Infective Germination: Granulomatous Inflammation: Lymph Node
Received: 20-Aug-2018 / Accepted Date: 17-Sep-2018 / Published Date: 24-Sep-2018
Abstract
A focal collection of inflammatory cells, chiefly histiocytes, macrophages, activated macrophages or epithelioid cells with giant cells (foreign body or langhans) and small lymphocytes at the site of a tissue infection may be designated as a “granuloma”. The non-infectious granulomas usually comprise of lymphadenitis secondary to conditions such as berylliosis, Hodgkin’s lymphoma, non-Hodgkin’s lymphoma, lymph nodes draining cancer or Crohn’s disease, sarcoid like reaction and sarcoidosis. Infective granulomatous lymphadenitis may be subdivided into “Suppurative lymphadenitis” which may be consequent to tularemia, cat scratch disease, yersinia enterocolitica and lympho-granuloma venereum. The specified aggregates may display a central necrosis or a centric abscess generally caused by the gram negative bacteria and or the chlamydial micro-organisms. Lymph nodes implicated in tularaemia and cat scratch disease usually incite the axillary and cervical lymph nodes. Yersinia micro-organisms may affect the mesenteric lymph nodes and lympho-granuloma venereum incriminates the inguinal lymph nodes.
Keywords: Granuloma; Lymph nodes; Infections; Microbiology
Introduction
A focal collection of inflammatory cells, chiefly histiocytes, macrophages, activated macrophages or epithelioid cells with giant cells (foreign body or langhans) and small lymphocytes at the site of a tissue infection may be designated as a “granuloma”. Granulomas may be generated with the amplification and ingress of monocytic myeloid precursors of the bone marrow into the peripheral circulation. Monocytes in the tissues may be designated as histiocytes. Stimulation of the histiocytes through the inherent immunity may elucidate the classic “epitheloid” countenance, contrary to the un-stimulated bean shaped histiocytes with well-defined cellular boundaries [1,2]. The dendritic (antigen presenting) cells along with the major histocompatibility complex II may configure an inherent and helper T cells (Th1) predominant immune adaptation, as the foreign particles may be incompetently ingested by the histiocytes. The molecules of the complement cascade C3a, C5a, Th1 along with the cytokines and chemokines (tumour necrosis factor-α, inteleukins 1, 6 and 17 with interferon gamma) may physiologically impel the histiocytes in order to engage and mobilize the macrophages to the location of injury within 24 to 48 h. The chronic inflammatory reaction and the subsequent tissue regeneration may be governed by the stimulated histiocytes. Histiocytes of two classes may appear; (a) One class may augment phagocytosis and stabilize the inflammation, (b) Other kind of histiocytes contribute in fibrosis, angiogenesis and reconstruction of the wound [1,2]. Granulomas may preponderantly be of two categories; (i) Foreign body granulomas displaying a histiocytic response to inanimate substances such as suture, talc or food matter, in the absence of an adaptive immune response. The foreign material may be discerned with the application of a light microscope along with a birefringence with polarized light, (ii) The immune granulomas may depict variable causative factors with a well demarcated morphology. The granulomas may be necrotizing with an acentric caseous, cheese like necrosis accompanied by a lymphohistiocytic palisade with a chronic inflammatory margin. The histology and immune histochemistry may delineate concrete causative organisms (such as the typical fungus-Coccidioides spp ). The non necrotising (naked granulomas) may be distinguished by focal aggregates of epitheloid histiocytes and giant cells accompanied by miniscule, marginal chronic inflammatory exudates (as depicted in sarcoid granulomas) [1,2].
Categorization of granulomas: Granulomatous inflammation of the lymph node may be codified into 2 major categories (Table 1).
| Non-infectious granulomatous disorders | Infectious granulomatous disorders | |
|---|---|---|
| Suppurative conditions | Non suppurative conditions | |
| Sarcoid lymphadenitis | Tularaemia | Tuberculosis |
| Sarcoid-like lymphadenitis | Cat scratch disease | Atypical mycobacterial infection |
| Berylliosis | Yersinia enterocolitica | BCG lymphadenitis |
| - | Lympho-granuloma venereum | Toxoplasma gondii infection (Piringer Kuchinka lymphadenitis) |
| - | Fungal granulomas | Leprosy (Mycobacterium leprae) |
| - | - | Syphilis |
| - | - | Brucellosis |
| - | - | Fungal infections (cryptococcosis, histoplasmosis, coccidiomycosis pneumocystis) |
Table 1: Types of granulomatous inflammation of the lymph node.
Analysis and Attributes
The non-infectious granulomas usually comprise of lymphadenitis secondary to conditions such as berylliosis, Hodgkin’s lymphoma, non- Hodgkin’s lymphoma, lymph nodes draining cancer or Crohn’s disease, sarcoid like reaction and sarcoidosis. Abscesses and central necrosis occurring within the granulomas is exceptional [1,2]. Infective granulomatous lymphadenitis may be subdivided into “Suppurative lymphadenitis” which may be consequent to tularemia, cat scratch disease, Yersinia enterocolitica and lympho-granuloma venereum. The specified aggregates may display a central necrosis or a centric abscess generally caused by the gram negative bacteria and or the chlamydial micro-organisms. Lymph nodes implicated in tularaemia and cat scratch disease usually incite the axillary and cervical lymph nodes. Yersinia micro-organisms may affect the mesenteric lymph nodes and lympho-granuloma venereum incriminates the inguinal lymph nodes [2,3]. “Non suppurative lymphadenitis” comprises of tuberculosis and Bacille Calmette Guerin (BCG) lymphadenitis. The specific lesions may depict non-suppurative, hypersensitivity type granulomas secondary to infection by a mycobacterium. Tuberculous lymphadenitis arises primarily in the cervical lymph node. The histiocytes are a predominant component of the granuloma with a minor contribution from small T lymphocytes, dendritic reticulum cells and peripheral B cells. The micro-organisms may be discovered in the necrotic zone by the Zeihl Neelsen stain. Toxoplasmosis may be an adjunctive non-suppurative protozoan infection (Toxoplasma gondii ) [3,4].
Sarcoidosis
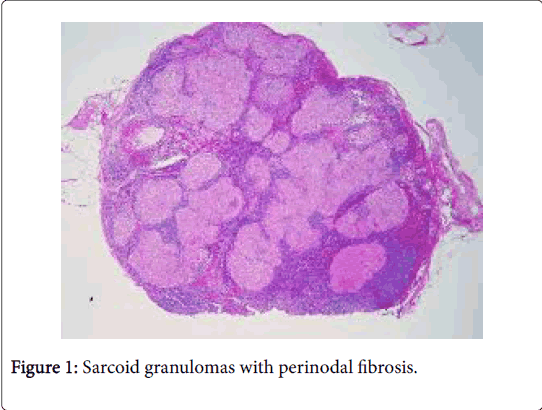
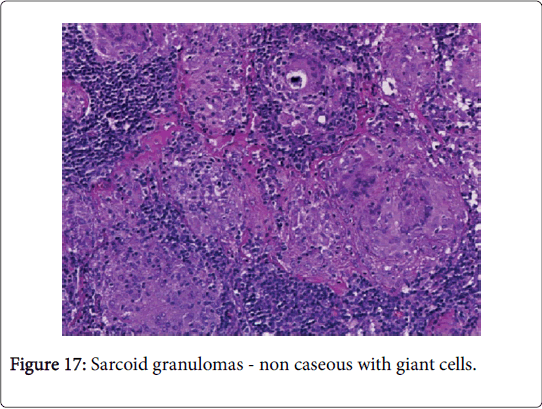
The latin description of sarcoidosis is “meaty chunk like lesion”. The aetiology of the disease is obscure. Sarcoidosis is a global disorder and no age or race is exempted. The severity of the disease may be exaggerated during winter and early spring [1]. The female to male ratio is 2:1 though the disorder is rare in children. The disease usually emerges between 20-40 years of age. The global annual incidence is reported at 8 cases/100000 individuals. Organs incriminated by the disorder may be the lungs, pulmonary hilar lymph nodes (80%), eyes (50%), skin (20%) and adjunctive lymph node groups. On a Polymerase Chain Reaction (PCR), mycobacterial DNA may be discerned in a majority (33-80%) of the sarcoid granulomas (Figure 1).
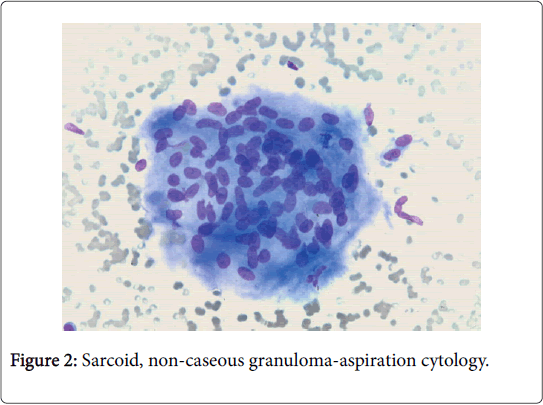
The clinical progression of the non-hereditary disorder is favorable. Multiple organs such as the lymph nodes of the pulmonary hilum, lung, eye, liver and skin may be implicated [2]. Variegated non caseous epithelioid cell granulomas may be enunciated in the lymph nodes. Complications such as blindness, respiratory distress and heart failure may supervene. Sarcoidosis may be concordant with mycobacterium tuberculosis, viral infections and autoimmune diseases [3,4] (Figure 2).
Clinical aspects: As the lungs, skin, eye, liver and heart are frequently implicated, the clinical symptoms may originate from the organ affected. Ocular involvement induces a blurred vision, photophobia and floaters in the eye. Nodules and plaques may emerge with dermatologic involvement, while respiratory lesions produce cough and respiratory discomfort. Cardiac involvement may frequently provoke arrhythmias. Sudden cardiac death may occur in 5%-10% instances. The pulmonary hilar lymph nodes (93.5%), cervical lymph nodes (12.2%), axillary lymph nodes (5.2%) and inguinal lymph nodes (3.3%) may be enlarged [1]. Co-existent functional hyperparathyroidism is invariable and erythema nodosum may precede or accompany the disorder. Bilateral lymph node enlargement, increased angiotensin converting enzyme, hypercalcemia, negative r tuberculin test, Bronchio-Alveolar Fluid (BAL) cytology, enhanced CD4+/CD8+ percentage and a positive Kveim test (an intra-dermal reaction to the inoculation of an extract of disease implicated human spleen) may be the conclusive investigations for sarcoidosis. Majority of instances (60%-85%) may depict a microscopic sarcoid like granuloma after 4-6 weeks of inoculation of the antigen. The sarcoid granulomas may be segregated from the epitheloid aggregates of tuberculosis, fungal infection, silicosis, berylliosis and Hodgkin’s lymphoma, on account of the typical well defined margin of the granulomas, lack of centric necrosis and with special stains such as Ziehl Neelsen (AFB) and silver impregnation techniques [2,4].
Histomorphology: The preliminary, involved lymph nodes depict follicular hyperplasia with sinus histiocytosis, resembling non-specific lymphadenitis. Subsequently, the histiocytes may decline and tiny epitheloid cell granulomas may emerge in the cortex. Distinctive granulomas constituted of epitheloid cells and multinucleated or langhans giant cells (few, tiny nuclei, as opposed to tubercular granulomas) may be dispersed within the lymph node. Occasionally, the granulomas may consolidate and the encompassing collagen fibers may induce fibrosis with hyalinization [2,3].
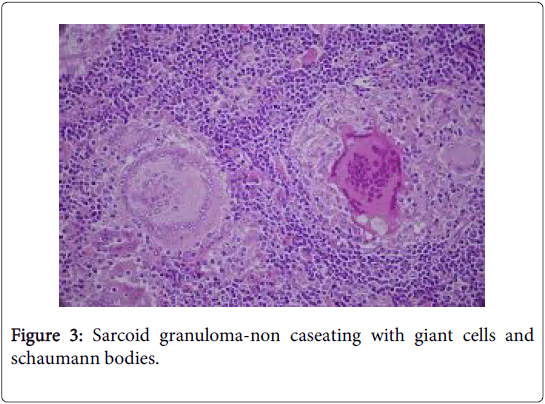
The granulomas usually lack neutrophils and loci of centric, tiny fibrinoid necrosis may be infrequent (hard granulomas). The CD4+/ CD8+ lymphocyte cell proportion (usually>3.5) may be amplified; thereby implying cell mediated immune hyperactivity. T lymphocytes, dendritic reticulum cells and macrophages are essential constituents of the granuloma [1]. T helper lymphocytes formulating the sarcoid granuloma along with the histiocytes may demonstrate immunereactive proliferation or activation of the Ki-67 antibody or interleukin 1 respectively (Figure 3).
A dysfunctional circulating T cell component is delineated with hyper-active B cells. A Human Leukocyte Antigen (HLA) linked immune response gene may be related to the disease susceptibility. An extra-nodal necrotizing variant may appear. Numerous inclusion bodies such as the Asteroid body (composed of calcium, silicon, phosphorus and aluminium), Schaumann body (round, concentric laminations of iron and calcium), calcium oxalate crystals or peculiar PAS positive inclusions, yellow or ovoid bodies with uncertain aetiology/pathogenesis and Hamazaki-Wesenberg bodies may be described in the cytoplasm of the giant cells [1,2].
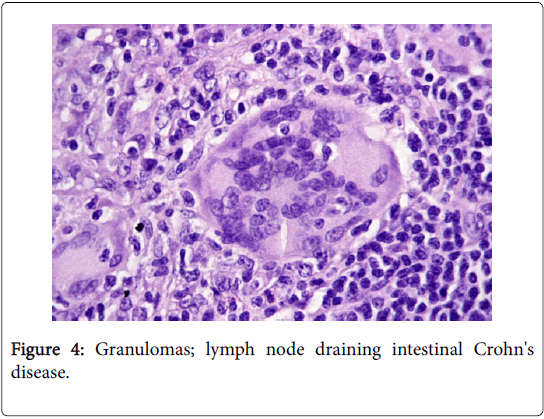
A non-caseating granuloma situated in the lymph node or skin, simulating the sarcoid granuloma on histology, may be described in tuberculosis, atypical mycobacteriosis (swimming pool granuloma), fungal infection, leprosy, syphilis, leishmanisis, brucellosis, tularaemia, chalazion, zirconium granuloma, berylliosis, Crohn’s disease, Hodgkin’s lymphoma and lymph nodes draining carcinoma [2,3] (Figure 4).
Sarcoid like reaction (Sarcoid like granuloma)
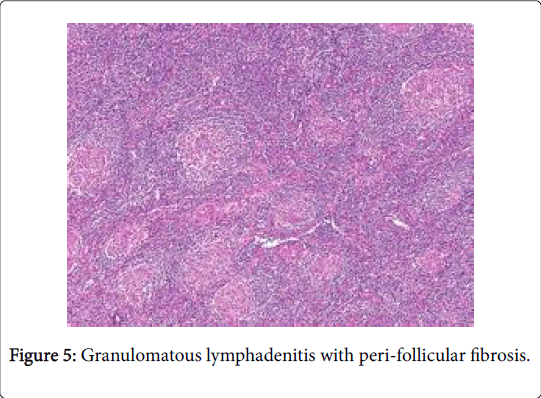
A sarcoid like reaction elucidates epitheloid granulomas identical to sarcoid nodules may be demonstrated in the regional lymph nodes with a concomitant disease. However, the granulomas may not imply the occurrence of systemic sarcoidosis [1] (Figures 5-7).
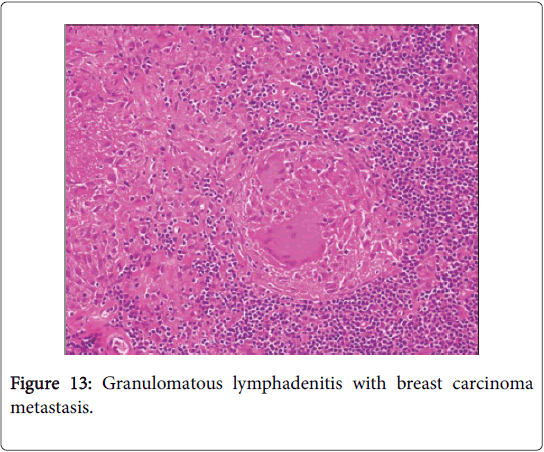
The co-existent disorders may be: carcinoma, toxoplasmosis, fungal infection, tuberculosis, atypical mycobacterial disease, pneumoconiosis, immune-compromised individuals (as in Crohn’s disease, primary biliary cirrhosis and Sjogren’s syndrome), extrinsic allergic inflammatory alveolitis (farmer’s lung) and anticancer chemotherapy besides chemical exposure (beryllium, zirconium, silicon, starch granules and pine pollen). Carcinoma of uterus, breast, lung and stomach may induce an identical response [1,2]. Cancer progression in combination with a sarcoid like reaction depicts a favorable outcome. An extensive, biological defense mechanism produced by continually stimulated lymph nodes to counter the metabolites and malignant disintegration, may initiate the reaction.
Clinical aspects: The concordant disease processes may dictate the nonspecific, constitutional symptoms. Lymph node enlargement of the pulmonary hilum may not be visible on chest x-ray. The tuberculin skin and Kveim test may be negative.
Histomorphology: Miniature, ill-defined granulomas comprising of a minimal epitheloid cell component along with small, mature lymphocyte may be disseminated in the lymph nodes. T lymphocytes, dendritic reticulum cells and macrophages may configure the granulomas. A CD4+/CD8+ percentage of 0.8 to 2.25 may be exhibited in the confluence of the granulomas [5]. The clinical picture and laboratory analysis may aid the distinction betwixt sarcoidosis and sarcoid like reaction.
Tularaemia (Ohara’s Disease)
A global gram negative bacterium cocco-bacillus, Franscisella tularensis generates an acute, infectious zoonosis labeled as tularaemia. The infection is devastating to rodents such as hare, mice, birds and dogs. The mode of transmission to humans may be variable such as a direct contact with an infected animal, consuming raw, uncooked meat of an infected rodent, bite of an infected arthropod, ingestion of contaminated water or food and the inhalation of infective aerosols [1].
The micro-organism may ingress through the mucous membrane or over the skin or by germination at the site of infection, may migrate to the lymph nodes via the lymphatic drainage and induce infection. A rise in the hemagglutinin titers may be elucidated. Person to person transference of the infection is unknown. An opportunistic or immune–compromised infection may appear with Franciscella species. Tularaemia along with small pox and anthrax has been classified as a “Category A” of most dangerous pathogens by the Centre of Disease Control (CDC) in 2001 [6].
Clinical aspects: The usual incubation period is 3-5 days and may extend from 1-21 days. The disease commences with high fever (38˚C-40˚C), chills, headache, generalized aches (particularly lumbar pain), sore throat, sweating, chills, malaise, exhaustion and gradual weight loss. Following bacterial dissemination pneumonia, septicaemia or meningitis may appear. Infections following ingestion of the bacteria may depict plague like symptoms. Tularaemia is clinically subcategorized as: surgical tularaemia, also referred to as the glandular, ulcero-glandular, oculo- glandular, oro-pharyngeal or the tonsillar tularaemia.
Glandular or ulcero-glandular sub-types are frequent. In the ulcero glandular disease, axillary lymph node enlargement predominates with mammalian vectors and the cervical or inguinal nodes may expand with the arthropod vectors [1]. The medical tularaemia is contingent to the initial route of entry of the pathogen and may be of the typhoid or latent types. Strains and species of the micro-organism may be discerned by culture, serum agglutination and skin tests or molecular biological techniques [6,7] .
Histomorphology: The fingers are commonly affected. The dermis is mildly oedematous with dilated capillaries and the lymphocytes may infiltrate the dermis. Numerous abscesses may appear at the site of antigen and genome inoculation. Multiple Monocytoid B cells (MBL) may abutt the abscess. The second week of infection displays dermal ulcers, with subsequent emergence of the dermal granuloma. F. tularensis antigen may be discovered in the skin from the 2nd to the 14th day. The centric necrotic zone of the granuloma may manifest the antigen, which may disappear after the configuration of the granuloma.
Lymph nodes in the right axilla and the right elbow are commonly affected with an exudation of yellow pus like material, accessible to culture and sensitivity. Histological sub-types of the nodal involvement may arise such as an abscess, abscess granuloma and granuloma. Numerous lymphoid follicles may appear up to the second week of infection with histiocytic cell aggregates in the sub-capsular sinus. The acute phase depicts an intense lymphadenitis with widespread necrosis and variable micro-abscesses with granulomas. Subsequently an abscess with mononuclear cells and central necrosis may configure (abscess form). Numerous Monocytoid B cells (MBLs) may be discerned abutting the abscess with primitive granulomas. Extending from the second week to the sixth week of the infection, innumerable miniature, cortical and para-cortical epitheloid granulomas with central necrosis may emerge [1,2,4].
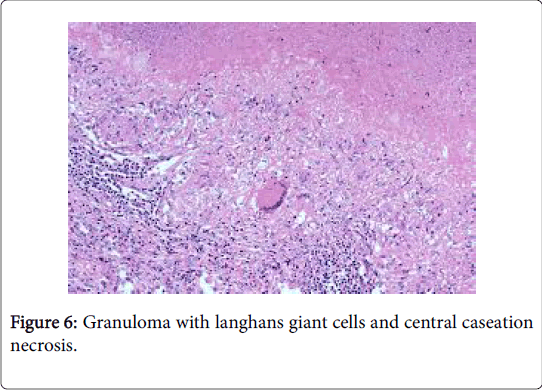
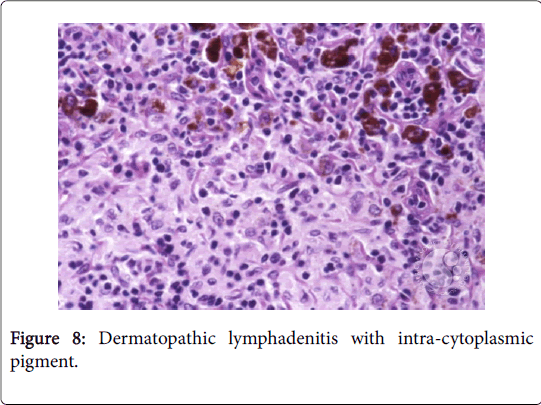
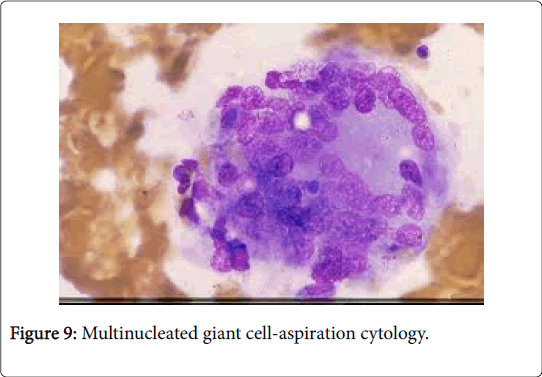
The granulomas then coalesce and conform enormous, variant lesions with a centric abscess (abscess-granuloma). A considerable multinucleated giant cell infiltrate may be discerned at the epitheloid cell perimeter. CD4+ lymphocytic cells may exceed the CD8+ lymphocytic cells, during the course of disease progression. B lymphocytes along with the CD 4+ lymphocytes may contribute to the configured granuloma. Following the 8th week of infection, the necrotic tissue converts into a homogenized caseous necrosis within the centric granuloma (granulomatous form). The preliminary skin lesions may be followed by the lymph node enlargement within one week .The microbial antigen may be discovered from the 7th day to the 92nd day within the lymph node. Following contact with an infected rodent, the contaminated skin of the finger inculcates the primary lesion into the regional lymph nodes, a condition designated as dermatopathic lymphadenitis [1,3] (Figures 8 and 9).
Cat scratch disease (Cat-scratch fever)
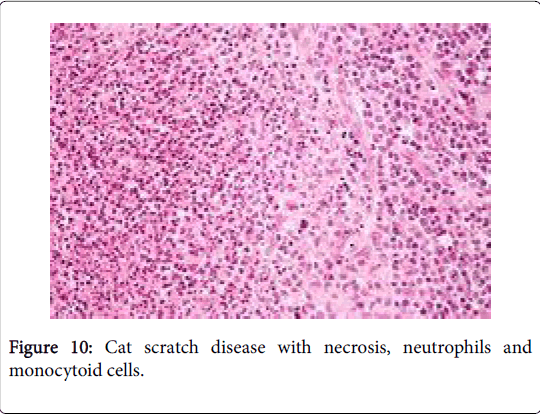
The fall to winter condition is an infectious disorder occurring due to a gram negative cocco-bacillary, pleomorphic, extracellular bacterium Bartonella henselae (B. henselae ) and /or B. Quintana . The site of inoculation is an invisible scratch or a bite by a wild male kitten. The primary cutaneous lesion appears with a regional (axillary or inguinal or cervical) suppurative lymph node enlargement. The lymph nodes may distend up to 3 cm in magnitude. Children and teens are usually affected, with an equivalent male to female proportion (M: F: 1:1). The annual prevalence of the disorder is the 9.3/100000 patients [1,2] (Figure 10).
Clinical aspects: A pustular or crusted red papule at the site of the skin inoculation arises between 7 to 12 days following contact. Histology of the skin displays dermal foci of necrosis, an encompassing mantle of histiocytes, multinucleated giant cells, lymphocytes and eosinophils. Erythema (20%-50%), vesicular blisters and abscesses may appear at the locus of the cat scratch/bite within 3-10 days. The upper extremities are commonly involved followed by the cervical and facial regions. A painful lymph node enlargement may ensue within one to three weeks usually in the cervical (33%), axillary (27%) or inguinal regions (18%). Systemic signs such as fever, malaise, headache and lymph node swelling may develop. Infrequent complications such as Parinaud’s syndrome (peri-auricular lymphadenopathy & granulomatous conjunctivitis, encephalitis, endocarditis, hepatitis and thrombocytopenic purpura) may be delineated. Kaposi sarcoma-like granuloma (epitheloid angiomatosis) may arise on the skin of the immune-compromised (AIDS) individuals [1,2]. The disease may be clinically suspected with: a) A history of contact with cats (exceptionally dogs), b) Examination of the site of abrasion, c) regional lymph node enlargement, d) discriminative histology of the lymph nodes, e) specific skin testing for cat –scratch disease. Immunehistochemical correlation and Polymerase Chain Reaction (PCR) may be advocated for the diagnosis of the disorder [3,4].
Histomorphology: The lymph nodes may described as: a) The early phase (nonspecific reactivity): Reactive follicular hyperplasia, histiocytic proliferation and expansion of lymphoid follicles may appear, the efferent lymphatic vessels, sub-capsular sinus and paracortical zone initially abound with immunoblasts, histiocytic cells , macrophages, phagocytosed bodies, neutrophils and plasma cells. b) The intermediate phase (micro-abscess formulation): Micro-abscesses with a centric necrosis, clustered neutrophils and a lack of epitheloid granuloma may emerge within the sub-capsular sinus. The lesion gradually extends from the cortex to the medulla. Monocytoid B cell (MBL) clusters may about the micro-abscess. The centric fibrinoid necrosis is comprised of neutrophilic aggregates and progressive suppuration [1,2].
The epitheloid cell granulomas may be configured by the enveloping macrophages with infrequent multinucleated or Langhans giant cells (stellate micro-abscess). The warthin starry silver stain may demonstrate tiny, curved gram negative bacilli congregated within an extensive zone of necrosis. The preliminary lesions within 15 days of disease inception may be teeming with bacteria. Immune histochemical staining of formalin fixed paraffin embedded sections with antibodies to B. henselae may be beneficial in determining the bacillus. A Polymerase Chain Reaction (PCR) may be employed to isolate the organism from the nodal histological sections or the aspirated pus. The diagnosis may also be confirmed by serology. c) The secondary phase may be described by a granulomatous abscess with a palisading of histiocytes where integration of the stellate microabscesses of varying magnitude produces an irregular, giant abscess (geographic abscess). The lymph node capsule may be fibrotic or considerably inflamed. The incidence of bacterial isolation may decline in the secondary phase [1,2].
Yersinia (Mesenteric) lymphadenitis
Yersinia enterocolitica and Yersinia pseudo-tuberculosis are miniature, gram negative, polymorphic coccoid or ovoid motile bacilli and conspicuous human pathogens. The genus yersinia may not be isolated from the normal human gastro-intestinal tract. The acute lymphadenitis engendered by Yersinia spp may appear as an acute febrile gastroenteritis with symptoms analogous to acute appendicitis. The disorder preponderantly appears in infants, children and young adults. Oral contamination ensues following consumption of infected meat, drinking water or adjunctive food-stuffs [1,3]. Following ingestion of the infected substances, the bacterial ingress may entrench into the mesenteric lymph nodes, by way of the lymphatic vessels of the intestine. The typically configured inflammatory lesion displays an expanded lymph node with induction of abdominal pain. Aside from the inflammation of mesenteric lymph nodes, acute appendicitis or terminal ileitis may be elucidated. Constitutional or specific symptoms may be negligible [2,4].
Clinical aspects: The infection produces a benign, self-limited disease that may clinically simulate acute appendicitis. The disease may present following an incubation of 24-48 h after ingestion of the bacterium, frequently with diarrhea (80%), right lower quadrant pain (50%), nausea, vomiting and fever (38˚C-39˚C) with flu like symptoms [1].
Infantile diarrhea, terminal ileitis, appendicitis and mesenteric lymphadenitis may appear in children and young adults. The instances may be misinterpreted as acute appendicitis, mesenteric lymphadenitis or terminal ileitis. Co-existent disorders such as arthritis, erythema nodosum, sepsis, Reiter’s syndrome, meningitis and cutaneous lesions may ensue in the adults [2,3]. Pseudo-infection with yersinia may arise in infants and is identical to the infection with Yersinia enterocolitica . Culture and sensitivity of the stool and vomit, histological analysis of the appendix and extracted lymph nodes with a serum antibody titer may be advocated. The micro-organism may be identified by Polymerase Chain Reaction (PCR). Therapeutic intervention may not be mandatory, however administration of antibiotics is beneficial [2,4].
Histomorphology: The identifiable, well demarcated lymph nodes of the ileum and cecum with elastic, soft consistency, a lack of distinctive necrosis and a magnitude of 1.5 cm may be apparent. a) The palpable lymph nodes of a preliminary Yersinia enterocolitica display a nonspecific reaction with compact aggregates of lymphocytes, immunoblasts and plasma cells in the dilated lymphatic sinuses. Cortical and para-cortical follicular hyperplasia may be evident with accumulation of large lymphocytes, elevated immunoblasts and plasma cells. b) The progressive or delayed phase enunciates a thickened, oedematous lymphoid capsule with an identical cellular exudate comprised of lymphocytes, immunoblasts and plasma cells, occupying the distended lymphatic sinuses [1,2].
Non-suppurative epitheloid cell granulomas may appear in the germinal centres. Suppuration of the centric epitheloid cell granulomas (central micro-abscesses) may ensue. The lesions may expand gradually to create spheroid micro-abscesses. A giant cell reaction may be absent. Epitheloid cell granulomas may be composed of epitheloid histiocytes along with dispersed, miniature lymphocytes and plasmacytoid monocytes [8]. The specific epitheloid cell granulomas of yersinia induced lymphadenitis lack the Monocytoid B cell (MBLs) effusion, in contrast to the granulomas accompanying the cat scratch disease or lympho-granuloma venereum infection. Gram negative acid fast diplo-bacilli may be recognized in the lesions. Lymphadenitis induced by Yersinia pseudo-tuberculosis exemplify an intense neutrophilic infiltrate and miniature granulomas with subsequent, disseminated micro-abscesses, centric suppuration and an envelope of plump histiocytes (suppurative granulomas), simulating the epitheloid aggregates of cat-scratch disease [1,2].
Infection with Yersinia enterocolitica may exceptionally depict granulomas identical to those induced by yersinia pseudo-tuberculosis. The appendiceal wall may be thickened with oedematous mucosa. Lesions of the appendix may display a trans-mural mixed inflammatory infiltrate along with multiple lymphoid follicles and epitheloid granulomas.
The preponderantly non-suppurative granulomas may be encompassed by T lymphocytes and plasmacytoid monocytes. As in the regional lymph node inflammation, the epitheloid granulomas may not incorporate Monocytoid B cells (MBLs) [8]. Occasionally, mild haemorrhage, catarrh and granulomas in the regional lymph nodes may be elucidated.
Tuberculous lymphadenitis
Tuberculosis is a chronic air borne infection engendered by Mycobacterium tuberculosis. The condition is reappearing globally, particularly in the developing countries.
Clinical aspects: The immensely virulent micro-organism may infect individuals with diminished immunity. With the primary infection, lymphadenitis may appear in the pulmonary, hilar and cervical lymph nodes. A frequent, clinically discernible lymphadenitis appears at the cervical region (scrofula), where a draining sinus may communicate with the skin (scrofuloderma).
Lymph nodes implicated by tuberculosis may be adherent and configure enormous multi-nodular masses which may be misinterpreted as metastatic carcinoma [9]. Lymphatic spread of M. tuberculosis may induce a tubercular meningitis and military tuberculosis. A definitive delineation of multifocal and adhesive tubercular lymphadenitis may be established by a lymph node biopsy, tuberculin reaction or a Polymerase Chain Reaction (PCR) [10].
Histomorphology: The organism M. tuberculosis proliferates within the alveolar macrophages, to configure a well characterized lesion in the lungs. The lesion is conventionally situated subjacent to the pleura of the upper lobes.
The broncho-pulmonary lymph nodes may be incriminated through the lymphatic vessels to articulate a classic “Ghon’s Complex” with lymphadenitis. Inhalation of the bacterium M. tuberculosis induces the primary infection which may terminate with a T cell mediated immune reaction that confers hypersensitivity against the organism. The inhaled micro-organism may be phagocytosed by the alveolar macrophages which may transfer the bacterium to the pulmonary hilar lymph nodes. In a specified period (few weeks), T cell mediated immunity may evolve by two methods–a) the configuration of epitheloid cell granulomas by the CD4+ lymphocytes and b) the emergence of the caseation rich granulomas by the CD8+ lymphocytes [1,2].
The microscopic picture varies from multiple, miniature epitheloid cell granulomas resembling sarcoid granulomas, to massive caseous necrotic aggregates enveloped by langhans giant cells, epitheloid cells and mature lymphocytes.
Epitheloid cell granulomas are generally encapsulated and proceed to a centric caseous necrosis which may ultimately heal. The lymphadenitis may be produced by the M tuberculosis infection and may comprise of the “Ghon’s complex” or a secondary organ tuberculosis [3,4].
Majority of the tubercular lymphadenitis (90%) emerge in the cervical and mediastinal lymph nodes. Non- specific lymphadenitis may occasionally be difficult to differentiate from the tubercular lymphadenitis, on account of the firm to tough, elastic consistency of the nodes. Inflammatory cells may ingress and gradually a periadenitis may emerge [11-13]. Following the articulation of abscesses, the lymph nodes expand and evolve into a soft, matted mass with caseous necrosis. The centric caseous necrosis encompassed by an epitheloid cell layer and an infrequent langerhans giant cell exudate is the typical histological elucidation of tubercular lymphadenitis. Lymphocytes and fibroblasts may be discerned at the perimeter of the scripted “tuberculosis nodule”, with an absence of plasma cells and constitutes the Type IV allergic response to the bacterium.
Tubercular lymphadenitis may be demarcated from sarcoid lymphadenitis by virtue of the presence of central caseous necrosis. Confirmation of the diagnosis necessitates demonstration of the organism by the special stains, bacillary cultures or a polymerase chain reaction (PCR) [1,3]. The centric, caseous coagulation necrosis may occasionally depict short rod shaped bacteria (mycobacterium tuberculosis) on Ziehl Neelsen stain. The polymerase chain reaction (PCR) may not always be capable of detecting the micro-organism. Calcification may accompany healing [9,10].
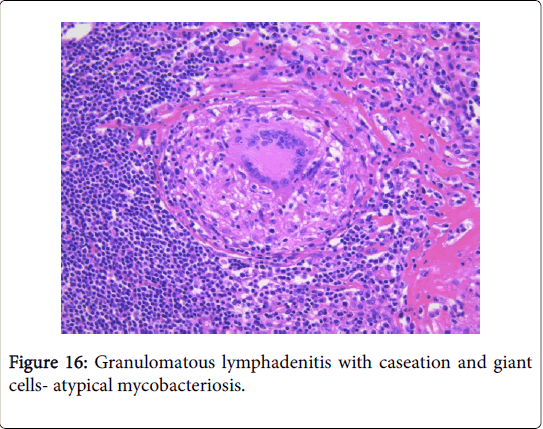
Atypical mycobacteriosis
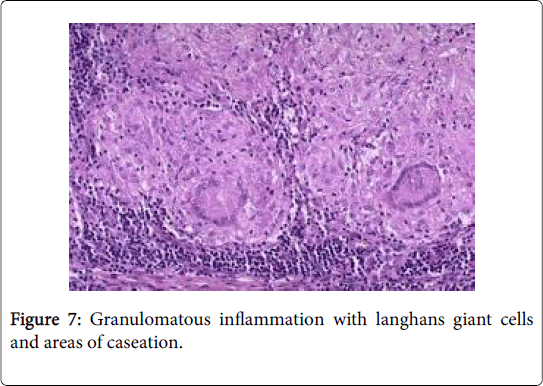
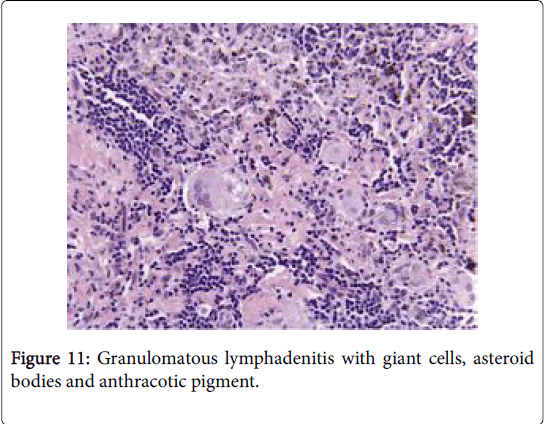
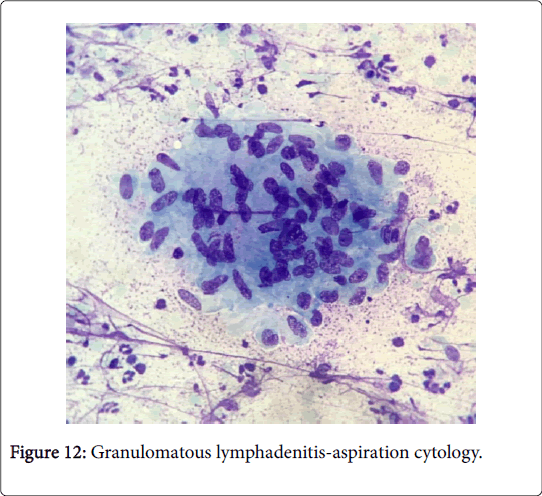
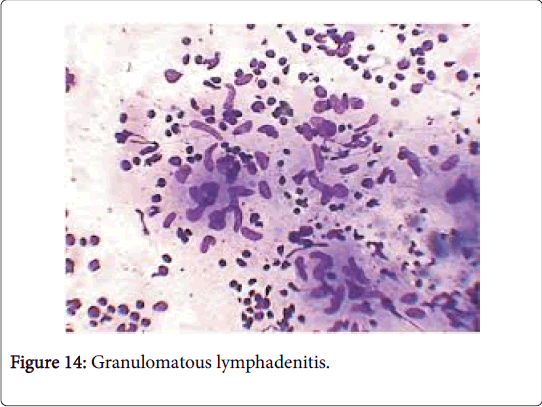
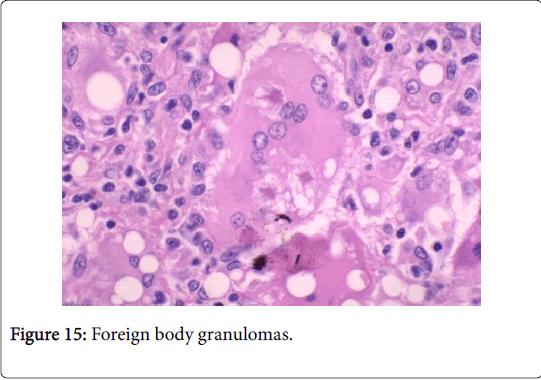
Frequently engenders of granulomatous lymphadenitis. Granulomatous disease with caseation in the cervical lymph node of a child, in the absence of pulmonary symptoms, is probably an atypical mycobacteriosis. Lymph nodes in the lateral mid -cervical zone may be implicated. The nodes may exhibit a purulent discharge in the absence of appurtenant therapy and healing may ensue with scarring and contractures [1] (Figures 11-15).
Microscopy: The host reaction may be similar to tuberculosis though extensive suppuration may obscure the granulomas. Ill defined (non-palisading), irregular or serpiginous granulomas may ensue. Each granulomatous and suppurative lymphadenitis particularly in a child or with an HIV infection mandates an evaluation with Ziehl Neelsen (AFB) stain. The diagnostic conclusion depends upon the cultural or the molecular attributes of the organism. Infection with a mycobacterium in patients with immune suppression may engender a florid spindle cell proliferation identical to that of a neoplasm (mycobacterial spindle cell pseudotumour) [1,3] (Figures 16 and 17).
BCG lymphadenitis (BCG histiocytosis)
The Bacille Calmette Guerin (BCG) vaccine is an effective immunestimulant which inhibits the dissemination of tubercular mycobacterium from the primary complex. The vaccine may be reinjected for tuberculin negative patients; however infants below 6 months of age require immunization only once in the absence the tuberculin tests. The vaccine (BCG) is a formulation of the weakest strain of Mycobacterium bovis with an attenuated virulence. Following vaccination, the bacillus migrates to the regional lymph node basin and disseminates to the systemic organs such as the lymph nodes, liver and spleen. The lesions may alleviate inevitably, in the absence of specific nodules [1,2]. Erythema and suppuration of the inoculation site may appear within ten days following the vaccination and signifies a tubercular infection. The lesions may recover in roughly three months [14-16].
Clinical aspects: A localized ulcer, configuration of an abscess and regional lymph node expansion may be probable complications to the vaccination. The frequently enlarged axillary and cervical lymph nodes may amplify up to 2-3 cm in diameter. The intra-dermal vaccines elucidate greater number of complications, rather than a percutaneous route of administration. The complication rate varies from 0.73%-22% in vaccinated children. Infants and youngsters may be prone to infections and lymphadenitis, which ensues within a week to nine months following vaccination [2,4]. Lymph node enlargement of three centimeter or more in magnitude, subsequent to a BCG vaccination, requires a surgical eradication. Lymph- nodes generally below the three centimeter diameter necessitate a follow-up. Conditions of congenital immune deficiency and patients with auto immune deficiency syndrome (AIDS & HIV) may depict a suppurative lymphadenitis with tuberculosis [17-20].
Histomorphology: The lymph node expansion is noticeably lesser than that of the tubercular lymphadenitis. Follicular hyperplasia with sinus histiocytosis may appear with non-specific lymph node enlargement in the preliminary phase. Subsequently, micro-nodules with epitheloid granulomas lacking necrosis or epitheloid granulomas with centric coagulation necrosis may be delineated. Langerhans giant cells are infrequent.
Toxoplasma Lymphadenitis
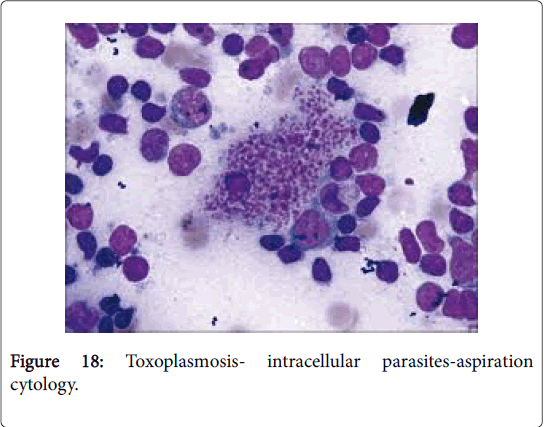
Toxoplasmosis, previously scripted as Piringer Kuchinka lymphadenitis, is a frequently occurring zoonosis of young women, secondary to infestation by a protozoan Toxoplasma gondii . The condition is uncommon in cold, dry regions (Figure 18).
Clinical aspects: The organism Toxoplasma gondii may parasitize numerous warm blooded animals particularly the humans and rodents which function as intermediate hosts while the cats may operate as the definitive hosts. Toxoplasmosis may clinically simulate an acute infectious mononucleosis with a mild fever [21]. The infection may be transmitted in humans through: a) ingestion of a parasitic cyst contained in undercooked meat; b) Presence of parasitic oocytes in the cat’s stool and c) a trans-placental transfer of the parasite from mother to foetus. In humans, the parasite Toxoplasma gondii may emerge in two conformations such as the tachyzoites, situated within the circulating macrophages and the bradyzoites, located within the intracellular cysts [1,2]. The patients may be asymptomatic. Following ingestion of oocytes or cysts, the symptoms usually ensue within five days to several weeks. The toxoplasma gondii infection may disseminate with immune deficiency such as in leukaemia, lymphoma, autoimmune deficiency syndrome (AIDS), the administration of immune suppressive therapy and in the foetus. Myocarditis, pneumonitis, chorioretinitis and encephalitis [2,4]may ensue and may be fatal. With transmission of infection in early pregnancy, the foetus may be critically impaired [22-25].
Histomorphology: Lymphadenitis due to toxoplasmosis may be determined on histology and confirmed by serology [1,2,4]. A limited, firm, moderate posterior cervical lymph node enlargement with fever is frequent in adult females. The lymph node architecture is well maintained. The three typical attributes on lymph node histology, though not always present are: a) a florid follicular hyperplasia with intense mitosis and phagocytosis of the nuclear debris, b) miniature granulomas chiefly comprising of aggregates of epitheloid cells situated at the perimeter and within the hyperplastic follicles, impinging upon and blurring the follicular margins and c) dilatation of marginal and cortical sinuses by proliferating monocytoid B cells (MBL). Immunoblasts and plasma cells may appear in the medullary cords [1,2]. Few granulomas may display necrosis or langhans giant cells. Prominent, expanded follicles with reactive germinal centre demonstrate an augmentation of centroblasts, macrophages and mitotic figures with numerous tingible body macrophages (Table 2).
| Bacterial Infection | Bartonella henselae, Brucella spp, Actinomyces spp, Chlamyadia trachomatis ( L1,L2L3 serovars), Coxiella burnetti, Francisella tularensis, Listeria monocytogenes, Malakoplakia (various bacteria), Mycobacterium tuberculosis, Non tuberculous mycobacteria, Yersinia granulomatosis |
| Fungal infection | Aspergillus spp, Blastomyces dermatitidis, Coccidioides spp, Cryptococcus spp, Histoplasma capsulatum, Mucorales, Sporothrix schenckii |
| Viral and parasitic infection | Cytomegalovirus, Epstein barr virus, Leishmania spp, Toxoplasma gondii |
| Auto-immune origin | Churg Strauss disease, Granulomatosis with polyangiitis, Sarcoid |
| Malignant origin (neoplasm) | Dendritic cell sarcoma, Erdheim Chester disease, Haemophagocytic lymphohistiocytosis, Histiocytic sarcoma, Hodgkin’s lymphoma, Interdigitating cell sarcoma, Langerhans cell histiocytosis, Langerhans cell sarcoma, Metastasis, Rosai Dorfman disease |
| Other | Foreign body reaction |
Table 2: Aetiological allocation of lymph node granulomas.
Monocytoid B cells (MBLs) with darkly staining nuclei may distend the lymphatic sinuses and abutting blood vessels. Monocytoid B cells (MBLs) may be exemplified in cat-scratch disease [2,3] and autoimmune deficiency syndrome (AIDS) although they are innumerable in lymphadenitis due to toxoplasmosis. Poorly delineated epitheloid cell aggregates may be found dispersed throughout the cortex, paracortex and sporadically within the germinal centers. Clearly enunciated granulomas may be lacking. Necrosis and fibrosis may be absent along with an exudation of neutrophils, and eosinophils. Lymphadenitis due to toxoplasmosis may be detected with the employment of specific serological assays such as Sabin Feldman dye test and the diagnostic histology, in contrast to the confirmatory immune-histochemistry or a Polymerase Chain Reaction (PCR). Immune-fluorescence with IgM may be reactive in the majority of the cases (97%). The condition requires a demarcation from Nodular Lymphocytic Predominance Hodgkin’s Disease (NLPHD) and adjunctive infectious diseases. Epitheloid cells contained within the germinal centers is a typical attribute of toxoplasmosis [3,4].
Fungal lymphadenitis
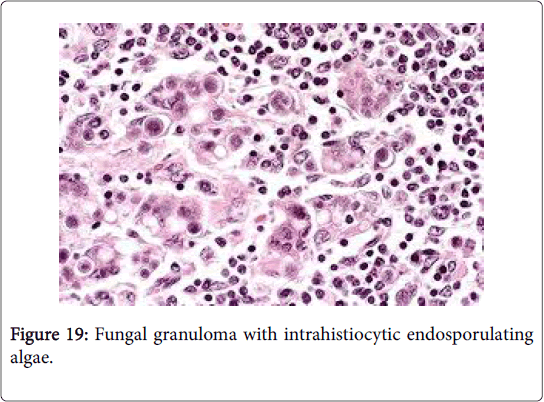
A chronic suppurative lymphadenitis, a granulomatous inflammation or a bilateral representation of the fungal aetiology is a frequent manifestation of lymph nodes incriminated by a fungal infection. Histoplasmosis is a crucial infection which may initiate extensive necrosis of the lymph node with prominent and diffuse hyperplasia of the sinus histiocytes. Adjunctive fungi which may provoke a lymphadenitis are blastomycosis, paracoccidiomycosis, coccidiomycosis and sporotrichosis (Figure 19).
Opportunistic infections such as cyptococcosis, aspergillosis, mucormycosis and candidiasis may also be elucidated in the lymph node [1,2]. The fungal organism may be demonstrated by the Gomori Methanamine Silver (GMS) and Periodic acid Schiff (PAS) Gridley stains. Fungal discovery and identification may also be possible by culture/sensitivity and molecular techniques [2-4].
Lympho-granuloma venereum
The micro-organism Chlamydia trachomatis with concordant serotypes L1, L2 and L3 may generate the sexually transmitted disease lympho granuloma venereum. A miniature (2-3 mm) painless, genital vesicle or ulcer may appear initially, disregarded and spontaneously alleviate within a brief period. Subsequently, a predominant inguinal lymph node enlargement ensues [1,2].
Histomorphology: The preliminary appearance is of a miniature, necrotic locus with neutrophilic infiltration followed by the typical aspect of the infection that is an expansive, necrotic foci which may amalgamate to conform stellate micro-abscesses [18]. Epitheloid cells, disseminated langhans giant cells and fibroblasts may successively emerge may laminate the wall of the abscess. Confluence of the abscesses may occur frequently and a cutaneous sinus tract may arise. Dense fibrosis with enveloping amorphous material and nodular aggregates ushers in the healing stage [2,4].
Identical histology may be elucidated in Cat scratch disease, a typical mycobacterium infection and tularaemia. A conclusive Frei skin test (a delayed hypersensitivity intra-dermal test employing the purified lympho-granuloma venerum antigen which indicates past or present chlamydial infection), complement fixation, molecular testing and immune-fluorescence may be accomplished in order to classify the disease [1,3,4] (Table 3).
| Features | Sarcoid like | Infective granuloma (tuberculosis like) |
|---|---|---|
| Association with metastasis | May or may not be associated with metastasis | Independent of the metastatic status |
| Granuloma | Simulating the sarcoid granuloma, small or large, may be confluent | Discrete granuloma like tuberculosis, with or without necrosis |
| Giant cells (Foreign body/Langhans) | +/- | +/- |
| Necrosis | Fibrinoid necrosis +/- | Caseous Necrosis +/- |
| Calcification | May be present | Generally absent |
| Asteroid bodies | May be present | Generally absent |
| AFB staining | Negative | +/- |
Table 3: Distinction of Sarcoid like and Infective Granuloma.
Brucellosis
Brucella abortus , B. melitensis and B. suis are the causative microorganisms of brucellosis. A food-borne infection occurring with the ingestion of milk and cheese exemplifies the disorder. Frequent clinical attributes may be fever, hepatomegaly and splenomegaly. Lymph node enlargement due to lymphadenitis may be moderate and infrequent [1,3].
Histomorphology: Primary manifestations may be nonspecific follicular hyperplasia and aggregates of epitheloid cells which may articulate massive non-caseating granulomas. A polymorphic infiltrate of eosinophils, plasma cells and immunoblasts may accompany the lymphoid pathology. Bacterial analysis with cultures, a Polymerase Chain Reaction (PCR) or amplifying agglutination titers with a serological assay may assist in isolating the organism [2,3].
Conclusion
A focal collection of inflammatory cells or epithelioid cells with giant cells (foreign body or langhans) aggregated at the site of tissue infection is labeled as a “granuloma”. The molecules of the complement cascade C3a, C5a, helper T cells (Th1) along with the cytokines and chemo-kines (tumour necrosis factor α, inteleukins1, 6, and 17 with interferon gamma) may engage and mobilize the macrophages to the location of granuloma formation. The granulomas may preponderantly be of two categories: a) foreign body granulomas displaying a histiocytic response to inanimate substances such as suture, talc or food matter, in the absence of an adaptive immune response; b) The immune granulomas may depict variable causative factors with a well demarcated morphology. Necrotizing granulomas with a centric caseous, cheese like necrosis accompanied by a lymphohistiocytic palisade with a chronic inflammatory margin may be elucidated.
The histology and immune histochemistry may delineate concrete causative organisms (Coccidioides spp ). The non necrotising (naked granulomas) may be distinguished by focal aggregates of epitheloid histiocytes and giant cells accompanied by miniscule, marginal chronic inflammatory exudates (sarcoid granulomas). The non-infectious granulomas usually comprise of lymphadenitis secondary to berylliosis , Hodgkin’s lymphoma, non-Hodgkin’s lymphoma, lymph nodes draining cancer or crohn’s disease, sarcoid like reaction and sarcoidosis. Infective granulomatous lymphadenitis may be subdivided into “Suppurative lymphadenitis” consequent to tularemia, cat scratch disease, yersinia enterocolitica and lympho-granuloma venereum, with a central necrosis or a centric abscess. “Non suppurative lymphadenitis” comprises of tuberculosis and Bacille Calmette Guerin (BCG) lymphadenitis with a non-suppurative, hypersensitivity type granuloma secondary to infection by a mycobacterium.
References
- Asano S (2012) Granulomatous Lymphadenitis. J Clin Exp Haematop 52: 1-16.
- Rosai J (2012) Rosai and Ackerman's Surgical Pathology. Elsevier 10: 1786.
- Ioachim HL, Medeiros LJ (2009) Ioachim's lymph node pathology. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins 4: 203-212.
- Silverberg SG, de Lellis RA (2006) Silverberg's principles and practice of surgical pathology and cytopathology. Elsevier Churchill Livingstone 4: 508-607.
- Kurata A, Terado Y, Schulz A, Fujioka Y, Franke FE (2005) Inflammatory cells in the formation of tumor-related sarcoid reactions. Hum Pathol 36: 564-554.
- Vogler AJ, Birdsell D, Price LB, Bowers JR, Beckstrom-Sternberg SM, et al. (2009) Phylogeography of Francisella tularensis: Global expansion of a highly fit clone. J Bacteriol 191: 2474-2484.
- Versage JL, Severin DD, Chu MC, Petersen JM (2003) Development of a multitarget real-time TaqMan PCR assay for enhanced detection of Francisella tularensis in complex specimens. J Clin Microbiol 41: 5492-5499.
- Kojima M, Morita Y, Shimizu K, Yoshida T, Yamada I, et al. (2007) Immunohistological findings of suppurative granulomas of Yersinia enterocolitica appendicitis: A report of two cases. Pathol Res Pract 203: 115-119.
- Sepkowitz KA (2006) One disease, two epidemics-AIDS at 25. N Engl J Med 354: 2411-2414.
- Polesky A, Grove W, Bhatia G (2005) Peripheral tuberculous lymphadenitis: Epidemiology, diagnosis, treatment, and outcome. Medicine 84: 350-362.
- Moore SW, Schneider JW, Schaaf HS (2003) Diagnostic aspects of cervical lymphadenopathy in children in the developing world : A study of 1877 surgical specimens. Paediatr Surg Intl 19: 240-244.
- Kraus M, Benharroch D, Kaplan D, Sion-Vardy N, Leiberman A et al. (1999) Mycobacterial cervical lymphadenitis the histological features of non-tuberculous mycobacterial infection. Histopathology 35: 534-538.
- Baughman RP, Lower EE, du Bois RM (2003) Sarcoidosis. Lancet 361: 1111-1118.
- du Bois RM, Goh N, McGrath D, Cullinan P (2003) Is there a role of micro-organisms in the pathogenesis of sarcoidosis? J Intern Med 253: 4-17.
- Cheuk W, Chan AK, Wong MC, Chan JK (2006) Confirmation of diagnosis of cat scratch disease by immunohistochemistry. Am J Surg Pathol 30: 274-275.
- Windsor JJ (2001) Cat scratch disease : Epidemiology, aetiology and treatment. Br J Biomed Sci 58: 101-110.
- Mabey D, Peeling RW (2002) Lymphogranuloma venereum. Sex Transm Infect 78: 90-92.
- Ellis J, Oyston PC, Green M, Titball RW (2002) Tularemia. Clin Microbiol Rev 15: 631-646.
- Lamps LW, Havens JM, Sjostedt A, Page DL, Scott MA (2004) Histologic and molecular diagnosis of tularemia: A potential bioterrorism agent endemic to North America. Mod Pathol 17: 489-495.
- Bastien P (2002) Molecular diagnosis of toxoplasmosis. Trans R Soc Trop Med Hyg 96: 205-215.
- Sehgal S, Goyal P, Ghosh S, Mittal D, Singh S (2014) Malignancy and granulomatosis: causality or coincidence? narrative systematic review. Iran J Pathol 9: 237-244.
- Caulfield AJ, Wengenack NL (2016) Diagnosis of active tuberculosis disease from microscopy to molecular techniques. J Clin Tuberc Other Mycobact Dis 4: 33-43.
- Hatipoglu N, Guvenc HB (2017) Peripheral tuberculous lymphadenitis: Clinical approach and medico surgical management.
- Baek SO, Ko HS, Han HH (2017) BCG vaccination-induced suppurative lymphadenitis: four signs to pay attention to. Int Wound J 14: 1385-1387.
Citation: Bajaj A (2018) Infective Germination: Granulomatous Inflammation: Lymph Node. J Biol Med Sci 2: 108.
Copyright: © 2018 Bajaj A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 23262
- [From(publication date): 0-2018 - Mar 06, 2026]
- Breakdown by view type
- HTML page views: 21912
- PDF downloads: 1350