Research Article Open Access
Induction of Antigen-Specific Cytotoxic T Lymphocytes by Chemoradiotherapy in Patients Receiving Wilms’ Tumor 1-Targetted Dendritic Cell Vaccinations for Pancreatic Cancer
| Shigetaka Shimodaira1*, Takashi Kobayashi2, Koichi Hirabayashi1, Kayo Horiuchi1, Terutsugu Koya1, Yumiko Mizuno1, Naoko Yamaoka1, Miki Yuzawa1,Shinsuke Ishikawa1, Yumiko Higuchi1, Kenji Sano3, Kenich Ito4, and Tomonobu Koizumi2 | |
| 1Center for Advanced Cell Therapy, Shinshu University Hospital, Matsumoto, Japan | |
| 2Shinshu Cancer Center, Shinshu University Hospital, Matsumoto, Japan | |
| 3Department of Laboratory, Shinshu University Hospital, Matsumoto, Japan | |
| 4Department of Breast and Endocrine Surgery, Shinshu University Hospital, Matsumoto, Japan | |
| Corresponding Author : | Shigetaka Shimodaira Center for Advanced Cell Therapy Shinshu University Hospital, Matsumoto, Japan Tel: +81-263-37-3580 Fax: +81-263-37-3027 E-mail: shimodai@shinshu-u.ac.jp |
| Received: July 06, 2015 Accepted: July 21, 2015 Published: July 24, 2015 | |
| Citation: Shimodaira S, Kobayashi T, Hirabayashi K, Horiuchi K, Koya T, et al. (2015) Induction of Antigen-Specific Cytotoxic T Lymphocytes by Chemoradiotherapy in Patients Receiving Wilms’ Tumor 1-Targetted Dendritic Cell Vaccinations for Pancreatic Cancer. OMICS J Radiol 4:196. doi:10.4172/2167-7964.1000196 | |
| Copyright: ©2015 Shimodaira S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Radiology
Abstract
Despite recent advances in cancer treatment, the prognosis of pancreatic cancer (PC) remains poor. Dendritic cells (DCs) play a central role in acquired immunity; therapeutic DC vaccinations have recently been developed for advanced PC. Here we present two cases of PC: inoperable PC localized to the pancreatic head (Case 1, stage IV) and local recurrence complicated by distant metastases following resection of the pancreas body and tail (Case 2, stage III). Both patients received DC vaccinations pulsed with human leukocyte antigen (HLA)-Class I/II-restricted Wilms’ tumor 1 (WT1) peptides during chemoradiotherapy. The induction of WT1 antigen-specific cytotoxic T cells (WT1-CTL) was markedly increased by chemoradiotherapy and was confirmed by measurement of WT1 tetramers and enzyme-linked immunosorbent spot (ELISpot) in both cases. WT1-CTL was found to persist at 1 year without additional DC vaccines in Case 1. In cases 1 and 2, the overall survival (OS) was 32.1 and 24.7 months, respectively, and progression-free survival (PFS) was 25.2 and 8.7 months, respectively. Adverse reactions due to the DC vaccination were tolerable even during chemoradiotherapy, resulting in disease stability. The findings of the present cases may form treatment strategies involving DC vaccination for PC.
|
Abstract
Despite recent advances in cancer treatment, the prognosis of pancreatic cancer (PC) remains poor. Dendritic cells (DCs) play a central role in acquired immunity; therapeutic DC vaccinations have recently been developed for advanced PC. Here we present two cases of PC: inoperable PC localized to the pancreatic head (Case 1, stage IV) and local recurrence complicated by distant metastases following resection of the pancreas body and tail (Case 2, stage III). Both patients received DC vaccinations pulsed with human leukocyte antigen (HLA)-Class I/II-restricted Wilms’ tumor 1 (WT1) peptides during chemoradiotherapy. The induction of WT1 antigen-specific cytotoxic T cells (WT1-CTL) was markedly increased by chemoradiotherapy and was confirmed by measurement of WT1 tetramers and enzyme-linked immunosorbent spot (ELISpot) in both cases. WT1-CTL was found to persist at 1 year without additional DC vaccines in Case 1. In cases 1 and 2, the overall survival (OS) was 32.1 and 24.7 months, respectively, and progression-free survival (PFS) was 25.2 and 8.7 months, respectively. Adverse reactions due to the DC vaccination were tolerable even during chemoradiotherapy, resulting in disease stability. The findings of the present cases may form treatment strategies involving DC vaccination for PC.
Keywords
Dendritic cells; Vaccination; Wilms’ tumor 1; Chemoradiotherapy; Tetramer analysis; ELISpot assay; Pancreatic cancer
Abbreviations
CT: Computed Tomography; DCs: Dendritic Dells; ELISpot: Enzyme-linked Immunosorbent Spot; HLA: Human Leukocyte Antigen; HIV: Human Immunodeficiency Virus; IFN: Interferon; mDCs: mature DCs; mAb: Monoclonal Antibodies; NK: Natural Killer; OS: Overall Survival; PC: Pancreatic Cancer; PBMCs: Peripheral Blood Mononuclear Cells; PET: Positron Emission Tomography; PFS: Progression-Free Survival; WT1: Wilms’ Tumor 1; WT1-CTL: Wilms’ Tumor 1 Antigen-Specific Cytotoxic T Cells
Introduction
Pancreatic cancer (PC) is the fourth leading cause of death due to cancer in the US, with an overall 5-year survival rate of 6% for all disease stages [1]. In Japan, the 5-year survival rate is approximately 5% and an increasingly common cause of cancer deaths [2]. Surgical resection (pancreaticoduodenectomy or distal pancreatectomy) is feasible in only a small proportion of patients with PC because of the frequency of advanced stage disease at diagnosis, resulting in minimal benefit in terms of survival [3]. Even after resection, recurrence occurs in majority of patients, contributing to a median survival time following PC diagnosis of approximately 18 months. Combination adjuvant chemo- and radiotherapy has been shown to improve disease-free survival and overall survival (OS) rates, suggesting that adjuvant chemotherapy would have significant survival benefit in patients with resected PC [4]. Chemotherapy with gemcitabine (2�¹2�¹-difluorodeoxycytidine, GEM) has been shown to increase survival compared with 5-FU treatment and has become established as the standard first line chemotherapy regimen for advanced PC [5]. However, majority of patients with advanced disease treated with GEM do not survive longer than 6 months. It has also been reported that tegafur-gimeracil-oteracil combination (S-1), an oral form of 5-FU, may improve the prognosis of patients with GEM-refractory PC as well as chemotherapy-naive PC patients [6,7]. Despite the development of novel chemotherapeutic drugs, effective treatment options for PC remain limited. Therefore, treatment modalities other than chemotherapy are urgently required for the treatment of patients with advanced PC.
Dendritic cell (DC)-based immunotherapy has recently been developed for a range of cancer types with DC vaccines primed with human leukocyte antigen (HLA) class I/II restricted Wilms’ tumor 1 (WT1) peptides, demonstrating strong therapeutic efficacy in a range of cancer types [8-13]. Studies have reported increased OS in patients with inoperable PC who received DC vaccination in combination with chemotherapeutic drugs such as GEM and S-1 [8,11,12]. Allogenic vaccines in PC induce T cell infiltration and aggregate formation, resulting in the induction of immunosuppressive regulatory mechanisms [14]. The efficacy of DC vaccination for PC may to be enhanced by off-target effects of GEM and S-1 [15]. The use of radiotherapy in the treatment for PC remains controversial; [4] however, chemoradiationhas been shown to be superior to radiation alone in cases of locally advanced PC [16]. Further, clinical trials of immune-maximizing therapies combining intensity-modulated radiotherapy or other forms of conformal radiotherapy with DC-based vaccines have reported response rates of over 50% in advanced cancers [17]. Therefore, the most efficacious combinations of DC vaccination with adjuvant chemotherapy and/or radiotherapy remain unclear. Here we present the cases of two patients with PC who received DC vaccination with HLA-Class I/II restricted WT1 peptides that indicate the utility of chemoradiotherapy in enhancing DC-induced cancer immunity. Materials and Methods
Preparation of autologous dendritic cell vaccine
Mature DCs (mDCs) were generated under Good gene, Cell & Tissue Manufacturing Practice conditions according to the “The Act on the Safety of Regenerative Medicine” in Japan introduced on November 25, 2014 [18]. Briefly, mononuclear cell-rich fractions were obtained by apheresis using the COM.TEC® cell separator (Fresenius Kabi Japan K.K., Tokyo, Japan). Immature DCs were generated by culturing adherent cells in AIM-V medium (Gibco, Gaithersburg, MD) containing granulocyte–macrophage colony-stimulating factor (50 ng/ml; Gentaur, Brussels, Belgium) and interleukin (IL)-4 (50 ng/ml; R&D Systems Inc., Minneapolis, MN) in a CO2 incubator equipped with a Cell Processing Isolator (H2O2-sterilizing system, Panasonic Corporation, Osaka, Japan) at the Cell Processing Center in Shinshu University Hospital. After 5 days of culture, immature DCs were differentiated into mDCs by stimulation with OK-432 (10 µg/ml; streptococcal preparation, Chugai Pharmaceutical Co, Ltd, Tokyo, Japan) and prostaglandin E2 (50 ng/ml; Daiichi Fine Chemical Co. LTD., Toyama, Japan) for 24 h [19]. Resultant mDCs were cryopreserved and stored until the day of administration. Cell culture supernatants were collected for sterility testing at the time of mDC freezing. The antigenic profiles of mDCs were determined using flow cytometry. mDCs were defined as CD11c+, CD14−, HLA-DR+, HLA-ABC+, CD80+, CD83+, CD86+, CD40+, and CCR7+ cells [19]. The criteria for DC vaccine administration were as follows: purity defined as a >90% proportion of CD11c+CD14−CD86+HLA-DR+>90% cells, >80% viability, mature DC phenotype, negative for bacterial and fungal infection after 14 days, endotoxin testing ≤ 0.05 EU/ml, and negative for mycoplasma.
For each vaccination, an aliquot of frozen mDCs was thawed immediately prior to clinical use and primed with 100 μg/ml of good manufacturing practice-grade WT1 peptide (NeoMPS Inc. San Diego, CA) containing 1–2 KE of OK-432. WT1 peptides contained HLA-A*02:01- or A*02:06-restricted peptides (126-134: RMFPNAPYL), HLA-A*24:02-restricted modified WT1 peptides (CYTWNQML, residue 235–243), and/or Class II peptides (332–347: KRYFKLSHLQMHSRKH) compatible with either DRB1*04:05, DRB1*08:03, DRB1*15:01, DRB1*15:02, DPB1*05:01, or DPB1*09:01 [12]. One course of seven biweekly sessions was performed with 1-3 × 107 DCs with 1-2KE of OK-432 injected intradermally at bilateral axillar and inguinal areas per session. The DC vaccination study was carried out at the Shinshu University Hospital and was approved by the Ethics Committee of Shinshu University School of Medicine (approval number 1199, December 2, 2008; 2704, April 8, 2014). Peripheral blood lymphocyte subsets
Peripheral blood lymphocyte phenotypes at the time of the first and seventh DC sessions were analyzed by flow cytometry (BD FACSCanto™ II, BD Biosciences, San Jose, CA). The following mouse anti-human monoclonal antibodies (mAb) were used to stain isolated lymphocytes: cluster of differentiation (CD)3 (SK7, BD Biosciences, San Jose, CA, USA), CD4 (SK3, BD Biosciences), CD8 (SK1, BD Biosciences), CD19 (4G7, BD Biosciences), CD25 (2A3, BD Biosciences), CD56 (NKH-1, Beckman Coulter, Inc. Brea CA, USA), CD127 (MB15-18C9, Miltenyi Biotec K.K., Tokyo, Japan), and HLA-DR (L243, BD Biosciences).
Tetramer analysis
Freshly isolated peripheral blood mononuclear cells (PBMCs) were stained with PE-conjugated human immunodeficiency virus (HIV)/HLA-A*24:02 tetramer as a negative control or PE-conjugated WT1 modified peptide/HLA-A*24:02 tetramer (MBL, Medical & Biological Laboratories Co., Ltd., Nagoya, Japan), allophycocyanin-conjugated anti-CD3 mAb, and fluorescein isothiocyanate-conjugated anti-CD8 mAb prior to analysis by flow cytometry (BD FACSCaliburTM) [20]. The presence of WT1 antigen-specific cytotoxic T cells (WT1-CTLs) was defined according to the following criterion: greater than 0.08% WT1-positive cells out of all CD8+ T cells with no evidence of false positive cells by tetramer assay.
Enzyme-linked immunosorbent spot assays
Enzyme-linked immunosorbent spot (ELISpot) assays were performed to measure WT1-specific interferon (IFN)-γ production by PBMCs using Human IFN-γ ELISpot PLUS kits (Mabtech, Nacka Strand, Sweden) according to the manufacturer’s instructions. Briefly, PBMCs, including effector and stimulator cells, isolated at each time point were cultured (1 × 106 cells/well) in the presence of either WT1126–134, WT1235–243, or WT1332–347 peptide with previously described modifications [20]. After 18 h incubation, spots were counted by an automated ELISPOT reader (Autoimmun Diagnostika, Strassberg, Germany).
The presence of WT1-specific CTLs was defined according to the following criteria: 1) at least 15 WT1-specific spots per 1 × 106 PBMCs and 2) at least 50% more WT1-specific spots than negative peptide (HIV peptide) spots. Case 1
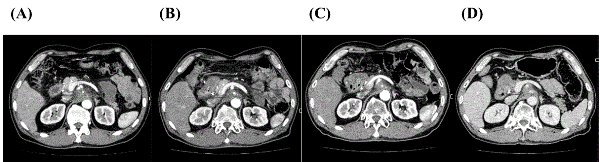
A 58-year-old male developed acute pancreatitis in August 2012 with a mass lesion of pancreas head subsequently detected by computed tomography (CT). He underwent endoscopic retrograde cholangiopancreatography with stent insertion and exfoliative cytodiagnosis (class IV) revealing localized advanced PC with involvement of the hepatic portal region from the superior mesenteric artery to the coeliac artery (Figure 1A), thereby contraindicating operative resection (Table 1).
Systemic chemotherapy with GEM and Elortinib was initiated but failed to achieve disease control according to the response evaluation criteria for solid tumors guideline. HLA-A*24:02 and DRB1*15:02 genotyping was compatible with WT1-Class I and II peptides, respectively (Table 1). One course (seven sessions, biweekly) of DC vaccination containing the modified WT1-Class I peptide (a total of 14.08 × 107 DCs) was administered from January to April 2013 with concurrent chemoradiotherapy comprising tomotherapy (45 Gy/25Fr) and S-1 chemotherapy. After one course of DC vaccination, CT scan indicated stable disease (SD) in Figure 1B. Skin reactions at intradermal injection sites and fever within 48 h of DC vaccination were tolerable, even during chemoradiotherapy. During maintenance chemotherapy with S-1, a second course of DC vaccination containing WT1-Class I/II peptides (a total of 21.48 × 107 DCs) was administered from December 2014 to March 2015. CT scan indicated SD before the second course of DC vaccination in Figure 1C. DC vaccination was found to be safe and tolerable (Table 2). S-1 chemotherapy was discontinued in April 2015 due to adverse effects on the skin with anaphoresis and frequent biliary tract infection. Follow-up CT imaging in June 2015 revealed persistence of the primary lesion without any evidence of recurrence indicating SD (Figure 1D). Progression-free survival (PFS) and OS times were 25.2 months and 32.1 months, respectively. Case 2
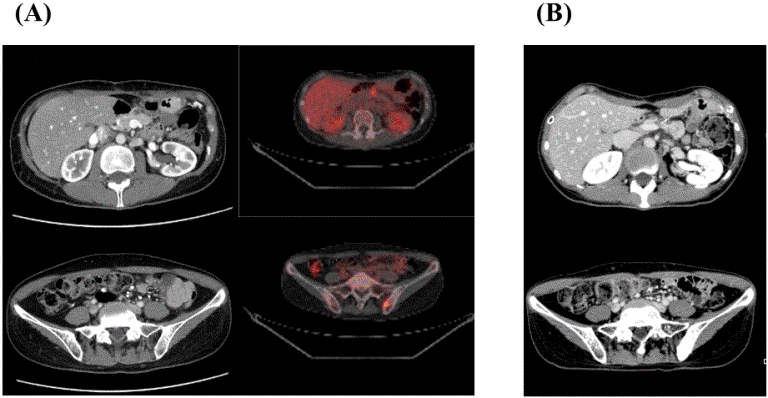
A 50-year-old female underwent resection of a cystic lesion at the pancreatic body and tail at another hospital in May 2013 with a pathological diagnosis of mucinous cystadenocarcinoma, T2N1M0 Stage III. Local recurrence with lymph node involvement and bone metastases (L5 and left iliac bone) were detected using CT (Figure 2A, left panels) and positron emission tomography (PET) imaging (Figure 2A, right panels) despite adjuvant S-1 in November 2013.
During the course of systemic chemotherapy with protein-bound paclitaxel and GEM for recurrent lesions, the patient was admitted to our hospital for DC vaccination. HLA-A, DRB1, and DPB1 genotyping indicated full compatibility with both WT1-Class I and II peptides (Table 1). One course (seven sessions) of DC vaccine pulsed with a combination of WT1-Class I/II peptides (a total of 24.70 × 107 DCs) was administered from July 2014 to October 2014 with concurrent 57.6 Gy/24Fr stereotactic external-beam radiation therapies (CyberKnife Radiosurgery) from July to September, 2014. Skin reactions at intradermal injection sites and fever within 48 h of DC vaccination were tolerable during radiotherapy (Table 2). Modified FOLFIRINOX at doses and intervals according to original regime administered after DC vaccination maintained SD status until April 2015 (Figure 2B), indicating a PFS time of 8.7 months. However, PET-CT imaging at this time revealed rapidly invasive gastric involvement and bone metastases (data not shown). The patient subsequently died of disseminated intravascular coagulation and organ failure because of cancer progression in June 2015. The OS time was 24.7 months after the initial diagnosis. Results
Peripheral blood lymphocyte subsets
Flow cytometry analysis was performed using 2 color- and 3 color-staining with lymphocyte gating to identify CD3+CD4+ helper T cells, CD3+CD8+ killer T cells, CD25+CD4+CD127+ regulatory T cells (Tregs), CD3+HLA-DR+ activated T cells, CD3-CD56+ natural killer (NK) cells, and CD19+ B cells (Table 3). In both cases, DC vaccination induced an increased proportion of CD3+CD8+ killer T cells and CD3+HLA-DR+ activated T cells in lymphocyte populations. While no increases in the proportions of Tregs or NK cells were observed following DC vaccination, a relative decrease in the proportion of CD3+CD4+ helper T compared with killer T cells and a lower proportion of B cells in the total lymphocyte population were observed. NK cells accounted for 47.0%–51.6% of lymphocytes during the second course of DC vaccination in Case 1. These findings indicate that activated killer T cells were induced by DC vaccination with no increase in peripherally-circulating Tregs during the DC vaccination course (Table 3).
Immune monitoring with tetramer analysis and enzyme-linked immunosorbent spot assay
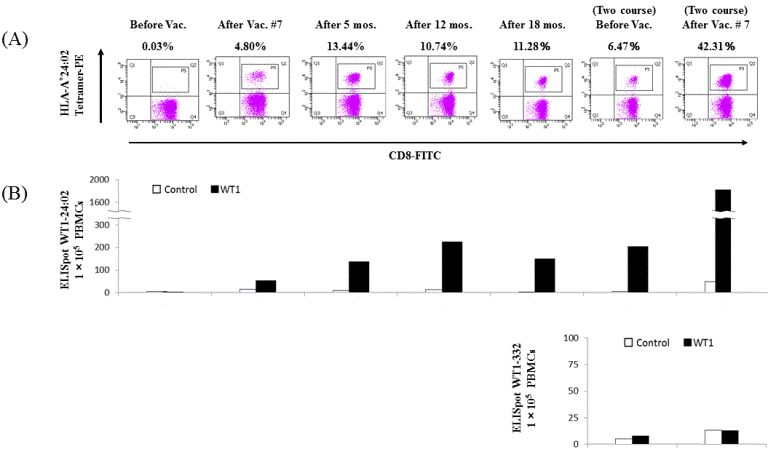
WT1-CTLs were determined by both WT1-peptide/HLA-A*24:02 tetramer analysis and/or IFN-γ-producing clones on ELISpot assays after one course of DC vaccination in both cases. In Case 1, the immune monitoring assay demonstrated that WT1-CTLs comprised 4.8% of the CD8+ T cell population (Figure 3A) with over 50 WT1-specific spots observed on ELISpot assays (Figure 3B) after one course of DC vaccination [21]. The number of WT1-CTLs was markedly increased 3 months after DC vaccination and persisted for over 1 year with S-1 chemotherapy without additional DC vaccines according to both tetramer analyses and ELISpot assays (Figures 3A and B). Further, WT1-CTLs comprised up to 45% of the total CD8+ T cell population, with nine times the number of IFN-γ-spots after the second session of DC vaccination pulsed with WT1 Class I/II peptides compared with that after the first session (Figure 3B). ELISpot assays using WT1-332 (HLA-Class II peptide) demonstrated low numbers and low specificity.
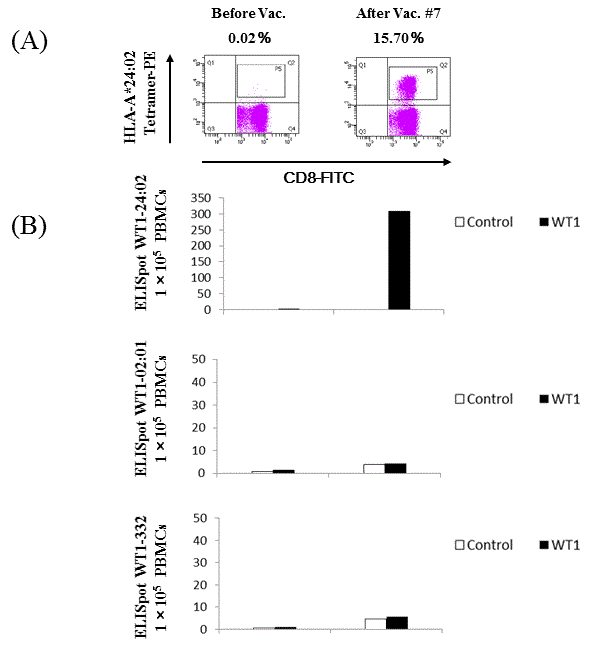
In Case 2, WT1-CTLs comprised over 15% of the total CD8+ T cell population according to the WT1-peptide/HLA-A*24:02 tetramer assay after the seventh session of WT1-Class I/II peptide-pulsed DC vaccination. Specific IFN-γ producing clones were confirmed by ELISpot assay in Case 2 (Figure 4). ELISpot assays using WT1-peptide/HLA-A*02:01 and WT1-332 (HLA-Class II peptide) failed to detect specific clones in vitro. Discussion
DCs may have antigen-specific bioactivity against tumor-associated antigens. DC vaccination during chemoradiation was found to be safe and tolerable in the two presented PC patients who received an average of 2-3 × 107 DCs during each of the seven sessions with only mild adverse reactions observed (Table 2). PFS and OS times indicated a survival benefit achieving stability of disease following DC vaccination and chemoradiation in both PC patients (Figures 1 and 2).
WT1 is reportedly a strongly immunogenic antigen [22] and HLA-A*24:02-restricted modified WT1 peptide may have considerable efficacy in promoting cancer immunity [23] as shown in Figures 3 and 4. The findings in these cases further confirmed that WT1-Class I/II peptides for DC vaccine prepared as previously described [12] were safe during chemoradiotherapy. Immunological monitoring of DC vaccination is an important validation tool in clinical studies and trials for proof of concept. Tetramer analysis and ELISpot assays are required to be reproducible and validated methods with simple, easily-reproducible protocols. Although the factors affecting the induction of WT1-CTLs have yet to be fully elucidated, the induction of immunosuppressive regulatory mechanisms in response to vaccination may play a major role [14]. Immune checkpoint inhibitors are rapidly being developed as chemotherapeutic agents [24]; however, radiotherapy allows the in situ modulation of Tregs and other suppressors in the tumoral microenvironment. Initial radiotherapy with maintenance chemotherapeutic drugs acting through off-target effects may have accelerated the development of acquired cancer immunity in both cases. Peripheral blood lymphocyte subpopulations demonstrated increased numbers of activated T cells without an increase in Tregs, following DC vaccination (Table 3). DC vaccines pulsed with WT1-Class II peptides, with a view to direct activation, may have induced the number of WT1-CTLs immunized with WT1-Class I peptides during the second DC vaccination course in Case 1 and Case 2 (Figures 3 and 4). Further studies are required to evaluate whether effecter memory T cell numbers prior to vaccination and exhausted marker of PD1-positive CTLs after DC vaccination may influence the efficacy of DC vaccination [12]. The development of combination therapies, potentially including immune checkpoint inhibitors, will improve the feasibility of personalized therapy for cancer patients [24]. Further clinical trials are required to evaluate the efficacy of combination therapies with chemoradiotherapy and determine the effect of strong induction of acquired immunity in response to DC vaccination on the prognosis of PC. Conclusion
DC vaccination with chemoradiotherapy was feasible and tolerable in PC patients. Strong induction of acquired immunity against cancer-associated antigens was detected by immune monitoring.
Acknowledgment
We thank Dr. Kojiro Tokutaka and Dr. Masahiro Kaneki for providing chemotherapeutic treatment and following up the patients.
Disclosure of Interest
All authors declare no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Abdominal Radiology
- AI in Radiology
- Breast Imaging
- Cardiovascular Radiology
- Chest Radiology
- Clinical Radiology
- CT Imaging
- Diagnostic Radiology
- Emergency Radiology
- Fluoroscopy Radiology
- General Radiology
- Genitourinary Radiology
- Interventional Radiology Techniques
- Mammography
- Minimal Invasive surgery
- Musculoskeletal Radiology
- Neuroradiology
- Neuroradiology Advances
- Oral and Maxillofacial Radiology
- Radiography
- Radiology Imaging
- Surgical Radiology
- Tele Radiology
- Therapeutic Radiology
Recommended Journals
Article Tools
Article Usage
- Total views: 14111
- [From(publication date):
August-2015 - Jul 19, 2025] - Breakdown by view type
- HTML page views : 9521
- PDF downloads : 4590
