46 years). The patients have been analyzed with regard to duration, location and disease activity, smoking habits, comorbidities, type of therapy, previous surgery, familiarity for IBD and CRP values. We isolated PBMCs to extract RNA for rt-PCR and proteins for Western Blotting.
Our results show a significant difference in the expression of the Inflammasome’s component in CD patients (Wilcoxon test and multivariate analysis). CD patients had increased expression of IL-1β (p=0.0012) and activation of the P2X7 receptor (p=0.00001) suggesting a role for the Inflammasome in the disease pathogenesis. The increased expression of ASC (p=0.0166) and NALP3 (p=0.0082) confirmed the capability of the Inflammasome to maintain disease activity.
Several features impacted on the Inflammasome's expression: disease’s location, comorbidities, therapy, smoking habits and elevated CRP values. Increased expression of IL-1β and ASC was especially found in ileal CD. Patients receiving immunotherapy showed a different Inflammasome activation compared to patients receiving with mesalazine but disease activity did not modify NALP proteins' expression. Increased CRP predicted increased P2X7 expression independently from other variables.
If our results will be confirmed at the mucosal level, P2X7 may be identified as a potential therapeutic target in CD and CD could be also classified as an auto-inflammatory diseases.
Keywords
Crohn’s disease; Inflammasome
Introduction
Crohn’s Disease can involve, in an irregular way, any segment of the gastrointestinal tract, from the mouth to the anus. The colon and the ileum are the most affected sections; other sections’ involvement, such as mouth, oesophagus, stomach and duodenum is really rare and it usually occurs in association to the ileum-colic involvement [1]. In genetically predisposed individuals, one or more environmental factors determine a loss of tolerance to antigens that are normally contained in the intestinal lumen with development of an uncontrolled activation of the mucosal immune system, amplified production of pro-inflammatory cytokines and loss of the normal balance of inflammatory mediators in the intestine. The inflammation is maintained by a chronic dis-regulation of the immune system of the gastrointestinal tract’s mucosa [2]. In vitro evidence indicate an increase in the number and state of activation of T lymphoid cells, intestinal macrophages, resulting in predominant cell-mediated response and release of cytokines, such as IL-12, IFN-γ, TNF-α, IL-21 IL-18. The increased release of these mediators is able to maintain and perpetuate, and in some cases to induce the intestinal inflammatory process [3]. Downline of the inflammatory cascade there is the inflammasome, an intracellular complex of proteins assembled in myeloid cells that plays a role in the innate immunity through the recognition of molecular damage. The inflammasome’s activation involves, ultimately, the formation of cytokines such as IL-1β, IL-18 and IL-33 that cover various inflammatory and regulatory functions of the immune system. These proteins are structured as a pro-protein not activated and must be cleaved by caspase-1, which it’s part of the complex activated by the inflammasome [4]. The overproduction of IL-1β is also the consequence of the mutation of nucleotide NOD dominion, as part of NALP3-inflammasome, that is considered accountable of some rare diseases "auto inflammatory" such as Muckle-Wells syndrome and the neonatal multi-system inflammatory disease [5]. In IBD, the alteration of intestinal epithelial barrier permits the strengthening and the activation of inflammasomes that detect the presence of "warning signal" [6]. A functional alteration of the inflammasome and the consequent reduction of cytokines such as IL-1β involves an alteration of the intestinal immune barrier against the commensal microbiota; it seems that this defective production of IL-1β process is the base of the onset of IBD [7]. The evidence of changes in Nod1 and NOD2 in 15-20% of cases of CD confirms in part this hypothesis. The inflammasomes (NALP3) are high molecular weight platforms localized in the cytosol of different cell types whose knowledge is still largely incomplete. They’re composed by a large number of oligomeric proteins whose exact composition depends on the subtype considered [8]. Another factor that can modulate the secretion of IL-1β was found to be known as the activation of the P2X7 receptor; this receptor is able, when activated, to provide for the synthesis by the resident intestinal cells of other inflammatory cytokines such as IL-2, IL-12, IL-18 and TNFα, which in turn promote the maturation of Th1 lymphocytes locoregional. The P2X7 receptor is also involved in the modulation of extracellular concentration of ATP, which, being a ubiquitous intracellular constituent and increase under conditions of tissue injury such as inflammation, hypoxia and ischemia [9], can also be considered an endogenous danger signal. The ATP-mediated activation of P2X7 receptors is also responsible for the increased permeability of the macrophage cells to K+ ions, expression of the inflammasome’s activation. It independently modulates the response to many cytokines also activating the mechanism of autophagy with the degradation of defective structures, proteins of long duration, playing a role in the homeostasis of the cells through the recycling of cytoplasmic structures to form new amino acid structures [10]. Some genetic loci related to the mechanism of autophagy have been identified in the CD, which modulate the secretion of pro-inflammatory cytokines through a process inflammasome-independent [11].
The inflammasome needs two signals for the secretion of IL-1β active and IL-18: the first that can get through toll-lik receptors (TLR) in response to pathogen associated molecular patterns (PAMPs), or IL-1β itself induces the transcription and transposition of the inactive forms in activated B cells (NF-kB). The second signal is related to the complete inflammasome’s activation and is required for the conversion of inactive forms in cytokine activated.
The spectrum of the activator molecules (PAMPs) in recent years has been greatly expanded: from well-defined pathogens, fungal, bacterial, viral, we have moved to include metabolic stimulus such as hyperglycemia, free fatty acids and ATP. Therefore, despite the inflammasome represents a model of the innate immune response, not just as a reaction to infections but also in response to metabolic signals of danger.
The mechanisms that explain the inflammasome’s activation are still the subject of debate [12]; available data support three models not reciprocally exclusive: The stimulation of the P2X7 receptor by extracellular ATP promotes an efflux of K+ ions and a gradual recruitment of the pore membrane Pannexin-1, enabling extracellular agonists of NALP3 the access to the cytosol and the activation of the NOD-like receptor (NLR) [13,14]; the channels "Pannexin-1" to allow the passage within the cell of high molecular weight PAMPs and DAMPs (danger associated molecular patterns) which involve activation of NALP3. However, forasmuch as the structural diversity of the agonists, it is unlikely a direct interaction of all NALP3 activators. The agonists forming particulate and crystalline structures (eg. monosodium urate, silica, β-amyloid) can be swallowed and cause damage to their physical properties that contain lysosomes, with subsequent release into the cytoplasm and activation of NALP3. The activation of NALP3 is based on the production of the radical 'oxygen species (ROS), secondary to the stimulation with all known agonists (including ATP), which represents one of the mechanisms of response to infection and cellular damage more conserved from the point of evolutionarily. ROS lead directly to the inflammasome’s activation by ligands specific proteins called thioredoxine.
The immune system responds to viral and bacterial infections through the production of potent inflammatory cytokines such as TNF-α, IFN-γ, IL-1β and IL-18. These cytokines stimulate neutrophils and macrophages to phagocytosis and the release of natural toxic substances such as oxygen and nitrogen radicals. Among these, IL-1β and IL-18 are important inducers of biological responses associated not only to infections, but also to inflammation and immune processes.
The main producers of IL-1β are circulating monocytes, tissue macrophages and dendritic cells.
B lymphocytes and NK cells produce IL-1β too, instead of fibroblasts and epithelial cells that generally do not produce the cytokine. The IL-1β is particularly effective in inducing the activation of nuclear factor-kB (NF-kB) and mitogen-activated protein kinase (MAPK) that regulate the transcription of pro-inflammatory genes. It stimulates the synthesis of other pro-inflammatory cytokines such as TNF and IL-6. It also regulates the differentiation of Th17 cells in the adaptive response. This last element is particularly relevant after the latest evidence of a central role for inflammation of the intestinal mucosa by the Th17 particularly in CD.
For certain, IL-1β is indirectly responsible for the acute phase response (mediated by IL-6), lowering of the threshold of pain, vasodilation and hypotension; promotes angiogenesis and is involved in metastatic tumor. Minimum doses of IL-1β release adrenocorticotropic hormone and the release of IL-6 induces the synthesis of acute phase proteins such as amyloid A and C-reactive protein stimulates cell adhesion and producing leukocytosis and thrombocytosis [15].
Molecules that would function as antagonists of IL-1β to obtain new molecules in addition to the current ones are in phase of study to block the effect of IL-1β, such as anakinra, which has a low half-life and require daily administration subcutaneous [16,17]. Between molecules in the process of production is shown good efficacy for oral inhibitor of caspase-1 VX-765, which was effective in blocking the production of IL-1β in monocytes in patients with fever and autoinflammatory family [18] and in animal models of rheumatoid arthritis. More recently rilonacept, a protein to long duration of action which binds the receptor for IL-1 and the cankinumab, a monoclonal anti-IL1β were developed with the aim of offering to the auto-inflammatory disease a therapeutic possibility with a better profile of administration [19].
Aim of the Study
The purpose of this study is to highlight how the inflammatory way, inflammasome-dependent, is crucial for the phenotypic expression of CD and should be considered adding to the "inflammatory ways" alternatives, such as that related to TNF-α. We wanted to evaluate the role of NALP3 inflammasome-in CD compared with control subjects and define different forms of the inflammasome’s proteins in relation to disease characteristics and patient (age, medication, smoking status). Understanding the role of NALP3-inflammasome in the CD could be used to develop potential targets for therapeutic interventions. In particular, the confirmation of the involvement of the inflammasome main receptor stimulation (P2X7) may justify the use of edicines already under study, which block the activation and may be an effective therapy for IBD [20]. The indirect objective is to highlight how the CD, in the light of inflammasome’s new knowledge, can be considered a disease of IL-1 correlated.
Patients and Statistical Methods
We selected 62 patients with CD related to the surgery of inflammatory bowel diseases and the Day Hospital Unit of Gastroenterology of the University Hospital of Ferrara; the diagnosis was made according to the diagnostic criteria in 2010 ECCO (European Crohn's and Colitis Organization): histological, radiological and clinical laboratory [21]. This cohort was compared with 58 healthy volunteers donors of blood components related to the Blood Transfusion Centre of the same company. Individuals with CD were stratified by some clinical features such as age, sex, smoking status, duration, localization and activation status of the disease, treatment taken, any previous surgical treatment and familiarity. For disease activity we used the Harvey-Bradshaw Index, used for the CD since 1980 began as a simplified version of the CDAI (Crohn's Disease Activity Index) to assess key aspects of the clinical condition of the patient. Finally we recorded values of C-reactive protein (CRP), to assess the inflammatory status of the general patient. Statistical analysis was performed using the χ2 test (for dichotomous variables) and the t-student test (for continuous variables), the comparison of the different variables in the two groups of patients sick vs. healthy patients with also construction of the scatter charts of the different variables in the two groups. The p-value of the test is calculated using the Kruskal-Wallis statistic, in the presence of a non-normal distribution of the variable in the two groups. Then we performed a multivariate analysis using as dependent variable the values of different cytokines compared with the independent variables consist of age, gender, smoking status, CRP, localization and activity of the disease, immunosuppressive therapy; the value of p <0.05 was considered significant. Analyses were performed using the software R firmware version 2.14 (R Development Core Team, 2010).
Laboratory Methods
We did a peripheral venous blood sample (3 ml tubes 7) to patients with CD and compared with the peripheral blood of healthy volunteers. We isolated peripheral mononuclear cells from the tube containing heparin after centrifugation. We isolated lympho-monocytes from which we proceeded to the subsequent extraction of RNA for Real-Time PCR, measurement of IL-1β was performed with an ELISA test. We prepared under biological hood for each sample, a 50 ml tube and three 15 ml, adding 5 ml of Ficoll Paque Plus in these last. Then we emptied the blood contained in the phials in the 50 ml tube and added to PBS until 21 ml; we layered the blood on Ficoll with a pipette (approximately 7 ml per tube). We centrifuged the sample for 20 minutes at 2100 rpm. After the centrifuge we took the ring of lympho-monocytes and put it in a 50 ml tube, adding PBS up to 30 ml. After a centrifugation at 1900 rpm for 10 minutes: re-suspension of the cells in 30 ml of PBS and centrifugation at 1700 rpm for 10 minutes; we eliminated the super-vessel with another washing with 30 ml of PBS and 1400 rpm for 10 minutes. We eliminated the super-vessel and the cells re-suspended in 10 ml PBS with centrifuge the cell suspension at 1100 rpm for 10 minutes. We re-suspended in 500 L of TRIZOL, to obtain RNA. The remaining content was re-suspended in saline sucrose + protease inhibitors (PMSF and Benzamidina) (in a volume ranging from 100 μl to 70 μl if the pellet is poor).
We finally transferred the samples in sterile eppendorf tubes and stored at -80°C.
Reagents
The 2',3'-(4-benzoyl-benzoyl)-ATP (BzATP), the bacterial endotoxin (LPS) extracted from Escherichia coli (serotype 055: B5), the benzamidina, the phenylmethylsulfonyl fluoride (PMSF) and l 'bovine serum albumin (BSA) were purchased from Sigma-Aldrich (Milan). The Ficoll was purchased from GE Healthcare (GE Healthcare, Milan, Italy), the RPMI-1640 from Lige Technologies (Gaithersburg, MD, USA), FCS, penicillin and streptomycin from Celbio (Milan).
Cell culture
Ficoll gradient purified the peripheral blood mononuclear cells (PBMCs). We used a part of these for the experiments in vitro stimulation; we retained the remaining part for analysis by Real Time-PCR. The PBMCs used for the in vitro stimulation were maintained in culture in RPMI-1640 in which it was added 10% heat-inactivated fetal bovine serum (FCS), 100 U/ml penicillin and 100 mg/ml streptomycin, at 37°C in presence of 5% CO2. We incubated the cells (5 × 105/ml) for 2 hours in the absence of stimulation or in the presence of 1 μg/ml LPS. We stimulated some cells after incubation with LPS with increasing concentrations of BzATP. We collected the supernatants cell, centrifuged them at 240 g for 10 minutes to remove any cells in suspension, and then frozen them at -20° until the moment came to analysis with ELISA. We lysed the cells in the wells instead using a saline solution of low ionic strength (300 mM sucrose, 1 mM K2HPO4, 1 mM MgSO4, 5.5 mM glucose and 20 mM Hepes, 1 mM CaCl2, pH 7.4 with KOH), in which we added protease inhibitors PMSF and benzamidina, in the presence of 0.1% Triton.
Measurement of the IL-1β secretion
We measured the concentration of IL-1β in cell supernatants using two ELISA kits R&D Systems (Minneapolis, MI, USA) that have a different sensitivity. We measured the IL-1β in 4 separate experimental conditions: (1) We kept the PBMCs in culture in absence of stimulation (control); (2) Stimulation with LPS (1μg/ml for 2 hours), inducer of transcription and translation of pro-IL-1β; (3) stimulation with LPS (1μg/ml for 2 hours) and incubation then with 30 µM BzATP, a selective agonist of the P2X7 receptor; (4) Stimulation with LPS and subsequent incubation with 100 µM BzATP.
Real-Time PCR
We extracted the RNA from PBMCs using Trizol (Invitrogen, Carlsbad, CA, USA) and analyzed through Real Time-PCR using the StepOne thermocycler (Apllied Biosystems, Monza, Italy). We used the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an endogenous control as expressed constitutively. We selected the primers and probes for GAPDH, P2X7, ASC and NALP3 from the Applied Biosystems Custom TaqMan Gene Expression Assay. We performed the relative quantification (RQ) of mRNA content of the studied genes using the ΔCT method. We normalized each reaction of Real Time-PCR to an endogenous control formed by the mRNA of a monocytic cell line, THP1, which we attributed the relative value QR=1.
Data analysis
We compared the RQ mRNA of ASC, NALP3, and the production of IL-1β of healthy controls with the ones of patients afflicted with CD. Then we carried out a comparative analysis of transcripts and IL-1β stratifying patients.
Results
We enrolled 62 patients afflicted with CD (36 females); mean aged 53.7 ± 12.5 years. The control group was formed by 58 subjects (38 females) healthy volunteers that are 46 ± 11.5 years mean age. In the control group there were no patients with inflammatory and/or immune disease and there wasn’t recently exposure to non-steroidal anti-inflammatory or immunosuppressive agents that would otherwise make the donor not eligible.
The clinical features of CD patients enrolled in the study are shown in Table 1.
| Total patients with CD (62) | ||
| Sex | Female (n(%)) | 36 (58.1%) |
| Male (n(%)) | 26 (41.9%) | |
| Age | ≤ 50 years (n(%)) | 20 (32.2%) |
| > 50 years (n(%)) | 42 (67.7%) | |
| Years of disease | ≤ 5 years (n(%)) | 16 (25.8%) |
| > 5 years (n(%)) | 46 (74.2%) | |
| Localization disease | Ileal (n(%)) | 19 (30.6%) |
| Ileo-cecal (n(%)) | 18 (29%) | |
| Colic (n(%)) | 16 (25.8%) | |
| Rectal (n(%)) | 9 (14.5%) | |
| Disease activity | Remission (n(%)) | 33 (53.2%) |
| Mild (n(%)) | 13 (21%) | |
| Moderate-severe (n(%)) | 16 (25.8%) | |
| Therapy | Mesalazine (n(%)) | 20 (32.3%) |
| Azathioprine (n(%)) | 16 (25.8%) | |
| Corticosteroid (n(%)) | 14 (22.6%) | |
| Anti-TNF-α (n(%)) | 10 (16.1%) | |
| None | 2 (3.2%) | |
| Previous intestinal surgery (n(%)) | 32 (51.6%) | |
| Smoking status (n(%)) | 24 (38.7%) | |
| Presence of autoimmune associated disorders (n(%)) |
19 (30.6%) | |
| Familiarity for IBD (n(%)) | 6 (9.7%) | |
| PCR pathologic (n(%)) | 16 (25.8%) | |
Table 1: Descriptive analysis of the population of patients with analyzed CD.
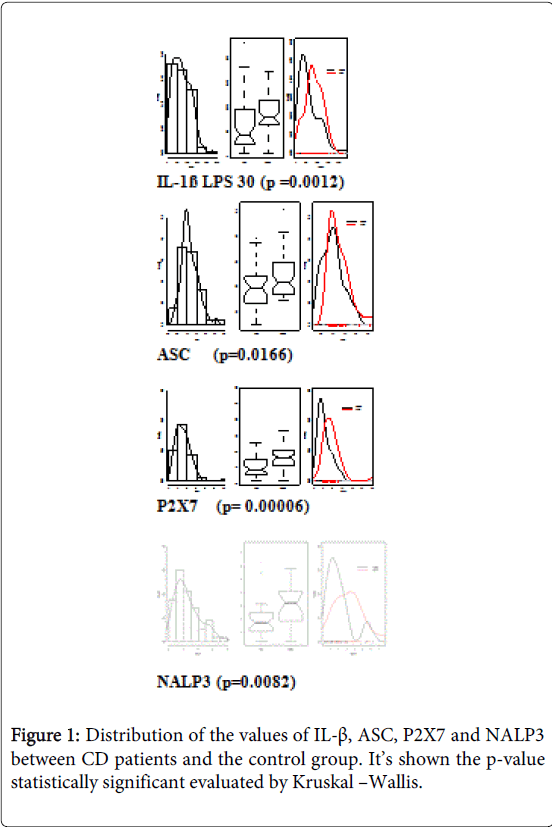
In the comparison of the values of IL-1β, inflammasome’s protein (ASC and NALP3) and the P2X7 receptor in healthy subjects and patients with CD we highlighted significant differences in the various parameters, we found higher values in patients with CD, expression of a different inflammasomic activation in the group of patients with CD (Figure 1). For values of IL-1β we compared the expression of the cytokine in baseline conditions, after a stimulus with a part of LPS, after a second stimulation with LPS and subsequent incubation with BzATP 30 µM, selective agonist of the P2X7 receptor and stimulation with LPS and subsequent incubation with BzATP 100 µM. Then we compared patients with CD and control population for each parameter.
Figure 1: Distribution of the values of IL-β, ASC, P2X7 and NALP3 between CD patients and the control group. It’s shown the p-value statistically significant evaluated by Kruskal –Wallis. Citation: Angelo Z, Riccardo B, Simonetta F, Incà Renata D, Carlo SG, et al. (2015) Inflammasome Activation P2X7-Dependent in Crohn’s Disease. J Gastrointest Dig Syst 5: 316. doi:10.4172/2161-069X.1000316 Page 4 of 7 J Gastrointest
In the evaluation of the various parameters of inflammation within the group of patients with CD we highlighted that the patients showed significant differences in where the disease has been localized, smoking status, PCR values, immunosuppressive therapy; in the case of patients receiving immunosuppressive therapy we found significant differences compared to both patients that are not receiving immunosuppressive therapy and in comparison of them with the healthy subjects (Table 2).
| p-value | |
| Localization of the disease | |
| IL-1β basal | 0.02795 |
| Smoking status | |
| ASC | 0.0386 |
| PCR values | |
| P2X7 | 0.0231 |
| Immunosuppressive therapy vs. not-immunosuppressive | |
| IL-1β BASAL | 0.0369 |
| IL-1β LPS30 | 0.0099 |
| IL-1β LPS100 | 0.0377 |
| P2X7 | 0.0073 |
| Not immunosuppressive therapy vs. healthy controls | |
| IL-1β BASAL | 0.00986 |
| ASC | 0.04344 |
| P2X7 | 0.00005 |
| Immunosuppressive therapy vs. healthy controls | |
| IL1β(LPS30) | 0,0002 |
| P2X7 | 0,002 |
Table 2: Evaluation of the different indices of inflammation in the group of patients with CD in relation to the location of disease, smoking status, CRP values, immunosuppressive therapy. Cytokines are reported with statistically significant differences (p<0.05).
Multivariate analysis showed that the levels of IL-1β - LPS30 are statistically dependent upon the localization of the disease in the ileum and upon not taking immunosuppressive therapy; the levels of ASC are dependent only upon the presence of smoking status, while those of P2X7, are dependent upon the plasma levels of PCR (Table 3).
| p-value | ||
| IL-1β(LPS30) | Ileal localization | 0.03127 |
| Not immunosuppressive therapy | 0.00351 | |
| ASC | Smoke | 0.0297 |
| P2X7 | PCR | 0.0225 |
Table 3: Parameters statistically significant results in independently influencing the different values of inflammatory cytokines in patients with CD
Discussion
The discovery of the inflammasome has changed the concept of innate immunity and permitted to reconsider the pathogenic processes of many inflammatory diseases. The P2X7-mediated production of IL-1β in immune cells is regulated by inflammatory stimuli that induce the transcription of the gene and production of the immature form of the cytokine. The subsequent activation of the P2X7 receptor by extracellular ATP facilitates the inflammasome’s activation and the production of mature IL-1β. The role of innate immunity in the pathogenesis of autoimmune and inflammatory diseases is not yet sufficiently clear and seems placed temporarily at an early stage of the process performing a function of priming required for the activation of the acquired immune response.
Our study highlights the correlation between the expression of P2X7 and ASC and confirms what already exists in the literature [22]: the P2X7 receptor (ATP dependent) is the most powerful stimulus for the activation of the inflammasome [23]. It isn’t possible to affirm their mutual dependency, but a certain importance in the inflammasomic process. On the other hand, the secretion of IL-1β has different ways that lead to the secretion and activation of the cytokine: we highlighted the presence of at least 3 inflammasomes involved in the production of IL-1 and then makes NALP3 only responsible of a part of the production of IL-1 [24]. ASC and NALP don’t have, in our analysis, significant correlation: this data, even if it’s difficult to interpret, given the small sample size, had been found in previous studies [25]. The expression of IL-1β does not present significant differences in the baseline condition measurement, because as already reported in patients with Crohn's disease, IL-1β isn’t constitutively increased [26]. Similarly, after partial stimulation with LPS there isn’t a significantly higher release of IL-1β: this data could be justified by the existence of two phases of activation, which priming with LPS is only the first step. It’s possible, although in view of the small numbers, it is an orientation, which in Crohn's disease is not altered the entire process of activation, but there is only a partial alteration of the cascade of the inflammasome. In this sense, the maturation phase of the IL-1β-NFkB dependent maintains its normal function .
As regards the data of IL-1β after the stimulation with LPS100, the curves trend of both cohorts suggests the presence of an exhaustion of the overall capacity of the conversion of IL-1β from or in absence of the precursor (pro-IL1β) or "rate limiting step" with the unavailability for exceeding the speed saturation. When the stimulation of the lymphocytes occurs with LPS and 30BZB clearly shows the increased expression of IL-1β in subjects with Crohn's disease. This data allows us to suppose that this cytokine, even after the recent evidence of his involvement in the translocation from Th1 to Th17, may be responsible for the pathogenesis of Crohn. The focus in the therapeutic environment exclusively to TNF, it would at least shared with IL-1β; as we did for other diseases (rheumatoid arthritis, auto-inflammatory diseases) it would be interesting to develop clinical trials for the use of anti-IL-1 medicines, which it is already validated for other indications (Anakinra, Ucituzimab etc.). In analogy with previous experimental studies, in patients with Crohn's disease, There was confirmation of increased activation of P2X7 as a driver of greater importance to the waterfall of the inflammasome. If this information would be confirmed in large records and with studies on the intestinal mucosa, P2X7 might show the mechanisms for the progression of the disease, making it more similar to autoinflammatory diseases, as well as to confirm the decisive role and not so well known of the inflammasome in the maintenance of inflammation in Crohn's disease.
In P2X7 is the same thing as we already expressed for IL-1β, that there are already at least three medicines tested in vitro, inhibiting this receptor that perhaps could be assumed for a clinical trial on Crohn. The significant data regarding ASC and NALP3 clearly confirm (despite in presence of the selection bias and small numbers) that the two main components of the structure of the inflammasome are activated and set the beginning of the disease process or the maintenance of itself. These data are similar in autoinflammatory diseases and autoimmune diseases (rheumatoid arthritis), in which, however, are less convincing. In our study, although the sample was not particularly large, there was some indications on the influence of the phenotypic expression of proteins of the inflammasome. The ileal localization if considered as a single variable or IL-1β and ASC multivariable (after LPS30 stimulus) presents significantly higher values: this seems to confirm other data in the NOD2 literature, that is the ileal disease presents a different cytokine and inflammatory pattern than other phenotypic presentations. It is not surprising that there is a role of increased expression of IL-1β in presence of associations with autoimmune diseases that may have a concomitant role in modifying the activation of the inflammasome. The recognition that cigarette smoke can mainly activate the inflammasome (in our analysis ASC in particular) could confirm the data reported in the literature: in particular, according to other authors would lead through the way of tools like receptor to activate ASC and NALP3 and then get a increased production of IL-1β [27].
In the comparison of different therapies, patients taking immunosuppressants have shown differences in all proteins expression involved in the inflammasome when compared with patients treated with mesalamine. In the comparison of pairwise therapies, the biggest difference was among patients taking only mesalazine compared to those who were taking azathioprine. On the other hand, this data appears justified in light of the fact that the therapy with anti-TNFα has a restricted target with minimal involvement of other cytokines even if there are data concerning the increasing of the production of IL-1 and IL-18 correlated to TNF in the psoriatic disease. The azathioprine therapy, on the contrary, reducing the whole lymphocyte activity is also directly involved in the reduction of cytokines related to them (IL-1). The disease activity (Harvey Bradshaw index) has not significantly altered the expression of any protein of the inflammasome: this data suggests that the difference compared to healthy ones cannot be correlated with the clinical activity but rather with the presence of the disease itself. The multivariate analysis of P2X7 confirmed that the values of C-reactive protein (CRP) increased may affect the increase of expression of this receptor independently of other variables. These are small numbers but it is definitely a confirmation that the systemic inflammatory response correlates with the activity of the inflammasome.
Understanding the importance of P2X7 also for Crohn's disease makes it important to start clinical trials with receptor antagonists medicines, already tested on rheumatoid arthritis [28].
The increased secretion of effector cytokines of the inflammasome IL-1β and IL-18 associated with intestinal inflammation and the risk of producing IBD is described for many years now. However, the regulatory role of NALP3 in intestinal inflammation still has controversial aspects. There are models in which the inflammasome is hyper-activated that hypo-enabled may lead to an alteration in the intestinal homeostasis: in the epithelium the inflammasome is essential for the regulation of the permeability and for the epithelial regeneration, however, the excessive activation of the inflammasome contributes to the intestinal inflammation in the lamina itself.
References
- Balfour Sartor R, Sandborn WJ (2005) Etiology and pathogenesis in Kirsner’s Inflammatory Bowel Diseases Saunders Edition Sixth Edition.
- Balfour Sartor R, Sandborn WJ (2005) Etiology and pathogenesis in Kirsner’s Inflammatory Bowel Diseases Saunders Edition Seventh Edition.
- Dinarello CA (2010) Anti-inflammatory Agents: Present and Future.Cell 140: 935-950.
- McDermott MF, Aksentijevich I (2002) The autoinflammatory syndromes.Curr Opin Allergy Clin Immunol 2: 511-516.
- McDermott MF, Aksentijevich I (2003) The autoinflammatory syndromes.Curr Opin Allergy Clin Immunol 3: 511.
- Haas SL, Ruether A, Singer MV, Schreiber S, Böcker U (2007) Functional P2X7 receptor polymorphisms (His155Tyr, Arg307Gln, Glu496Ala) in patients with Crohn's disease.Scand J Immunol 65: 166-170.
- Zaki MH, Lamkanfi M, Kanneganti TD (2011) The Nlrp3 inflammasome: contributions to intestinal homeostasis.Trends Immunol 32: 171-179.
- Slavova N, Drescher A, Visekruna A, Dullat S, Kroesen AJ, et al. (2010) NALP expression in Paneth cells provides a novel track in IBD signaling.Langenbecks Arch Surg 395: 351-357.
- Bodin P, Burnstock G (2001) Purinergic signalling: ATP release.Neurochem Res 26: 959-969.
- CriÅŸan TO, Plantinga TS, van de Veerdonk FL, FarcaÅŸ MF, Stoffels M, et al. (2011) Inflammasome-independent modulation of cytokine response by autophagy in human cells.PLoS One 6: e18666.
- CriÅŸan TO, Plantinga TS, van de Veerdonk FL, FarcaÅŸ MF, Stoffels M, et al. (2011) Inflammasome-independent modulation of cytokine response by autophagy in human cells.PLoS One 6: e18666.
- Martinon F, Agostini L, Meylan E, Tschopp J (2004) Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome.Curr Biol 14: 1929-1934.
- Xing L, Schwarz EM, Boyce BF (2005) Osteoclast precursors, RANKL/RANK, and immunology.Immunol Rev 208: 19-29.
- Lechtenberg BC, Mace PD, Ried SJ (2014) Structural mechanisms in NLR inflammasome signaling.Curr Opin Struct Biol 29: 17-25.
- Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, et al. (2008) The NALP3 inflammasome is involved in the innate immune response to amyloid-beta.Nat Immunol 9: 857-865.
- Martinon F, Gaide O, Pétrilli V, Mayor A, Tschopp J (2007) NALP inflammasomes: a central role in innate immunity.Semin Immunopathol 29: 213-229.
- McCulloch CA, Downey GP, El-Gabalawy H (2006) Signalling platforms that modulate the inflammatory response: new targets for drug development.Nat Rev Drug Discov 5: 864-876.
- Stack JH, Beaumont K, Larsen PD, Straley KS, Henkel GW, et al. (2005) IL-converting enzyme/caspase-1 inhibitor VX-765 blocks the hypersensitive response to an inflammatory stimulus in monocyte from familial cold autoinflammatory syndrome patients. J Immunol 175:2630-2634.
- Mitroulis I, Skendros P, Ritis K (2010) Targeting IL-1beta in disease; the expanding role of NLRP3 inflammasome.Eur J Intern Med 21: 157-163.
- Romagnoli R, Baraldi PG, Cruz-Lopez O, Lopez-Cara C, Preti D, et al. (2008) The P2X7 receptor as a therapeutic target.Expert Opin Ther Targets 12: 647-661.
- Magro F, Langner C, Driessen A, Ensari A, Geboes K, et al. (2013) European Society of Pathology (ESP); European Crohn's and Colitis Organisation (ECCO). European consensus on the histopathology of inflammatory bowel disease. J Crohns Colitis 7:827-825.
- Riteau N, Gasse P, Fauconnier L, Gombault A, Couegnat M, et al. (2010) Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis.Am J Respir Crit Care Med 182: 774-783.
- Qu Y, Franchi L, Nunez G, Dubyak GR (2007) Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages.J Immunol 179: 1913-1925.
- González-Navajas JM, Lee J, David M, Raz E (2012) Immunomodulatory functions of type I interferons.Nat Rev Immunol 12: 125-135.
- Ozkurede VU, Franchi L (2012) Immunology in clinic review series; focus on autoinflammatory diseases: role of inflammasomes in autoinflammatory syndromes.Clin Exp Immunol 167: 382-390.
- Goldbach-Mansky R (2012) Immunology in clinic review series; focus on autoinflammatory diseases: update on monogenic autoinflammatory diseases: the role of interleukin (IL)-1 and an emerging role for cytokines beyond IL-1.Clin Exp Immunol 167: 391-404.
- Mortaz E, Henricks PA, Kraneveld AD, Givi ME, Garssen J, et al. (2011) Cigarette smoke induces the release of CXCL-8 from human bronchial epithelial cells via TLRs and induction of the inflammasome.Biochim Biophys Acta 1812: 1104-1110.
- Stock TC, Bloom BJ, Wei N, Ishaq S, Park W, et al. (2012) Efficacy and safety of CE-224,535, an antagonist of P2X7 receptor, in treatment of patients with rheumatoid arthritis inadequately controlled by methotrexate.J Rheumatol 39: 720-727.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 14056
- [From(publication date):
August-2015 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 9666
- PDF downloads : 4390