Review Article Open Access
Infected Mandibular Fractures: Risk Factors and Management
Ehab Abdelfadil1*, Ahmed S Salem2, Samah I Mourad2and Fouad A Al-Belasy2
1Graduate Institute of Medical Science, China Medical University Taichung City, Taiwan, China
2Department of Oral and Maxillofacial Surgery, Mansoura University, Mansoura, Egypt
- *Corresponding Author:
- Ehab Abdelfadil
Graduate Institute of Medical Science
China Medical University Taichung City
Taiwan
Tel: +01004403518
Fax: +2-050-226-0173
E-mail: ehabident@yahoo.com
Received Date: March 28, 2013; Accepted Date: May 15, 2013; Published Date: May 18, 2013
Citation: Abdelfadil E, Salem AS, Mourad SI, Al-Belasy FA (2013) Infected Mandibular Fractures: Risk Factors and Management. J Oral Hyg Health 1:102. doi: 10.4172/2332-0702.1000102
Copyright: © 2013 Abdelfadil E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Oral Hygiene & Health
Abstract
Postoperative infection is one of the most commonly encountered complications after treatment of jaw fractures. Mandibular fractures are reported to be associated with the highest rate of infections among other maxillofacial fractures. Different factors can increase the risk of infection, including, for example, the patient systemic condition, nature of injury, time of medical care, and type of treatment utilized. This article was aimed to review these risk factors and to highlight the management of the infected mandibular fractures.
Keywords
Mandible; Fracture; Infection
Abbreviations
FB: Foreign Body; NB: Necrotic Bone
Introduction
Mandibular fractures account for the majority of maxillofacial traumatic injuries [1,2,3]. A particular interest is given to mandibular fractures owing to the diversity of locations, severity of fractures, and the availability of diff erent treatment modalities [4,5]. Infection of jaw fractures represents the most commonly encountered postoperative complication and mandibular fractures are reported to be associated with the highest rate of infections among other maxillofacial fractures [6,7]. Th is may be attributed to its increased cortical structure and its location in a contaminated environment [8]. Alpert et al. [9] applied the term “infected mandibular fracture” whenever there is “frank purulent drainage from the fracture site, either intraoral or through extraoral fi stula in chronic cases or as associated facial cellulitis in acute presentation”. Th is postoperative infection is perhaps diffi cult to determine whether it arises from the injury itself or from the treatment [10]. Th e incidence of postoperative infection encountered with mandibular fractures varies widely among studies and ranges from 0% to 25% (Table 1). Such discrepancy suggests the involvement of multiple contributing variables or risk factors. Th is article was aimed to present a review of these risk factors and to highlight the management of infected mandibular fractures based on diff erent reports from the literature.
| Author | Patients [n] | Infection | |
|---|---|---|---|
| n | % | ||
| Bolourian R et al. [60] | 31 | 0 | 0 |
| Mehra P et al. [34] | 133 | 3 | 2.3 |
| Fox A et al. [47] | 68 | 2 | 2.9 |
| Adeyemo W et al. [100] | 314 | 12 | 3.8 |
| Lamphier J et al. [7] | 594 | 34 | 5.7 |
| deMatos FB et al. [46] | 126 | 10 | 7.9 |
| Moreno et al. [42] | 232 | 19 | 8.2 |
| Ramakrishnan J et al. [55] | 83 | 8 | 9.6 |
| Lovato C et al. [87] | 150 | 18 | 12 |
| Czerwinski et al. [8] | 181 | 25 | 13.8 |
| Ellis E 3rd [56] | 402 | 75 | 18.7 |
| Malanchuk et al. [11] | 789 | 195 | 24.7 |
Table 1: Incidence of Postoperative Infection in Mandibular Fractures.
Risk Factors
Trauma-related factors
Malanchuk and Kopchak [11] reported that severe combined trauma does not contribute to the development of infection in mandibular fracture. He explained this fi nding by the fact that such patients usually favor an early and adequate medical care as well as prophylactic antibiotics. In contrast, Zachariades et al. [12] reported that comminution, gross displacement, and compound fractures are all factors that can contribute to the development of infection in mandibular fractures. In a study be Ellis et al. [13], 4 out of 6 infections encountered with mandibular fracture were associated with fractures having 2 to 4 fragments suggesting an association between severity of trauma and infection. Gordon et al. [14] studied the relation between severity of mandibular fractures as measured by the UCLA Mandibular Injury Severity Score (MISS) [15] and patient health status with postoperative infl ammatory complications. Th ey concluded that a higher MISS score was signifi cantly associated with an increased risk of infl ammatory complications.
Severe traumatic injuries such as those caused by gun-shots are oft en associated with increased bone fragmentation and soft tissue disruption, [13] which could be easily linked to wound contamination and subsequent infection. Such injuries basically necessitate prolonged operative time particularly in patients with compromised medical status and in procedures involving vascularized free fl aps transfer. However, the association between prolonged operative time and the risk of infection seems to be trivial. In a study of 225 patients who were surgically treated for mandibular fractures, van den Bergh et al. [16] reported a mean operative time of 102.2 min with an incidence of postoperative infection accounting for only 2.6%.
Patient-related factors
Virulence of the microorganism, host resistance are the most important patient-related factors linked to the development of infection. However, many other factors have been considered. Aging is suggested to be a potential risk factor for postoperative infection [17]. In pediatric patients, mandibular fractures are most common, though a variable incidence of maxillofacial trauma has been reported [18,19]. The immature immune system of children may contribute to the decreased resistance to infection. However, a relatively low incidence rate of complications has been reported. This low incidence has been attributed to the high osteogenic potential of the pediatric mandible [20,21]. Eskitascioglu et al. [22] reported only 3% infection rate in a retrospective study that involved 235 pediatric patients with mandibular fractures. Upon calculating the incidence of infection within all the encountered complications, they found the incidence to be as high as 35% [22]. Stone et al. [23], on the contrary, reported no association between age and the incidence of postoperative infection. However, aging is known to be associated with impaired or at least delayed wound healing and the situation may not be the same when dealing with infection in children [24].
Aging is usually associated with systemic diseases, and both can contribute to increased risk of infection [25,26]. Gordon et al. [14] reported a significant association between aging and postoperative inflammatory complications and suggested that the associated positive medical history to be the cause. Malanchuk and Kopchak [11] reported that infection rate in patients with systemic disease was as high as 42.7% when compared to only 22.4% in healthy individuals. He also reported in the same study that infection rate increased from 9.4% in patients younger than 20 years to 55.5% in patients older than 60 years. Increased risk for postoperative infection of mandibular fractures was reported in patients with AIDS, diabetes mellitus, tuberculosis, and drug abuse [11,27,28].
Substance abuse has been linked to increased postoperative complications rate [29,30]. Serena-Gomez [29] reported post surgical complications in patients with substance abuse (smoking, alcohol, and drugs) to be as high as 3.6 folds compared to non-substance abuse patients. Biller et al. [31] also reported a considerable increase of postoperative infection in patients with substance abuse. Smoking has been suggested to have a considerable role in the development of infection, wound dehiscence, and compromised osseous tissue regeneration [32-35]. The function of cellular and humoral immune system is affected by smoking, although the exact underlying mechanisms are not fully understood [36]. Smoking is reported to retard bone healing, adversely affect bone mineral density, and even to increase the risk of osteomyelitis [37,38]. These effects were evident in Benson et al. [39] study that involved 43 patients with infected mandibular fractures. Most of those patients were smoking more than one pack of cigarettes each day [39]. Serena-Gomez [29] also reported that 37.5% of patients treated for mandibular fractures with postoperative infections were smokers. However, bone fragility, increased incidence of fracture, and retarded bone healing secondary to smoking have been suggested to be independent of the decreased bone mineral density [40]. While the negative effect of alcohol on bone healing is well known due to impaired nutrition, the effect on infection is not clear [41]. However, in the Serena-Gomez [29] study, postoperative infection occurred in 18 alcohol abusers compared to that in 15 smokers.
Pre-surgical and post surgical contamination of the fractured site, and hence the incidence of infection, is greatly influenced by the patient dental condition and oral hygiene [42,12]. Oral hygiene is greatly influenced by patient compliance, which would affect the treatment type to be used. An example is rigid fixation, which has the main advantage of immediate jaw mobilization or at least shortening of the maxillomandibular fixation (MMF) period [43]. Rigid fixation requires adequate postoperative care to prevent po complications especially infection, which cannot be guaranteed in noncompliant patients [44]. It is also critical for patients to comply to the treatment method for the recommended time, otherwise increased risk of infection may result [23]. Eskitascioglu et al. [22] correlated the increased rate of complications encountered in the 12 to 16 years old patients with mandibular fractures to weak oral hygiene measures.
Time between fracture and treatment
Early treatment, within the first few hours after trauma, is said to be associated with fewer rates of postoperative infections. Delayed treatment (1-2 weeks after trauma) is also said to be accompanied with increased risk of infection [42,34]. Malanchuk and Kopchak [11] in a study of 334 patients reported a significant association between delayed treatment (more than 7 days) and the development of infection. Czerwinski et al. [8] in a retrospective study of 177 patients with mandibular fractures found that delaying treatment for more than 72 hours does not significantly increase the risk of infection. Other studies also reported that delayed treatment has no significance on the incidence of postoperative complications (Table 2) [8,44-47]. How delayed treatment would influence the incidence of infection is not clear. However, Beckers [48] described a case of infected mandibular fracture that had a third molar in the line of fracture with radiographic evidence of chronic pericoronal infection, and suggested that infection could have been prevented if the delayed antibiotic administration was avoided. Therefore, in certain situations, delayed “medical treatment” may increase the incidence of infection. This might explain the association between delayed treatment and the incidence of complications described by Malanchuk and Kopchak [11].
| Author | Patients [n] | Delayed time | Significance on infection? | |
|---|---|---|---|---|
| Total | Infection | |||
| Czerwinski et al. [8] | 181 | 25 [14%] | 72h | No |
| Fox A et al. [47] | 68 | 2 [2.9%] | Mean 7.2 days | No |
| Lucca M et al. [44] | 92 | 6 [6.5%] | 48h | No |
| Malanchunk et al. [11] | 789 | 195 [24.7%] | First day 2-3 days 4-7 days >7 days | Yes |
| deMatos FB et al. [46] | 126 | 10 [7.9%] | Mean 5.4 days | No |
| Biller[31] | 84 | 11 [13%] | 3 days | No |
Table 2: Effect of Delayed Treatment on Incidence of Postoperative Infection.
Tooth in the line of fracture
Although the tooth in the line of fracture may interfere with reduction and/or occlusion, the greatest concern is usually directed towards the possibility of inducing infection. Even with clinically sound teeth, contamination can still occur through the involved periodontal ligament, which renders all fractures in the tooth-bearing area open or compound. Additionally, the socket forms a huge channel for bacterial invasion, which is usually difficult to control especially when MMF is used [49,50]. Yet, removal of the erupted or partially bony impacted teeth during treatment of mandibular fractures was reported to contribute to wound dehiscence even when care is taken to minimize tension during flap closure [34]. Wound dehiscence and plate exposure are often associated with contamination and clinical mobility that may necessitate plate removal [51,52]. Therefore, the ideal handling of teeth in fracture lines has always been a controversial issue. Different options were described ranging from routine removal of the tooth in all cases to routine preservation of sound teeth. However, most surgeons agree to the concept of removing the tooth only if presented with loss of vitality, root fracture, loosening, or when interfering with fracture reduction or occlusion [34,47,53,54]. When a decision is taken to leave the tooth in the fracture line, it has been suggested to closely check for its vitality after fracture consolidation to perform endodontic treatment whenever loss of vitality is noted [11].
Mehra et al. [34] reported that leaving third molars at fracture site increases the risk of infection. He justifies its removal if open reduction and internal fixation is to be used, as long as enough bone can be left in the lingual and inferior buccal aspects to ensure adequate bone contact and subsequent bone healing. On the other hand, preservation of teeth in the line of fracture has been described to add to the reduction stability [47]. This may be true in some situations, where removal of the tooth may result in a defect that can reduce the bone to bone contact and hence significantly reduce fracture stability.
Ramakrishnan et al. [55], in a study of 83 patients with angle fractures, reported that the presence of third molar teeth had no significant impact on postoperative complications. Moreover, he found that selective removal of these teeth following the standard guidelines may not decrease postoperative complication rates. Malanchuk and Kopchak [11], in a study of 789 patients with mandibular fracture, reported a 25% infection rate in cases with teeth in the fractured site. However, this high infection rate was not significantly different when compared to cases that developed infection with no teeth in the fracture line (22%). Similar results were also reported by Ramakrishnan et al. [55]. In a study by Ellis [56], the incidence of infection related to presence or absence of teeth in the line of angle fractures was not statistically different. Moreover, there was no significance related to either retaining or removing the teeth involved in the fracture line. Cabrini Gabrielli et al. [57] also reported an insignificant difference between an infection rate of 7.14% after third molars in the fracture line were removed and 11.9% when they were retained. Impacted teeth in the fracture line usually present less concern compared to erupted teeth. Baykul et al. [45] reported that removal of asymptomatic impacted third molars with no history of previous infection results in additional trauma that can increase the risk of infection and displacement of bony fragments.
Open Versus Closed Treatment
Whether the type of treatment is a significant determinant factor for the development of postoperative infection is controversial. Compared to open reduction, closed reduction is reported to be associated with lower rates of postoperative complications [7]. The higher incidence of postoperative complications with open reduction has been ascribed to exposure of the fracture site as well as the hardware to the oral cavity flora [55] Acero et al. [58] reported that, 100% of retrieved titanium plates that were intraorally exposed had contamination compared to 36% of plates removed 3 months postoperatively in a control group. However, some studies reported minimal infection rates with open reduction techniques. Erol et al. [1] reported a very low rate of infection following internal fixation accounting only for 1.1%. Gordon et al. [14] also showed no significant difference between closed and open reduction considering the risk of postoperative inflammatory complications. These conflicting reports may support the multifactorial etiology of postoperative infection in mandibular fractures.
Moreno et al. [42] reported an association between postoperative infection and the severity of the fracture rather than the type of treatment used. In this study, they described the complications encountered in 323 patients treated for mandibular fractures with different treatment modalities (intermaxillary fixation, 2.0 mm mini-plates, AO 2.4 and 2.7 mm systems), and found that there was no significant difference in the postoperative infection rate among the different types of treatment used. However, apart from the trauma itself, a more invasive surgical treatment with wide exposure is usually required for severe traumatic injuries. This in turn decreases vascularity owing to periosteal elevation and increases the possibility of wound dehiscence and contamination. Rasubala et al. [59] compared the healing process in plated and nonplated mandibular fractures in rats. He found that the healing process was delayed for 1 week in the plated group. He attributed this finding to the surgical trauma and stripping of the periosteum, which plays an important role by providing the osteogenic progenitor cells in the early stages of bone healing.
Extensive periosteal stripping may decrease the resistance to infection.(50) Proceeding from the superiority of limited periosteal elevation on the healing outcomes, some surgeons utilized an approach that combines both internal fixation and MMF dispensing with the advantage of immediate jaw mobilization. Bolourian et al. [60] described a treatment approach that utilized the use of 2.0 mm miniplate fixation placed transorally at Champy’s line of ideal osteosynthesis [61] accompanied with 2 weeks of MMF and none of the 44 patients developed any complication including infection. In a similar study, Chritah et al. [62] utilized a transoral 2.0 mm locking miniplate fixation combined with 1 week of MMF and no postoperative infection was encountered. This approach combines the advantages of less periosteal stripping owing to the use of a single osteosynthesis plate, reinforcement of the tension band, better soft tissue healing, and reduced possibility of wound dehiscence that may predispose to the development of infection [7,54,60]. These previous studies provide clues that periosteum elevation is an important factor to be considered during treatment of mandibular fractures and corroborate the fact that local vascularity is a determinant factor of the capacity of wound healing [63].
Rigidity of Fixation
While fracture instability is known to retard bone healing through interfering with proliferation of capillaries across the gap, [64] the relationship between the rigidity of fixation and infection remains less defined. However, inadequate stability and interfragmentary mobility is reported to be associated with a greater tendency of infection [42,57]. Interfragmentary movement has been suggested to introduce microorganisms into the fracture site [65]. Alpert [50] stated that macro movement breaks up the capillary ingrowth into the fracture hematoma and pumps in nonpathogenic oral flora through the periodontal ligament [49]. Stone et al. [23] reported that 20% of the overall postoperative infection occurred in patients treated with open reduction and internal fixation with wire osteosynthesis (in addition to MMF for 4 to 6 weeks) compared to only 6.3% when open reduction with rigid internal fixation was used. This seems a little bit confusing as to whether wire osteosynthesis and MMF compared to rigid internal fixation provide less rigidity to a degree that leads to increased risk of infection. Stone [23], however, mentioned that virtually all the patients who developed postoperative infections following open reduction with wire osteosynthesis released their MMF prematurely against medical advice. Fracture stability can be also greatly influenced by the experience of the operator [66]. A loose internal fixation device acts as a foreign body and hence induces infection [50]. Consequently, errors arising from poor plate adaptation, screw-holes drilling, or screw placement can result in interfragmentary mobility that increases the risk of infection.
Soft tissue infection and wound contamination are often considered as important factors regarding fracture severity and expected complications [67]. Infection results in a hypoxic environment, which may lead to fibrous union without bone formation [68]. A high association between infection and nonunion has been reported. Mathog et al. [69] reported that 17 out of 25 cases of nonunion were associated with infection. Malanchuk and Kopchak [11] augmented this finding in a study of 195 infected mandibles and showed that 55% of the infected cases developed nonunion secondary to infection.
In a study including 32 patients with oblique infected mandibular fractures, Ghanem et al. [54] used a single 2.3 mm reconstruction plate and reported no postoperative complications. These authors [54] compared two groups; one with the 2.3mm reconstruction plate fixed with 3 screws on each side and another group with the plate fixed with 2 screws on each side followed by MMF. They reported a higher rate of bone formation in the first group as revealed by postoperative follow up radiographs.
A balance between interfragmentary micromovement and macromovement determines whether the vascular ingrowth will be stimulated or broken down [50]. For successful treatment, the osteosynthesis device must provide adequate stability, which controls the interfragmentary movements without necessarily preventing it completely. Interfragmentary micro movements are reported to help fracture healing by stimulating external callus formation [10,54,50]. However, in conventional mandibular fractures, rigid fixation, functionally stable fixation, or even nonsurgical treatment including only observation and soft diet are all viable treatment options [70,71]. Ogasawara et al. [72] reported a case of pathological fracture resulting from osteomyelitis that was treated with only intermaxillary elastic guidance. The authors attributed the bony union to prevention of displacement of the fractured segments.
The use of 2 mini plates for mandibular angle fracture is reported to be superior to single plating technique [73]. In a comparative study of a single versus 2 non-compression plates for treatment of mandibular fractures, Danda [74] reported similar complications rates regarding wound dehiscence and infection, and concluded that the use of 2 plates has no advantages over the single-plate technique.
Titanium Versus Biodegradable Plates
The indications for removal of titanium plates are infection, inflammation, exposure, palpation, nonunion, pain, device failure and denture discomfort [58,75,76]. Although infection may be associated with an increased risk of nonunion, bone healing was reported to occur when infection was present. However, plate removal becomes inevitable for successful resolution of infection when it loosens [50,40].
Titanium is well known of its biocompatibility [58]. Theologie- Lygidakis et al. [77] studied the morphological and chemical changes of retrieved titanium osteosynthesis plates as well as the adjacent soft tissues, and reported no electrochemical changes of titanium nor titanium deposits in the soft tissues. However, they reported mild chronic inflammation of the adjacent tissues that could not be attributed to the titanium plates. In another study, Langford et al. [78] found titanium in the soft tissues up to 13 years postoperatively. However, most titanium lied extracellularly with no evidence of inflammatory response or giant cell reactions.
The biodegradable bone plates present today posses the biocompatibility, rigidity and strength necessary to provide undisturbed bone healing [79]. The degradation of biodegradable bone plates is initiated by an inflammatory process. However, when intense inflammation is induced, secondary infection occasionally occurs [80]. Laine et al. [81], however, surveyed 163 patients who had 329 orthognathic osteotomies fixed with bioresorbable devices and found that only 1 patient (0.6%) had infection. Despite the difference between traumatic fractures and orthognathic osteotomies, Laine et al. [81] study indicates that a bioresorbable device by itself is unlikely to induce infection. Lee et al. [82] and Bhatt et al. [83] reported no significant difference in complications encountered with titanium plates when compared to biodegradable plates in 2 studies that respectively involved 91 and 40 patients treated for mandibular fractures (Table 3). These studies support the concept of biocompatibility of the bioabsorbable devices and further disclaim any association between such devices and the risk of postoperative infection.
| Author | Patients | Infection | Significance? | |||||
|---|---|---|---|---|---|---|---|---|
| n | Titanium | Biodegradable | Titanium | Biodegradable | ||||
| n | % | n | % | |||||
| Lee H et al. [82] | 91 | 44 | 47 | 1 | 2.27 | 2 | 4.25 | No |
| Bhatt K et al. [83] | 40 | 21 | 19 | 1 | 4.7 | 0 | 0 | No |
Table 3: Incidence of Infection with Titanium versus Biodegradable Bone Plates.
The Value of Antibiotics
Some maxillofacial surgeons favor the prophylactic use of antibiotics. Moreno et al. [42] reported the use of broad spectrum antibiotic as a prophylactic measure in almost all patients with mandibular fractures. Albeit this measure was started from the time of admission to hospital, infection was the most common postoperative complication (8.2%). A similar protocol was followed by Fox et al. [47] and only 2.9% infection rate was encountered. Van den Bergh [16] used postoperative prophylactic antibiotics for 1 week and reported only 2.6% post operative infection rate.
The importance of postoperative antibiotic administration has also been questioned. Abubaker et al. [84] evaluated the value of postoperative prophylactic antibiotics in a randomized, double-blinded and placebo controlled clinical study. He found no benefit of postoperative prophylactic antibiotics in reducing the incidence of infection. The same results were reported by Miles [85] (Table 4). However, Mehra et al. [34] reported only 1.8% postoperative infection rate in a series of 163 mandibular angle fractures after using a prophylactic antibiotic protocol consisting of intravenous administration of penicillin G (or clindamycin in case of penicillin-allergy) in addition to postoperative oral antibiotics for 7 days and chlorhexidine mouth wash for 2 weeks. Furr et al. [86] found no correlation between antibiotic administration and long-term postoperative infections. They found that 83% of patients who developed infections actually received antibiotics at some point in the treatment course.
| Author | Patients | Infection | Significance? | |||||
|---|---|---|---|---|---|---|---|---|
| Total | Ab† | No Ab | Ab | No Ab | ||||
| n | % | n | % | |||||
| Abubaker et al. [84] | 30 | 14 | 16 | 2 | 14.3 | 2 | 12.5 | No |
| Miles [85] | 181 | 81 | 100 | 8 | 9.9 | 14 | 14 | No |
Table 4: Effect of Postoperative Antibiotics Administration on the Incidence of Infection.
Lovato et al. [87] compared perioperative antibiotic regimen (no more than 24 hours postoperatively) versus extended postoperative regimen (from 1 to 10 days postoperatively). They found no significant difference between the 2 groups with respect to the development of infection. Malanchuk and Kopchak [11] reported that the type of antibiotic used can influence the risk of infection in cases with mandibular fractures. They described a lower infection rate associated with the use of lincosamides, which are known to accumulate in bone tissues.
Management
Besides bacteria, infection of mandibular fractures can originate from inadequate interfragment stability, foreign bodies, loose hardware, tooth in the line of fracture and necrotic bone fragment [50]. Therefore, careful examination is necessary to find out possible implicated factors to initiate the appropriate treatment accordingly. In a case report described by Thurnwald [65], he suggested that early use of antibiotics, appropriate oral hygiene and a supportive bandage could prevent infection of compound mandibular fractures. However, whether a supportive bandage could actually aid in decreasing interfragment mobility till fracture treatment is undertaken is questionable. Early intervention is perhaps the adequate approach to achieve this. Another important issue that could help in the management of initially infected fractures is adequate irrigation. Wound irrigation is an essential maneuver in all surgical procedures. Some authors advocated the use of pulsatile pressure saline/antibiotic irrigation in an attempt to decrease contamination prior to reduction [88].
For management of infected compound mandibular fractures 72 hours after trauma, Maloney et al. [89] suggested the use of MMF and intravenous antibiotics until infection is resolved before open reduction is performed. The same concept was also suggested by Michelet et al. [43]. Beckers [48] described the use of bone plates for treatment of initially infected mandibular fractures and reported noncomplicated healing in 14 out of 19 patients treated in his study. In the remaining 5 patients, although infection persisted for approximately 10 days postoperatively, fracture healing was achieved albeit plates were exposed. Postsurgical infections can be successfully treated with localized intraoral incision and drainage in addition to antibiotic therapy [34,47,82]. In many cases, resolution of infection can be achieved through intravenous administration of antibiotics [90].
As long as the fixation is rigid, fracture healing can still be expected even though resolution of infection is incomplete [91]. Lamphier et al. [7] stated that whenever wound dehiscence is encountered, daily irrigation is imperative to prevent infection development. Fox et al. [47] reported a case of mandibular angle fracture treated with mini plates that developed infection 4 weeks after surgery. Although infection resolved in response to incision and drainage plus a 10-day antibiotic therapy combined with chlorhexidine rinse, the incision failed to heal and plate removal was required. Cases resistant to conventional treatment or those with failed internal fixation devices often necessitate the removal of the osteosynthesis material. [6,42,50] This is usually followed by debridement to healthy bleeding bone and restabilization with rigid fixation if bone healing was not achieved [92]. If a defect has developed, autogenous bone grafts might be needed [69].
Osteosynthesis screws must be placed in healthy bones [43]. The bone viability of severely infected fractures, e.g. pathological mandibular fractures due to osteomyelitis, cannot be guaranteed and limited healing or union capacity is expected. This may frequently necessitate resection of the pathologically involved tissue until normal healthy bone is encountered. This may subsequently require vascularized bone grafting [93,94]. This scenario shifts the situation from a simple traumatic injury to a more complicated one that requires a reconstructive surgery.
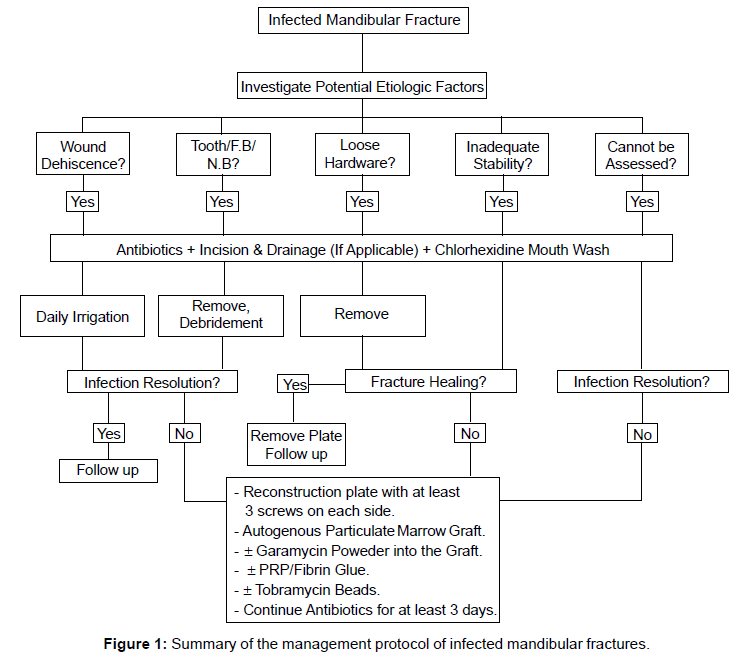
Recently, Benson et al. [39] and Alpert et al. [9] advocated the use of a more aggressive approach to successfully treat the infected mandibular fractures. This approach involved the use of antibiotics, aggressive debridement and trimming to healthy bone and rigid internal fixation with 2.4 or 2.7 mm reconstruction plate with at least 3 bicortical screws on each side. In addition, immediate autogenous particulate marrow bone grafting was used. Incorporation of gentamycin powder into the bone graft or the use of tobramycin beads has been also suggested. Unsuccessful outcome using this approach was reported to be associated with medically compromised patients. Figure 1 summarizes a protocol for management of infected mandibular fractures advocated by some authors [6,9,37,50,54,56].
Discussion
The management of infection is one of the most common and occasionally intriguing jobs in medical practice. Infection superimposed on jaw fractures may be somewhat more challenging. A number of factors have been implicated as causative to this infection. Accompanying soft tissue lacerations, degree of bone fragmentation, the healing capacity of tissues, and the immune system status are some of several factors that play a role. Therefore, a thoughtful assessment of these potential factors becomes imperative for better outcome.
Despite the diversity of the available management protocols described for maxillofacial traumatic injuries, patients are often managed differently. While some severe traumatic injuries were successfully treated with a low incidence of postoperative infections in one study [11], severe injuries were a considerable risk of less favorable outcomes in four studies [13,14,22,42]. This implied that even the greatest care may not prevent complications and one or more of the involved factors may pass uncorrected during the treatment course. However, patient-related factors cannot be ignored. Apart from the systemic condition of the patient, the effects of smoking, alcohol or drug abuse on how tissues react against injuries and infection may shift the treatment outcomes towards unpredictable prognosis.
Oral hygiene is a key factor to eliminate and prevent postoperative infection. For patients, the immediate post-traumatic few days are usually associated with the greatest inconvenience encountered throughout the whole treatment course. During this period, patients suffer pain, edema, and difficult chewing. Therefore, they usually try to mitigate against discomfort by restricting mandibular movements with subsequent inadequacy or absence of oral hygiene measures.
Morbidity associated with teeth in the line of fracture is debatable [95,96]. However, as long as the tooth in the line of fracture is clinically and radio graphically sound, there is no increased risk with regard to the development of infection when the tooth is retained. Tooth removal may add to the severity of the situation in both open and closed reduction protocols. Some displacement and imperfect union may be encountered with closed reduction. Although this can be corrected by physiologic bone remodeling, the need ensues for increasing the MMF period, which is the main disadvantage of the closed reduction technique. On the other hand, depending on tooth position, removal of the tooth in the fracture line with an open reduction protocol my result in a defect that sometimes necessitates a bone graft with an increase in the complexity and cost of treatment.
The basic concept of better bone vascularity with less periosteum elevation is probably true with respect to bone healing. However, its role in infection may be less defined. Comminuted fractures are the most complicated among all fracture patterns [96]. They are classified as open fractures in most cases, [97] and owing to the bone fragmentation, they are highly susceptible to compromised vascularity when treated with open reduction and internal fixation [98]. Despite these facts, the traditional closed treatment for comminuted fractures has been changed and the standard of care now is open reduction and internal fixation [13]. Li et al. [99] reported only 2 postoperative infections in a series of 21 patients treated for comminuted fractures with open reduction and internal fixation (in addition to MMF). He attributed these infections to involvement of untreated impacted tooth in one case and to mobility of segments in the other case. No nonunion complications were reported. They pinpointed that despite the disruption of the covering soft tissues by wires, screws, and bone plates, the excellent blood supply to the face allows small fragments of bone to combine and heal when open reduction of comminuted fracture is used [99]. An important factor that is often overlooked with respect to the mandibular fractures is the lingual periosteum. In most cases, the lingual periosteum is intact, even in severe traumatic injuries, and it is extremely rare to be compromised in any way during treatment of the fracture. In comminuted fractures, the lingual periosteum plays a critical role in the treatment outcomes, and hence, it is essential to be maintained if at all possible [97].
Bilkay et al. [100] examined the role of periosteum on callus formation. The results of both subperiosteal and supraperiosteal dissections were similar with respect to callus formation and maturation at 3 and 8 weeks, respectively. When periosteum was elevated both buccally and lingually, immature callus formation was noted at 8 weeks. Therefore, with the exception of severely compromised patients, the potential risk of infection should not be considered a limiting factor against the use of rigid internal fixation.
Antibiotics are a basic and instrumental adjunct in the management of infection. However, with respect to mandibular fractures, preoperative and postoperative antibiotics and strict oral hygiene measures combined with chlorhexidine mouthwashes have their reliable role in the prevention of postoperative infection.
References
- Erol B, Tanrikulu R, Görgün B (2004) Maxillofacial fractures. Analysis of demographic distribution and treatment in 2901 patients (25-year experience). J Craniomaxillofac Surg 32: 308-313.
- Adebayo ET, Ajike OS, Adekeye EO (2003) Analysis of the pattern of maxillofacial fractures in Kaduna, Nigeria. Br J Oral Maxillofac Surg 41: 396-400.
- Maliska MC, Lima Júnior SM, Gil JN (2009) Analysis of 185 maxillofacial fractures in the state of Santa Catarina, Brazil. Braz Oral Res 23: 268-274.
- Gear AJ, Apasova E, Schmitz JP, Schubert W (2005) Treatment modalities for mandibular angle fractures. J Oral Maxillofac Surg 63: 655-663.
- Dawson KH, Chigurupati R (2002) Fixation of mandibular fractures: a tincture of science. Ann R Australas Coll Dent Surg 16: 118-122.
- Murthy AS, Lehman JA Jr (2005) Symptomatic plate removal in maxillofacial trauma: a review of 76 cases. Ann Plast Surg 55: 603-607.
- Lamphier J, Ziccardi V, Ruvo A, Janel M (2003) Complications of mandibular fractures in an urban teaching center. J Oral Maxillofac Surg 61: 745-749.
- Czerwinski M, Parker WL, Correa JA, Williams HB (2008) Effect of treatment delay on mandibular fracture infection rate. Plast Reconstr Surg 122: 881-885.
- Alpert B, Kushner GM, Tiwana PS (2008) Contemporary management of infected mandibular fractures. Craniomaxillofac Trauma Reconstr 1: 25-29.
- Marsh DR, Li G (1999) The biology of fracture healing: optimising outcome. Br Med Bull 55: 856-869.
- Malanchuk VO, Kopchak AV (2007) Risk factors for development of infection in patients with mandibular fractures located in the tooth-bearing area. J Craniomaxillofac Surg 35: 57-62.
- Zachariades N, Papademetriou I (1995) Complications of treatment of mandibular fractures with compression plates. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 79: 150-153.
- Ellis E 3rd, Muniz O, Anand K (2003) Treatment considerations for comminuted mandibular fractures. J Oral Maxillofac Surg 61: 861-870.
- Gordon PE, Lawler ME, Kaban LB, Dodson TB (2011) Mandibular fracture severity and patient health status are associated with postoperative inflammatory complications. J Oral Maxillofac Surg 69: 2191-2197.
- Shetty V, Atchison K, Der-Matirosian C, Wang J, Belin TR (2007) The mandible injury severity score: development and validity. J Oral Maxillofac Surg 65: 663-670.
- van den Bergh B, Heymans MW, Duvekot F, Forouzanfar T (2012) Treatment and complications of mandibular fractures: a 10-year analysis. J Craniomaxillofac Surg 40: e108-e111.
- Malik MH, Harwood P, Diggle P, Khan SA (2004) Factors affecting rates of infection and nonunion in intramedullary nailing. J Bone Joint Surg Br 86: 556-560.
- Haug RH, Foss J (2000) Maxillofacial injuries in the pediatric patient. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 90: 126-134.
- Rahman RA, Ramli R, Rahman NA, Hussaini HM, Idrus SM, et al. (2007) Maxillofacial trauma of pediatric patients in Malaysia: a retrospective study from 1999 to 2001 in three hospitals. Int J Pediatr Otorhinolaryngol 71: 929-936.
- Amaratunga NA (1988) Mandibular fractures in children--a study of clinical aspects, treatment needs, and complications. J Oral Maxillofac Surg 46: 637-640.
- Rémi M, Christine MC, Gael P, Soizick P, Joseph-André J (2003) Mandibular fractures in children: long term results. Int J Pediatr Otorhinolaryngol 67: 25-30.
- Eskitascioglu T, Ozyazgan I, Coruh A, Gunay GK, Yuksel E (2009) Retrospective analysis of two hundred thirty-five pediatric mandibular fracture cases. Ann Plast Surg 63: 522-530.
- Stone IE, Dodson TB, Bays RA (1993) Risk factors for infection following operative treatment of mandibular fractures: a multivariate analysis. Plast Reconstr Surg 91: 64-68.
- Guo S, Dipietro LA (2010) Factors affecting wound healing. J Dent Res 89: 219-229.
- Chandra RK (1989) Nutritional regulation of immunity and risk of infection in old age. Immunology 67: 141-147.
- Bertoni AG, Saydah S, Brancati FL (2001) Diabetes and the risk of infection-related mortality in the U.S. Diabetes Care 24: 1044-1049.
- Senel FC, Jessen GS, Melo MD, Obeid G (2007) Infection following treatment of mandible fractures: the role of immunosuppression and polysubstance abuse. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 103: 38-42.
- Schmidt B, Kearns G, Perrott D, Kaban LB (1995) Infection following treatment of mandibular fractures in human immunodeficiency virus seropositive patients. J Oral Maxillofac Surg 53: 1134-1139.
- Serena-Gómez E, Passeri LA (2008) Complications of mandible fractures related to substance abuse. J Oral Maxillofac Surg 66: 2028-2034.
- Passeri LA, Ellis E 3rd, Sinn DP (1993) Relationship of substance abuse to complications with mandibular fractures. J Oral Maxillofac Surg 51: 22-25.
- Biller JA, Pletcher SD, Goldberg AN, Murr AH (2005) Complications and the time to repair of mandible fractures. Laryngoscope 115: 769-772.
- Bui P, Demian N, Beetar P (2009) Infection rate in mandibular angle fractures treated with a 2.0-mm 8-hole curved strut plate. J Oral Maxillofac Surg 67: 804-808.
- Zheng LW, Ma L, Cheung LK (2008) Changes in blood perfusion and bone healing induced by nicotine during distraction osteogenesis. Bone 43: 355-361.
- Mehra P, Murad H (2008) Internal fixation of mandibular angle fractures: a comparison of 2 techniques. J Oral Maxillofac Surg 66: 2254-2260.
- Xu M, Scott JE, Liu KZ, Bishop HR, Renaud DE, et al. (2008) The influence of nicotine on granulocytic differentiation - inhibition of the oxidative burst and bacterial killing and increased matrix metalloproteinase-9 release. BMC Cell Biol 9: 19.
- Arcavi L, Benowitz NL (2004) Cigarette smoking and infection. Arch Intern Med 164: 2206-2216.
- Sloan A, Hussain I, Maqsood M, Eremin O, El-Sheemy M (2010) The effects of smoking on fracture healing. Surgeon 8: 111-116.
- Castillo RC, Bosse MJ, MacKenzie EJ, Patterson BM (2005) Impact of smoking on fracture healing and risk of complications in limb-threatening open tibia fractures. J Orthop Trauma 19: 151-157.
- Benson PD, Marshall MK, Engelstad ME, Kushner GM, Alpert B (2006) The use of immediate bone grafting in reconstruction of clinically infected mandibular fractures: bone grafts in the presence of pus. J Oral Maxillofac Surg 64: 122-126.
- Wong PK, Christie JJ, Wark JD (2007) The effects of smoking on bone health. Clin Sci (Lond) 113: 233-241.
- Chakkalakal DA (2005) Alcohol-induced bone loss and deficient bone repair. Alcohol Clin Exp Res 29: 2077-2090.
- Moreno JC, Fernández A, Ortiz JA, Montalvo JJ (2000) Complication rates associated with different treatments for mandibular fractures. J Oral Maxillofac Surg 58: 273-280.
- Michelet FX, Deymes J, Dessus B (1973) Osteosynthesis with miniaturized screwed plates in maxillo-facial surgery. J Maxillofac Surg 1: 79-84.
- Lucca M, Shastri K, McKenzie W, Kraus J, Finkelman M, et al. (2010) Comparison of treatment outcomes associated with early versus late treatment of mandible fractures: a retrospective chart review and analysis. J Oral Maxillofac Surg 68: 2484-2488.
- Baykul T, Erdem E, Dolanmaz D, Alkan A (2004) Impacted tooth in mandibular fracture line: treatment with closed reduction. J Oral Maxillofac Surg 62: 289-291.
- de Matos FP, Arnez MF, Sverzut CE, Trivellato AE (2010) A retrospective study of mandibular fracture in a 40-month period. Int J Oral Maxillofac Surg 39: 10-15.
- Fox AJ, Kellman RM (2003) Mandibular angle fractures: two-miniplate fixation and complications. Arch Facial Plast Surg 5: 464-469.
- Beckers HL (1979) Treatment of initially infected mandibular fractures with bone plates. J Oral Surg 37: 310-313.
- Schilli W (1977) Compression osteosynthesis. J Oral Surg 35: 802-808.
- Alpert B (1998) Management of the complications of mandibular fracture treatment. Operative Techniques in Plastic and Reconstructive Surgery. 5: 325-333.
- Webb LS, Makhijani S, Khanna M, Burstein MJ, Falk AN, et al. (2009) A comparison of outcomes between immediate and delayed repair of mandibular fractures. Can J Plast Surg 17: 124-126.
- Acero J, Calderon J, Salmeron JI, Verdaguer JJ, Concejo C, et al. (1999) The behaviour of titanium as a biomaterial: microscopy study of plates and surrounding tissues in facial osteosynthesis. J Craniomaxillofac Surg 27: 117-123.
- BRADLEY RL (1965) TREATMENT OF FRACTURED MANDIBLE. Am Surg 31: 289-290.
- Ghanem WA, Elhayes KA, Saad K (2011) The management of unstable oblique infected mandibular fractures with a 2.3 mm mandibular osteosynthesis reconstruction bone plate. J Craniomaxillofac Surg 39: 600-605.
- Ramakrishnan J, Shingleton A, Reeves D, Key JM, Vural E (2009) The effects of molar tooth involvement in mandibular angle fractures treated with rigid fixation. Otolaryngol Head Neck Surg 140: 845-848.
- Ellis E 3rd (2002) Outcomes of patients with teeth in the line of mandibular angle fractures treated with stable internal fixation. J Oral Maxillofac Surg 60: 863-865.
- Cabrini Gabrielli MA, Real Gabrielli MF, Marcantonio E, Hochuli-Vieira E (2003) Fixation of mandibular fractures with 2.0-mm miniplates: review of 191 cases. J Oral Maxillofac Surg 61: 430-436.
- Acero J, Calderon J, Salmeron JI, Verdaguer JJ, Concejo C, et al. (1999) The behaviour of titanium as a biomaterial: microscopy study of plates and surrounding tissues in facial osteosynthesis. J Craniomaxillofac Surg 27: 117-123.
- Rasubala L, Yoshikawa H, Islam AA, Nagata K, Iijima T, et al. (2004) Comparison of the healing process in plated and non-plated fractures of the mandible in rats. Br J Oral Maxillofac Surg 42: 315-322.
- Bolourian R, Lazow S, Berger J (2002) Transoral 2.0-mm miniplate fixation of mandibular fractures plus 2 weeks' maxillomandibular fixation: a prospective study. J Oral Maxillofac Surg 60: 167-170.
- Champy M, Loddé JP, Schmitt R, Jaeger JH, Muster D (1978) Mandibular osteosynthesis by miniature screwed plates via a buccal approach. J Maxillofac Surg 6: 14-21.
- Chritah A, Lazow SK, Berger JR (2005) Transoral 2.0-mm locking miniplate fixation of mandibular fractures plus 1 week of maxillomandibular fixation: a prospective study. J Oral Maxillofac Surg 63: 1737-1741.
- Hausman MR, Rinker BD (2004) Intractable wounds and infections: the role of impaired vascularity and advanced surgical methods for treatment. Am J Surg 187: 44S-55S.
- Rhinelander FW (1975) Minimal internal fixation of tibial fractures. Clin Orthop Relat Res : 188-220.
- Thurnwald GA, Macleod AW (1994) An infected mandibular fracture. Case report. Aust Dent J 39: 276-278.
- Kearns GJ, Perrott DH, Kaban LB (1994) Rigid fixation of mandibular fractures: does operator experience reduce complications? J Oral Maxillofac Surg 52: 226-231.
- Shetty V, Atchison K, Der-Martirosian C, Wang J, Belin TR (2003) Determinants of surgical decisions about mandible fractures. J Oral Maxillofac Surg 61: 808-813.
- Ellis E 3rd (1996) Complications of rigid internal fixation for mandibular fractures. J Craniomaxillofac Trauma 2: 32-39.
- Mathog RH, Toma V, Clayman L, Wolf S (2000) Nonunion of the mandible: an analysis of contributing factors. J Oral Maxillofac Surg 58: 746-752.
- Ellis E III (2002) Selection of Internal Fixation Devices for Mandibular Fractures: How Much Fixation Is Enough? Seminars Plastic Surg. 16: 229-240.
- Ghazal G, Jaquiéry C, Hammer B (2004) Non-surgical treatment of mandibular fractures--survey of 28 patients. Int J Oral Maxillofac Surg 33: 141-145.
- Ogasawara T, Sano K, Hatsusegawa C, Miyauchi K, Nakamura M, et al. (2008) Pathological fracture of the mandible resulting from osteomyelitis successfully treated with only intermaxillary elastic guiding. Int J Oral Maxillofac Surg 37: 581-583.
- Levy FE, Smith RW, Odland RM, Marentette LJ (1991) Monocortical miniplate fixation of mandibular angle fractures. Arch Otolaryngol Head Neck Surg 117: 149-154.
- Danda AK (2010) Comparison of a single noncompression miniplate versus 2 noncompression miniplates in the treatment of mandibular angle fractures: a prospective, randomized clinical trial. J Oral Maxillofac Surg 68: 1565-1567.
- Rallis G, Mourouzis C, Papakosta V, Papanastasiou G, Zachariades N (2006) Reasons for miniplate removal following maxillofacial trauma: a 4-year study. J Craniomaxillofac Surg 34: 435-439.
- Mosbah MR, Oloyede D, Koppel DA, Moos KF, Stenhouse D (2003) Miniplate removal in trauma and orthognathic surgery--a retrospective study. Int J Oral Maxillofac Surg 32: 148-151.
- Theologie-Lygidakis N, Iatrou I, Eliades G, Papanikolaou S (2007) A retrieval study on morphological and chemical changes of titanium osteosynthesis plates and adjacent tissues. J Craniomaxillofac Surg 35: 168-176.
- Langford RJ, Frame JW (2002) Tissue changes adjacent to titanium plates in patients. J Craniomaxillofac Surg 30: 103-107.
- Yerit KC, Hainich S, Turhani D, Klug C, Wittwer G, et al. (2005) Stability of biodegradable implants in treatment of mandibular fractures. Plast Reconstr Surg 115: 1863-1870.
- Turvey TA, Proffit WP, Phillips C (2011) Biodegradable fixation for craniomaxillofacial surgery: a 10-year experience involving 761 operations and 745 patients. Int J Oral Maxillofac Surg 40: 244-249.
- Laine P, Kontio R, Lindqvist C, Suuronen R (2004) Are there any complications with bioabsorbable fixation devices? A 10 year review in orthognathic surgery. Int J Oral Maxillofac Surg 33: 240-244.
- Lee HB, Oh JS, Kim SG, Kim HK, Moon SY, et al. (2010) Comparison of titanium and biodegradable miniplates for fixation of mandibular fractures. J Oral Maxillofac Surg 68: 2065-2069.
- Bhatt K, Roychoudhury A, Bhutia O, Trikha A, Seith A, et al. (2010) Equivalence randomized controlled trial of bioresorbable versus titanium miniplates in treatment of mandibular fracture: a pilot study. J Oral Maxillofac Surg 68: 1842-1848.
- Abubaker AO, Rollert MK (2001) Postoperative antibiotic prophylaxis in mandibular fractures: A preliminary randomized, double-blind, and placebo-controlled clinical study. J Oral Maxillofac Surg 59: 1415-1419.
- Miles BA, Potter JK, Ellis E 3rd (2006) The efficacy of postoperative antibiotic regimens in the open treatment of mandibular fractures: a prospective randomized trial. J Oral Maxillofac Surg 64: 576-582.
- Furr AM, Schweinfurth JM, May WL (2006) Factors associated with long-term complications after repair of mandibular fractures. Laryngoscope 116: 427-430.
- Lovato C, Wagner JD (2009) Infection rates following perioperative prophylactic antibiotics versus postoperative extended regimen prophylactic antibiotics in surgical management of mandibular fractures. J Oral Maxillofac Surg. 67: 827-832.
- Betts NJ, Cocolis PK Jr, Beanland D (1996) Using pulsatile pressure saline/antibiotic irrigation before reduction and fixation of infected mandibular fractures: literature review and report of two cases. Compend.Contin.Educ.Dent. 17: 871-882.
- Maloney PL, Lincoln RE, Coyne CP (2001) A protocol for the management of compound mandibular fractures based on the time from injury to treatment. J Oral Maxillofac Surg 59: 879-884.
- Agarwal S, Gupta A, Grevious M, Reid RR (2009) Use of resorbable implants for mandibular fixation: a systematic review. J Craniofac Surg 20: 331-339.
- Koury M, Ellis E 3rd (1992) Rigid internal fixation for the treatment of infected mandibular fractures. J Oral Maxillofac Surg 50: 434-443.
- Klotch D (1995) Application of reconstruction plates to the mandible. 6: 89-96.
- Coletti D, Ord RA (2008) Treatment rationale for pathological fractures of the mandible: a series of 44 fractures. Int J Oral Maxillofac Surg 37: 215-222.
- Kato H, Matsuoka K, Kato N, Ohkubo T (2005) Mandibular osteomyelitis and fracture successfully treated with vascularised iliac bone graft in a patient with pycnodysostosis. Br J Plast Surg 58: 263-266.
- Neal DC, Wagner WF, Alpert B (1978) Morbidity associated with teeth in the line of mandibular fractures. J Oral Surg 36: 859-862.
- Finn RA (1996) Treatment of comminuted mandibular fractures by closed reduction. J Oral Maxillofac Surg 54: 320-327.
- Futran, Neal D (2008) Management of comminuted mandible fractures. Operative Techniques in Otolaryngology-Head and Neck Surgery. 19: 113-116.
- Kelly JF (1977) Management of war injuries to the jaw and related structures. Washington DC, US Government Printing Office.
- Li Z, Li ZB (2011) Clinical characteristics and treatment of multiple site comminuted mandible fractures. J Craniomaxillofac Surg 39: 296-299.
- Bilkay U, Celik N, Bilkay U, Görken C, Alper M, et al. (2000) The role of periosteum and different dissection types on callus formation: quantitative analyses with scintigraphy in a rabbit mandible model.. Ann Plast Surg 45: 48-53.
Relevant Topics
- Advanced Bleeding Gums
- Advanced Receeding Gums
- Bleeding Gums
- Children’s Oral Health
- Coronal Fracture
- Dental Anestheia and Sedation
- Dental Plaque
- Dental Radiology
- Dentistry and Diabetes
- Fluoride Treatments
- Gum Cancer
- Gum Infection
- Occlusal Splint
- Oral and Maxillofacial Pathology
- Oral Hygiene
- Oral Hygiene Blogs
- Oral Hygiene Case Reports
- Oral Hygiene Practice
- Oral Leukoplakia
- Oral Microbiome
- Oral Rehydration
- Oral Surgery Special Issue
- Orthodontistry
- Periodontal Disease Management
- Periodontistry
- Root Canal Treatment
- Tele-Dentistry
Recommended Journals
Article Tools
Article Usage
- Total views: 27716
- [From(publication date):
July-2013 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 22363
- PDF downloads : 5353