Induced Activation of the Procoagulant Pathways and the Pathogenesis of Ischemia, Venous Thrombosis
Received: 29-Jul-2022 / Manuscript No. asoa-22-77591 / Editor assigned: 01-Aug-2022 / PreQC No. asoa-22-77591 (PQ) / Reviewed: 15-Aug-2022 / QC No. asoa-22-77591 / Revised: 18-Aug-2022 / Manuscript No. asoa-22-77591 (R) / Accepted Date: 22-Aug-2022 / Published Date: 25-Aug-2022
Abstract
A pathway is outlined through which diminished levels of oxygen activate the transcription factor early growth response-1 (Egr-1) leading to de novo transcription/translation of tissue factor in mononuclear phagocytes and smooth muscle cells, which eventuates in vascular fibrin deposition. The procoagulant response is magnified by concomitant suppression of fibrinolysis by hypoxia-mediated upregulation of plasminogen activator inhibitor-1. These data add a new facet to the biology of thrombosis associated with hypoxemia/stasis and imply that interference with mechanisms causing Egr-1 activation in response to oxygen deprivation might prevent vascular fibrin deposition occurring in ischemia without directly interfering with other pro/anticoagulant pathways.
Keywords
Atherosclerosis; Tissue factor; Hypoxia; Ischemia; Egr-1; PAI-1
Introduction
In fact, the intact, healthy vessel wall has very low levels of tissue factor with an increasing gradient toward the adventitia. The cell type most uniformly accepted as being capable of expressing substantive amounts of tissue factor within the intravascular space in response to environmental stimuli is the mononuclear phagocyte, although poly morphonuclear leukocytes may also contribute, and endothelial cells have been shown to produce tissue factor in selected settings [1]. Thus it may not be surprising that a brief period of hypoxemia and/or stasis alone in a normal vessel are not sufficient to trigger fibrin formation.
Nonetheless, links between thrombosis and hypoxemia/ stasis have remained strong even in the absence of mechanistic explanations. Studies in a canine model of limb immobilization showed stasis to be associated with a rapid fall in venous oxygen tension to virtually undetectable levels in the affected extremity [2]. Furthermore, hypoxemia was most severe in proximity to venous valve cusps, and nascent thrombi appeared to form on the apparently intact vessel wall at the parietal aspect of the valve cusp (this occurred during the period of hypoxemia). These accumulations of fibrin contained peripheral blood cells and erythrocytes and were speculated to result from hypoxemia-induced perturbation of the vessel wall. In fact, when untraumatized canine leg veins were exposed in vivo to intermittent periods of hypoxemia and reoxygenation, venous thrombi were shown to occur [3].
Therefore, although in selected circumstances hypoxemia alone is sufficient to cause venous thrombosis, blood stasis results in ischemia that is associated with a myriad of changes in the vascular microenvironment, including diminished aerobic metabolism and accumulation of waste products. Thus the ischemic environment induces cell stress on many levels in addition to a contribution resulting from diminished blood oxygen. Another setting in which hypoxia has been considered to have a central role concerns vascular perturbation in response to high altitude, termed hypobaric hypoxia. Mountain climbing and other activities at high altitude are associated with vascular dysfunction, both prothrombotic events and increased vascular permeability (high altitude pulmonary and cerebral edema), and these have been recently documented in graphic detail in the lay press.
In the setting of ischemia, the most striking tissue injury occurs during reperfusion when leukocytes pour into the previously unperfused zone. We propose that mechanisms underlying pathologic events whose consequences are exaggerated during reperfusion are set in motion during the previous period of hypoxemia. An example of this principle is the critical period during organ preservation, when hypoxemia and stasis prime the graft vasculature for damage during reperfusion.15 Such tissue injury occurring as soon as blood flow to the graft is reestablished is limited by strategies to diminish preservationinduced vascular dysfunction with more optimal preservation solutions during the period of organ storage. Our brief review will focus on mechanisms through which hypoxemia promotes fibrin formation in vivo and the results of recent studies to elucidate the bases for such activation of the procoagulant pathway [4].
A Model of Hypoxia-Induced Fibrin Deposition
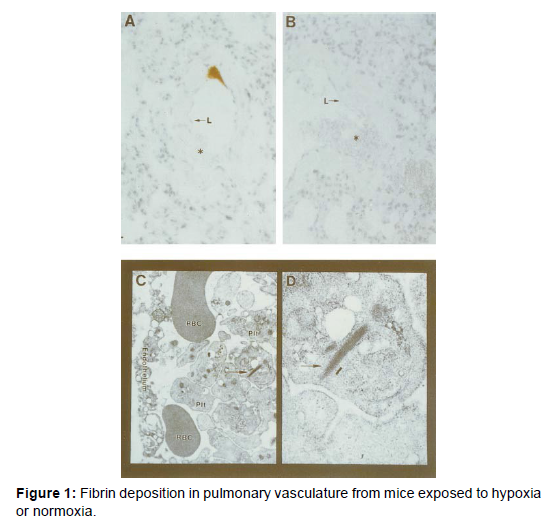
We have developed a model system to examine the effects of hypoxia on intravascular thrombus formation, and this system has also recently been adapted by others in studies of mechanisms of hypoxiainduced thrombosis. In this model, analysis of fibrin formation can employ 3 complementary techniques: morphological and immunoblotting studies with monospecific polyclonal antibody to a neo- epitope in fibrin gamma gamma chain dimers (other investigators have used antibody to human fibrin beta chains with similar ability to detect murine fibrin), electron microscopy, and deposition of radio iodinated fibrinogen. After exposure to hypoxia, vascular fibrin deposition occurs within approximately 6 hours. Immunostaining shows close association of fibrin deposits with the vessel wall (Figure 1A), compared with lack of fibrin deposits in normoxic controls (Figure 1B). Electron microscopy shows that immune reactive fibrin deposits from hypoxemic lung vasculature display ultrastructural properties of fibrin, especially the 22-nm periodicity (Figures 1C and 1D). Consistent with these observations, immunoblotting using fibrinspecific antibody and extracts from hypoxic lung treated with plasmin showed an immunoreactive band, whereas none was seen in normoxic controls or in hypoxic mice that had been pretreated with either hirudin or blocking antibody to tissue factor. These data indicate that hypoxia/ hypoxemia triggers a pathway leading to fibrin deposition in the lung.
Hypoxia-Associated Expression of Tissue Factor
Because blocking antibody to tissue factor suppressed fibrin accumulation in hypoxic murine lung, it was likely that oxygen deprivation caused increased expression of tissue factor in a compartment exposed to circulating blood. One possibility was that polymorphonuclear leukocytes (PMN) became adherent to the hypoxemic vessel wall due to translocation of Pselectin to the cell surface, cytokine mediated up regulation of intercellular adhesion molecule-1 expression. Subsequent leukocyte activation could generate reactive oxygen intermediates, damaging the vessel wall and causing exposure of tissue factor in sub endothelial layers of the vessel wall. However, antibody-induced depletion of PMNs had no effect on fibrin accumulation [5-9]. Another mechanism could be in- creased vascular permeability due to the direct effect of hypoxia on the endothelium or indirectly via hemodynamic changes (for example, pulmonary hypertension). However, increased vascular leakage in response to hypoxia occurs at later times and is not substantially different within 8 hours of being subject to the oxygen-deficient environ- ment, at which time fibrin deposition was already evident. In contrast, antibodyinduced depletion of monocytes did suppress fibrin deposition in hypoxic lung, suggesting that this cell type was likely to have an integral role in activation of the procoagulant pathway in this setting. This evidence led us to predict that monocytes were triggering the procoagulant pathway, probably by up regulating tissue factor, and platelets were subsequently amplifying procoagulant events.
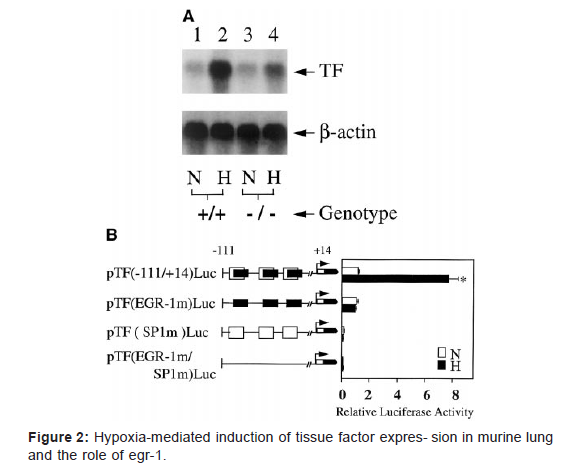
To test this hypothesis, tissue factor expression in hypoxic murine lung was analyzed. Northern analysis showed ≈20-fold increased tissue factor transcripts in hypoxic lung from wild type animals compared with normoxic controls (Figure 2A). Immunohistochemical studies demonstrated increased tissue factor in hypoxic lung, colocalized with mononuclear phagocytes from wild-type mice, compared with normoxic controls. In vitro studies confirmed that mononuclear phagocytes placed in an hypoxic environment demonstrate transcriptional up- regulation of tissue factor mRNA [10]. These data implicating tissue factor expression in hypoxia-driven thrombosis are especially provocative, given recent data showing the important role tissue factor plays in maintaining normal hemostasis. When tissue factor is expressed at very low levels in genetically altered mice, they exhibit a hemorrhagic tendency.
In depth exploration of the mechanism by which hypoxia increases tissue factor transcription led to the identification of Egr- 1 as the primary driving motif underlying hypoxia-induced tissue factor transcription. These data are in concordance with recent studies identifying an association between Egr-1 and vascular injury [11-14]. Using double- stranded DNA probes from the tissue factor promoter, electrophoretic mobility shift assays with extracts from mononuclear phagocytes showed an apparent increase in Egr DNA binding activity with no change in the DNA binding activity for Sp1 sites. The most striking data were obtained using consensus probes for Sp1 or Egr- 120; nuclear extracts showed no increase in the gel shift band with Sp1 in response to hypoxia. In contrast, similar experiments with the radiolabeled consensus Egr probe showed a gel shift band (supershifted with anti–Egr-1 antibody) in nuclear extracts from hypoxic cultures, al- though not in normoxic counterparts. Finally, transient transfection experiments were performed with constructs promoter-reporter gene constructs derived from the serum response region of the tissue factor promoter (Figure 2B). Increased expression of the luciferase reporter was seen with the wild-type construct, in which Sp1 and Egr-1 sites were intact, whereas mutational inactivation of Egr-1 sites blocked hypoxia-enhanced gene expression. Inactivation of Sp1 sites prevented both basal and hypoxia-induced expression of luciferase. Transfection studies with various promoter-luciferase reporter constructs showed that only constructs with an intact Egr site displayed increased expression in hypoxia.20 These data emphasized the role of Egr-1 in hypoxia-modulated expression of tissue factor.
In vivo studies confirmed the biological importance of Egr-1- driven tissue factor transcription by hypoxia; ho- mozygous Egr-1 null animals placed in a hypoxic environ- ment showed a much smaller increment in tissue factor mRNA, with virtually no change in tissue factor antigen (Figure 2A) and virtually no fibrin deposition compared with their counterparts left in a normoxic environment. Studies are in progress to trace the sequence of events increasing Egr-1 activation in hypoxia, and preliminary results indicate that oxygen deprivation enhances de novo Egr-1 synthesis and that this is driven by binding of ternary complex factor to ets/SRE sites in the Egr-1 promoter [15].
Hypoxia-Associated Suppression of Fibrinolysis
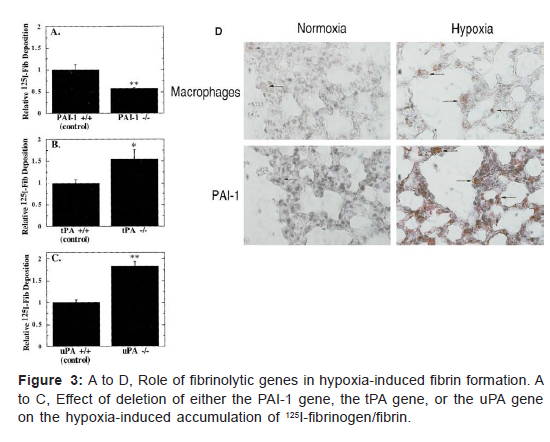
The unusual presence of fibrin within the intravascular space in hypoxic mice, even with an apparently intact endothelial cell lining, suggested that in addition to enhanced procoagulant activity, oxygen deprivation might also be associated with diminished fibrin removal. Our first studies evaluating possible hypoxia-associated suppression of anticoagulant mechanisms focused on thrombomodulin. However, hypoxia-mediate suppression of thrombomodulin expression was not observed until at least 24 hours subjecting cultures to oxygen deprivation, and fibrin deposition had already occurred in the pulmonary vasculature by this time. In contrast, analysis of the fibrinolytic system has shown coordinated enhanced expression of plasminogen activator inhibitor-1 (PAI-1) and suppression of plasminogen activators. Use of mice deficient in PAI-1, urokinase type plasminogen activator (uPA), or tissue-type plasminogen activator (tPA) provided definitive evidence for the relevance of suppressed fibrinolysis to hypoxia-induced fibrin deposition. Whereas PAI-1 null mice showed no increase in fibrin formation on exposure to hypoxia, uPA or tPA deficient mice showed a strong potentiation of fibrin deposition (Figures 3A-3C). Thus PAI-1 overexpression is likely to be an important factor preventing normally active fibrinolytic mechanisms.
From removing fibrin deposits formed in hypoxemic vasculature. In concordance with these data, mice placed under conditions of normobaric hypoxia showed increased levels of PAI-1 transcripts in the lung and, at the protein level, increased PAI-1 antigen and activity levels com- pared with normoxic controls. Immunocytochemical analysis of cells overexpressing PAI-1 in hypoxic lung pointed to an important contribution of mononuclear phagocytes18 (Figure 3D). Further studies on hypoxic lung showed a time-dependent decrease in transcripts for tissue- type urokinase-type-plasminogen activators, lending sup- port to the concept that net fibrinolytic activity of the hypoxic lung was decreased, thereby facilitating fibrin accumulation. The results of in vitro studies using trans- formed murine macrophages (RAW cells) as a model system for mononuclear phagocytes pointed to a strong effect of hypoxia on mononuclear phagocytes. RAW cells showed a 6-fold increase in PAI-1 transcription (by densitometry analysis of nuclear run blots) after placement in hypoxia. In addition to an increased rate of transcription of the PAI-1 message, there was also an apparent increase in the stability of the PAI-1 message under conditions of hypoxia. These 2 observations, the increased rate of PAI- 1 mRNA transcription and the increased stability of the PAI-1 mRNA after transcription, contributed to the marked increase in PAI-1 mRNA in hypoxic cells, which was observed by Northern analysis (Figure 4).
Conclusion
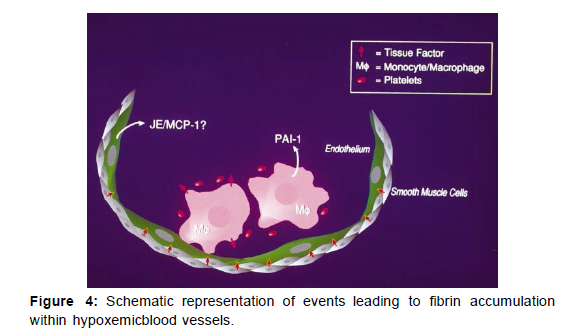
This initial picture of hypoxia-associated triggering of the procoagulant pathway raises many questions. For example, by what mechanisms are monocytes attracted and retained in hypoxemic vasculature? In a previous study, we observed that hypoxia increased transcripts for the macro- phage chemoattractant JE/MCP-1 in cultured endothelial cells and that JE/MCP-1 antigen was increased in hypoxic lung. Thus it is possible that endothelial production of JE/ MCP-1 draws in macrophages and serves as a cofactor in their activation and retention. JE/MCP-1 produced by hypoxic endothelium could also have an integral role in increased expression of tissue factor levels observed in smooth muscle cells in hypoxemic vasculature. Aside from these questions of the basic biology of the procoagulant response, recognition of the relative vulnerability of the lung to hypoxemiaassociated fibrin deposition in the mouse may also provide important insights. Although reasons for this are unclear, the combination of the lung’s rich vasculature and the intensity of the vasoconstrictor response to hypoxia, in contrast to vasodilation in the systemic circulation, may serve to magnify activation of coagulation. Taken together, the series of events causing expression of tissue factor in the hypoxemic vasculature, especially in mononuclear phagocytes and smooth muscle cells, provides a new biologic context to consider mechanisms underlying and possible interventions to prevent pathologic thrombosis in settings of oxygen scarcity.
References
- Zavodni AE, Wasserman BA, McClelland RL, Gomes AS, Folsom AR, et al. (2014) Carotid artery plaque morphology and composition in relation to incident cardiovascular events: the Multi‐Ethnic Study of Atherosclerosis (MESA). Radiol 271: 381-389.
- Polonsky TS, McClelland RL, Jorgensen NW, Bild DE, Burke GL, et al. (2010) Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA 303: 1610-1616.
- Kajinami K, Akao H, Polisecki E, Schaefer EJ (2005)Pharmacogenomics of statin responsiveness.Am J Cardiol 96: 65-70.
- Kataoka Y, John J, Wolski K, Uno K, Puri R, et al. (2015) Atheroma progression in hyporesponders to statin therapy. Arterioscler Thromb Vasc Biol 35: 990-995.
- Arad Y, Goodman KJ, Roth M, Newstein D, Guerci AD (2005) Coronary calcification, coronary disease risk factors, C‐reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. J Am Coll Cardiol 46: 158-165.
- Adamson S, Leitinger N (2011) phenotypic modulation of macrophages in response to plaque lipids. Curr Opin Lipidol 22: 335-342.
- Fu P, Birukov KG (2009) Oxidized phospholipids in control of inflammation and endothelial barrier. Transl Res 153: 166-176.
- Lehti S (2018) Extracellular lipid accumulates in human carotid arteries as distinct three-dimensional structures with pro inflammatory properties. Am J Pathol 188: 525-538.
- Westerterp M (2017) Cholesterol accumulation in dendritic cells links the inflammasome to acquired immunity. Cell Metab. 25: 1294-1304.
- Wityk RJ, Lehman D, Klag M, Coresh J, Ahn H, et al. (1996) Race and sex differences in the distribution of cerebral atherosclerosis.Stroke 27: 1974-1980.
- Dichgans M, Pulit SL, Rosand J (2019) Stroke genetics: discovery, biology, and clinical applications. Lancet Neurol 18: 587-599.
- Shafi S, Ansari HR, Bahitham W, Aouabdi S (2019) The Impact of Natural Antioxidants on the Regenerative Potential of Vascular Cells. Front Cardiovascu Med 6: 1-28.
- Ala-Korpela M (2019) The culprit is the carrier, not the loads: cholesterol, triglycerides and Apo lipoprotein B in atherosclerosis and coronary heart disease. Int J Epidemiol 48: 1389-1392.
- Esper RJ, Nordaby RA (2019) cardiovascular events, diabetes and guidelines: the virtue of simplicity. Cardiovasc Diabetol 18:42.
- Hansson GK, Libby P, Tabas I (2015) Inflammation and plaque vulnerability. J Intern Med 278: 483-493.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Yan SF, Mackman N, Kisiel W, Stern D, Pinsky D (2022) Induced Activation of the Procoagulant Pathways and the Pathogenesis of Ischemia, Venous Thrombosis. Atheroscler Open Access 7: 182.
Copyright: © 2022 Yan SF, et al.. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 1861
- [From(publication date): 0-2022 - Mar 31, 2025]
- Breakdown by view type
- HTML page views: 1408
- PDF downloads: 453