Research Article Open Access
Indices from Chrysicthys nigrodigitatus (Lacépède, 1803), Tilapia guineensis (Bleeker, 1862), Clarias gariepinus (Burchell, 1822) and Clarias anguillaris (Linnaeus, 1758) as Bioindicators of Organic Pollution in Ogun River, Southwest Nigeria
Olufelo OS1, Olufade OB2, Matilukuro YA2 Efuntoye MO2and Sowunmi AA1*
1Department of Plant Science and Applied Zoology, Olabisi Onabanjo University, Ago-Iwoye. Nigeria
2Department of Microbiology, Olabisi Onabanjo University, Ago-Iwoye, Nigeria
3Hydrobiology and Fisheries Unit, Department of Zoology, University of Ibadan, Ibadan, Nigeria
- *Corresponding Author:
- Sowunmi AA
Hydrobiology and Fisheries Unit
Department of Zoology, University of Ibadan
Ibadan, Nigeria
Tel: +234- 8054454529
E-mail: aa.sowunmi@mail.ui.edu.ng
Received date March 05, 2016; Accepted date May 16, 2016; Published date June 05, 2016
Citation: Olufelo OS, Olufade OB, Matilukuro YA, Efuntoye MO, Sowunmi AA (2016) Indices from Chrysicthys nigrodigitatus (Lacépède, 1803), Tilapia guineensis (Bleeker, 1862), Clarias gariepinus (Burchell, 1822) and Clarias anguillaris (Linnaeus, 1758) as Bioindicators of Organic Pollution in Ogun River, Southwest Nigeria. J Fisheries Livest Prod 4:187. doi: 10.4172/2332-2608.1000187
Copyright: © 2016 Olufelo OS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Fisheries & Livestock Production
Abstract
The current health status of Ogun river Southwest Nigeria was investigated using body and derived indices from Chrysicthys nigrodigitatus, Tilapia guineensis, Clarias gariepinus and Clarias anguillaris. Quality of effluents from the two abattoir located at Isheri-Olofin and Lafenwa, and the adjacent receiving water of R. Ogun were evaluated. Physico-chemical parameters exceeded the NESREA (2011) national criteria for effluent and USEPA (2002) for aquatic life. NH3-N and PO43 indicated elevated levels of organic pollution but trace metal contents has direct implication for aquatic life. Reduction in growth indices of total length, standard length, total body weight and fecundity were observed in all the species compared with previous records. Derived indices of gonadosomatic index and Fultons’ condition factor (K) exhibited similar pattern. Liversomatic index (LSI) were elevated indicative of disruption in body mechanism of the fish species arising from pollution. The remaining indices of gonadal index (GI), visceral somatic index (VSI) and percentage dress out (PDO) were significant and first reports any species from Ogun river.
Keywords
Stress; Fishes; Abattoir wastes; River health; Fecundity
Introduction
The use of finfishes as indicators of aquatic quality and health have been suggested because many observed physiological and anatomical changes, often manifest a form of stress compensating response [1]. These compensating responses classified from primary to tertiary responses; usually divert energy from critical processes .
Ogun River is one of the perennial rivers found in southwest Nigeria. It rises at approximately 8°41’N, 3°28’E with altitude of 380 m flowing for 320 km southwards into Lagos lagoon at approximately 6°35’N, 3°25’E. It has a total drainage area of about 21,800 km2. Detailed information on hydrology had been provided by Sydenham [2,3]. The degradation the river health increased with the construction of abattoirs at Lafenwa and Isheri-Olofin along the river course with capacity to process 300-320 cows and 40-50 goats/rams daily. The offal and processing wastes are washed directly into river system daily.
Previous studies on the river were limited and scattered covering composition and zonation of resident fish species [2,4]; hydrology [2,5]; food and feeding [5]; reproduction [6]; faunal changes [7], bioaccumuluation [8] and physico-chemistry [9,10]. Presented here is use of various indices from Chrysicthys nigrodigitatus (Lacépède, 1803), Tilapia guineensis (Bleeker, 1862), Clarias gariepinus (Burchell, 1822) and Clarias anguillaris (Linnaeus, 1758), four economically important fish species, as bioindicators of aquatic ecosystem health and the physico-chemistry of Ogun river and abattoir wastes from Lafenwa and Isheri-Olofin Southwest Nigeria.
The river rises in Oyo State near Shaki at coordinates 8°41′0′N 3°28′0′E and flows through Ogun State into Lagos State. The river is crossed by the Ikere Gorge Dam in the Iseyin local government area of Oyo State. The reservoir capacity is 690 million cubic metres (560,000 acre·ft).
Materials and Methods
Ch. nigrodigitatus, T. guineensis, C. gariepinus and C. anguillaris were collected between June and December 2004 directly from fisher folks after landing and transported live or dying to laboratory at Olabisi Onabanjo University, Ago-Iwoye. A total of 200 individuals were collected. Total Length (TL), Standard Length (SL), Total Body Weight (TBW) and Gutted Weight (GtW) were determined on each individual. Sexes were determined by combination of external features and dissection. Liver and gonads were separated and weighed. Ovaries were stored in Gilson’s fluid for three weeks to liberate individual eggs for determination of absolute fecundity [11]. The following indices were calculated for each individual: (1) length-weight relationship as W=aLb (2) Gonadosomatic index (GSI)=gw.tbw-1.100 (3) Gonadal Index (GI)=WO/TLb (4) Fulton’s condition factor (K)=100 tbw/TL3 (5) Liversomatic Index (LSI)=lw/tbw.100 (6) Visceral-Somatic-Index (VSI)=(tbw-gtw)/tbw.100 and (7) Percentage Dress Out (PDO)=gtw/ tbw.100; where tbw=W=total body weight, gw=gonad weight. gtw=gutted weight, lw=liver weight, TL=L=total length, WO=ovary weight, b=slope of regression line of log10 tbw and log10 TL computed using the least square method, a=the intercept [9,12-17]. The relationship among sexes for all body indices was explored using heteroscedastic t-test. Temperature, pH, Total Dissolved Solids (TDS), conductivity, Dissolved Oxygen (DO), Biochemical Oxygen Demand (BOD), nitrate-nitrogen NO3-N, ammonia–nitrogen (NH3-H), phosphate (PO4 3-), copper (Cu), iron (Fe), lead (Pb), manganese (Mn), mercury (Hg) and zinc (Zn) on momentary samples of abattoir effluent and Ogun river using combination of in situ and laboratory standard methods.
Results
Summary of morphometrics, body and derived indices from collected Ch. nigrodigitatus T. guineensis, C. gariepinus and C. anguillaris are presented on Tables 1-4. Lower mean TL=21.05±5.40 and GtW=85.68±86.41 were recorded in female Ch. nigrodigitatus (n=19) compared with lower SL, TBW, GdW and Lwt the males (n=31) (Table 1). Derived indices of GSI, K, GI, and VSI recorded higher values in Ch. nigrodigitatus females compared with LSI and PDO with higher respective values of 0.75 ± 0.53 and 102.02 ± 136.20 from the males. Mean SL, TBW, GdW, Lwt, GSI, K, GI and VSI values from females and TL, GtW and PDO from males were higher compared with total means. Reduced mean LSI was observed from the separate sexes compared with the pooled (0.95 ± 0.56) value. Mean values of TL, SL, TBW, GtW, GdW and Lwt were higher in T. guineensis females (n=32) (Table 2). Derived indices of K=4.88 ± 1.78 and VSI=119.91 ± 91.31 were higher in males (n=18) while GSI (1.45 ± 1.10), LSI (0.97 ± 0.47) and PDO (56.60 ± 23.76). C. gariepinus recorded higher mean values in females (n=15) for body measurement but higher values in males (n=35) for only LSI (0.74 ± 0.40) (Table 3). Contrary to observations in other fishes C. anguillaris recorded higher mean values in the males (n=25) for all body measurements except GdW which was higher in females (6.75 ± 13.21; n=25). All derived indices were higher in females except VSI which recorded a higher value of 20.01 ± 10.84 in males (Table 4).
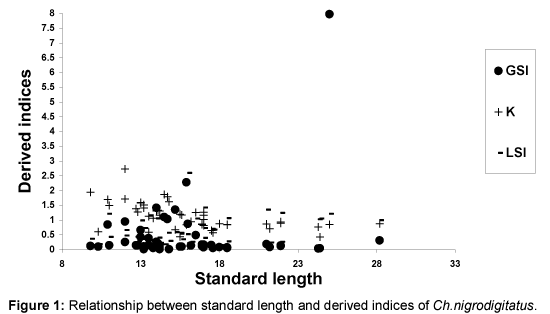
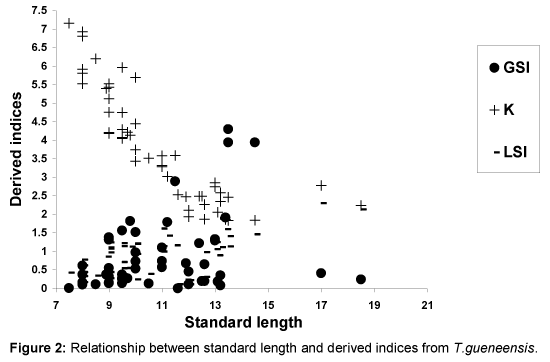
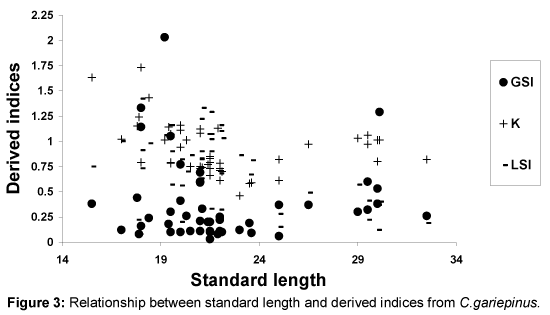
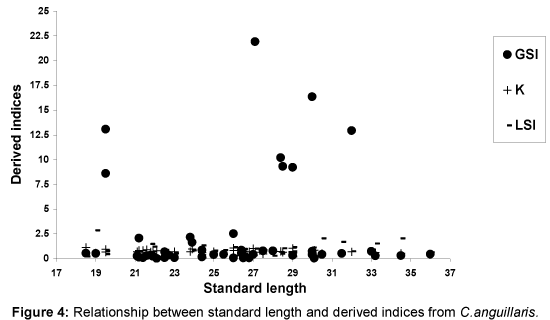
Relationships between SL and derived indices (GSI, K and LSI) are presented as Figures 1-4. Derived indices of GSI, K and LSI exhibited comparable direct, but moderate relationships with standard length (Figures 1 and 3) in Ch. nigrodigitatus and C. gariepinus. Strong direct relationships between SL with GSI and LSI in T. guineensis were depicted while K exhibited strong inverse relationship (Figure 2). The relationships between SL and derived indices were moderately direct for C. gariepinus (Figure 3). C. anguillaris exhibited irregular relationships (Figure 4).
| Parameter | Male | Female | Total |
|---|---|---|---|
| N | 31 | 19 | 50 |
| Total length (TL) (cm) | 22.82 ± 4.86 | 21.05 ± 5.40 | 22.15 ± 5.09 |
| Standard length (SL) (cm) | 16.06 ± 3.70 | 16.17 ± 4.45 | 16.10 ± 3.96 |
| Total body weight TBW) (g) | 115.24 ± 46.89 | 121.04 ± 89.44 | 117.45 ± 65.52 |
| Gutted weight (GtW) (g) | 96.26 ± 46.28 | 85.68 ± 86.41 | 92.24 ± 63.89 |
| Gonad weight (GdW) (g) | 0.13 ± 0.10 | 1.90 ± 5.76 | 0.80 ± 3.69 |
| Liver weight (Lwt) (g) | 0.70 ± 0.66 | 0.86 ± 1.15 | 0.76 ± 0.86 |
| Absolute fecundity | 2267.47 ± 8853.54 | ||
| Gonadosomatic Index (GSI) | 0.15 ± 0.17 | 1.00 ± 1.17 | 0.47 ± 1.17 |
| Condition Factor (K) | 1.01 ± 0.39 | 1.20 ± 0.53 | 1.11 ± 10.39 |
| Liversomatic Index (LSI) | 0.65 ± 0.53 | 0.59 ± 0.34 | 0.85 ± 0.56 |
| Gonadal Index (GI) | 4.37 ± 11.97 | 13.16 ± 21.94 | 7.71 ± 16.83 |
| Visceral somatic Index (VSI) | 35.45 ± 35.50 | 72.23 ± 51.31 | 49.04 ± 45.43 |
| Percentage dress out (PDO)(%) | 78.02 ± 17.08 | 65.59 ± 16.95 | 72.16 ± 18.48 |
Table 1:Body indices of Ch. nigrodigitatus from Ogunriver.
| Parameter | Male | Female | Total |
|---|---|---|---|
| n | 18 | 32 | 50 |
| Total length (TL) (cm) | 13.57 ± 3.71 | 14.26 ± 2.76 | 14.01 ± 3.12 |
| Standard length (SL) (cm) | 10.58 ± 2.79 | 11.09 ± 2.06 | 10.91 ± 2.33 |
| Total body weight TBW) (g) | 94.42 ± 52.73 | 98.27 ± 40.66 | 96.89 ± 44.88 |
| Gutted weight (GtW) (g) | 58.11 ± 54.38 | 69.58 ± 49.09 | 65.45 ± 50.81 |
| Gonad weight (GdW) (g) | 0.15 ± 0.16 | 1.29 ± 1.31 | 0.88 ± 1.18 |
| Liver weight (Lwt) (g) | 0.15 ± 0.16 | 1.07 ± 1.15 | 0.99 ± 1.25 |
| Absolute fecundity | 2734.90 ± 6280.19 | ||
| Gonadosomatic Index (GSI) | 0.15 ± 0.09 | 1.25 ± 1.10 | 0.85 ± 1.02 |
| Condition Factor (K) | 4.78 ± 1.78 | 3.63 ± 1.35 | 3.83 ± 1.53 |
| Liversomatic Index (LSI) | 0.66 ± 0.59 | 0.98 ± 0.47 | 0.86 ± 0.54 |
| Gonadal Index (GI) | 0.03 ± 0.19 | 0.27 ± 0.26 | 0.19 ± 0.24 |
| Visceral somatic Index (VSI) | 119.81 ± 91.21 | 68.06 ± 52.81 | 86.57 ± 72.62 |
| Percentage dress out (PDO) (%) | 54.60 ± 23.76 | 63.89 ± 17.46 | 60.54 ± 20.50 |
Table 2:Body indices of T. guineensis from Ogunriver.
| Parameter | Male | Female | Total |
|---|---|---|---|
| n | 35 | 15 | 50 |
| Total length (TL) (cm) | 23.79 ± 3.70 | 27.11 ± 5.79 | 24.78 ± 4.63 |
| Standard length (SL) (cm) | 21.38 ± 3.15 | 23.70 ± 4.88 | 22.08 ± 3.85 |
| Total body weight TBW) (g) | 117.58 ± 68.01 | 228.02 ± 134.73 | 150.71 ± 104.92 |
| Gutted weight (GtW) (g) | 67.40 ± 43.78 | 179.37 ± 130.97 | 100.99 ± 94.43 |
| Gonad weight (GdW) (g) | 0.25 ± 0.29 | 1.52 ± 1.19 | 0.63 ± 0.90 |
| Liver weight (Lwt) (g) | 0.76 ± 0.39 | 1.17 ± 0.43 | 0.88 ± 0.44 |
| Absolute fecundity | 4230.21 ± 7230.12 | ||
| Gonadosomatic Index (GSI) | 0.19 ± 0.14 | 0.75 ± 0.50 | 0.36 ± 0.39 |
| Condition Factor (K) | 0.86 ± 0.25 | 1.05 ± 0.73 | 0.91 ± 0.26 |
| Liversomatic Index (LSI) | 0.74 ± 0.40 | 0.63 ± 0.28 | 0.71 ± 0.37 |
| Gonadal Index (GI) | 0.002 ± 0.004 | 0.005 ± 0.00 | 0.001 ± 0.00 |
| Visceral somatic Index (VSI) | 89.68 ± 91.09 | 46.34 ± 36.32 | 76.68 ± 80.85 |
| Percentage dress out (PDO) (%) | 58.07 ± 13.57 | 71.88 ± 15.57 | 62.21 ± 15.43 |
Table 3:Body indices of C. gariepinus from Ogunriver.
| Parameter | Male | Female | Total |
|---|---|---|---|
| n | 25 | 25 | 50 |
| Total length (TL) (cm) | 30.69 ± 5.56 | 29.35 ± 4.44 | 30.05 ± 5.05 |
| Standard length (SL) (cm) | 26.75 ± 4.71 | 25.36 ± 3.68 | 26.08 ± 4.26 |
| Total body weight TBW) (g) | 196.26 ± 89.44 | 197.07 ± 86.09 | 196.81 ± 89.56 |
| Gutted weight (GtW) (g) | 160.94 ± 79.29 | 158.18 ± 80.19 | 159.61 ± 78.10 |
| Gonad weight (GdW) (g) | 5.081 ± 2.65 | 6.75 ± 13.21 | 5.88 ± 12.82 |
| Liver weight (Lwt) (g) | 1.48 ± 1.59 | 1.45 ± 0.82 | 1.46 ± 1.27 |
| Absolute fecundity | 7878.10 ± 10719.78 | ||
| Gonadosomatic Index (GSI) | 2.39 ± 5.51 | 2.53 ± 4.12 | 2.64 ± 4.84 |
| Condition Factor (K) | 0.65 ± 0.15 | 0.71 ± 0.18 | 0.68 ± 0.17 |
| Liversomatic Index (LSI) | 0.78 ± 0.57 | 0.83 ± 0.55 | 0.78 ± 0.56 |
| Gonadal Index (GI) | 1.72 ± 4.26 | 2.25 ± 4.32 | 1.97 ± 4.25 |
| Visceral somatic Index (VSI) | 19.01 ± 10.84 | 17.09 ± 28.95 | 18.09 ± 21.31 |
| Percentage dress out (PDO) (%) | 80.68 ± 10.84 | 82.90 ± 28.95 | 81.90 ± 21.31 |
Table 4:Body indices of C.anguillarisfrom Ogunriver.
Significant difference between sexes was observed only for GI in C. gariepinus (Table 5). Regression equations for males, females and the pooled sexes of all the species are presented below:
Ch. nigrodigitatus
W=- 0.2554 L1.6788 (r2=0.3445) male
W=-0.5869 L1.9784 (r2=0.6504) female
W=-0.3076 L1.7354 (r2=0.4322) pooled
T. guineensis
W=0.4679 L1.3138 (r2=0.7997) male
W=0.5765 L1.2153 (r2=0.6306) female
W=0.5238 L1.2621 (r2=0.7140) pooled
C. gariepinus
W=-0. 5934 L1.9168 (r2=0.5893) male
W=-1.5593 L2.7001 (r2=0.8986) female
W=2.700 L-0.4012 (r2=0.0178) pooled
C. anguillaris
W=-1.2463 L2.3592 (r2=0.8079) male
W=-1.8028 L2.7578 (r2=0.7447) female
W=-1.4306 L2.4931 (r2=0.7676) pooled
The values of exponent b from C. anguillaris in all cases ranged between 2.3592-2.7578, with the females (b=2.7578) closest to 3, the ideal b value. The values from females C. gariepinus (b=2.7001) also close to the ideal value; but the b values for males and pooled varying significantly. b values for Ch. nigrodigitatus (1.6788-1.9784) and T. guineensis (1.2153-1.3138) the ideal value.
| Parameters | Ch. nigrodigitatus | T. guineensis | C. gariepinus | C.anguillaris |
|---|---|---|---|---|
| Total length (TL) | 1.1664 | -0.6886 | -2.0497 | 0.9398 |
| Standard length (SL) | -0.0938 | 0.6699 | -1.741 | 1.1708 |
| Total body weight TBW) | -0.2614 | -0.2685 | -3.0143 | 0.1986 |
| Gutted weight (GtW) | -0.7707 | -0.7409 | -3.2342 | 0.2264 |
| Gonad weight (GdW) | -1.3356 | -4.798 | -4.0386 | -0.4554 |
| Liver weight (Lwt) | -0.5703 | -0.6164 | -3.1128 | 0.0887 |
| Gonadosomatic Index (GSI) | -2.045 | -5.5876 | -4.1733 | -0.1025 |
| Condition Factor (K) | -1.8228 | 1.1392 | -2.5426 | -1.2878 |
| Liversomatic Index (LSI) | 0.4708 | -1.9875 | 1.0541 | -0.6675 |
| Gonadal Index (GI) | -1.6051 | -5.1989 | 2.7583 | -0.4321 |
| Visceral somatic Index (VSI) | -0.0399 | 0.6431 | -3.2342 | 0.3057 |
| Percentage dress out (PDO) | -0.071 | -1.6537 | -2.9835 | -0.3057 |
Table 5:Comparison of body and derived indices between sexes t0.05=2.021 t0.01=2.704.
Summary of physico-chemistry of abattoir wastes and river Ogun at Lafenwa and Isheri-Olofin respectively is presented on Table 6. Temperature of 31°C and 29.5°C and pH of 6.8 and 7.8 were recorded at the respective locations. Isheri-Olofin recorded higher Total Dissolved Solids (TDS) (1700 mg/l) and conductivity (220 mg/l) compared with Lafenwa (600 mg/l and 147.5 mg/l). DO concentration from water (7.20 and 3.14 mg/l) compared with higher BOD values from the wastes (15.6 and 13.92 mg/l). Nutrient parameters of nitratenitrogen (NO3-N), ammonia-nitrogen (NH3-N) and phosphate (PO4 3-) differ greatly between the two locations. NO3-N and NH3-N was higher at Isheri-Olofin, but PO4 3- concentration was higher (22.23 mg/l) at Lafenwa. Concentrations of trace metals were elevated at Isheri-Olofin. Zinc (Zn) had the highest concentration of 18.25 mg/l and mercury (Hg) the least of 0.089 mg/l. Concentrations of Manganese (Mn) recorded the least 0.074 mg/l and Iron (Fe) the highest of 0.195 mg/l at Lafenwa. Copper (Cu), Lead (Pb) and Mercury (Hg) were not detected in the effluent from Lafenwa.
Discussion
The presence of unwanted materials in aquatic environment has been known to cause subtle or robust damages to aquatic life forms effects of which can be used to detect spatio-temoral changes and distribution of the pollutants [18-22]. This is because the ultimate effect is biotic integration into body mechanisms.
| Parameters | Abattoir waste | Ogun river | NESREA -2011 effluent limits | NESREA -2011 Fisheries limits | USEPA (2002) | |||
|---|---|---|---|---|---|---|---|---|
| Lafenwa | Isheri-Olofin | Lafenwa | Isheri-Olofin | 1CMC | 2CCC | |||
| Tempearture (°C) | 31 | na | 27.5 | 30 | n.s | n.s | 35 | 35 |
| pH | 6.8 | 7.8 | 6.9 | 6.2 | 6.5-8.5 | 6.5-8.5 | 6.5-9 | 6.5-9 |
| Total Dissolved Solids (TDS) (mg/l) | 600 | 1700 | 250 | 126 | 2000 | Ns | 250 | 250 |
| Conductivity (µS¯1cm¯1) | 147.5 | 220 | 85.4 | 280 | n.s | ns | ns | ns |
| Dissolved oxygen (DO)(mg/l) | 2.44 | 2.08 | 7.2 | 3.14 | 4 | 3 | 3 | |
| Biochemical Oxygen Demand (BOD)(mg/l) | 15.6 | 13.92 | 4.66 | 7.52 | 6 | 3 | ns | ns |
| Nitrate-Nitrogen (NO3-N) (mg/l) | 0.018 | 133.1 | 0.015 | 94.7 | 40 | 9.1 | 1 | 1 |
| Ammonia-Nitrogen (NH3-N)(mg/l) | 25.28 | 59.92 | 22.5 | 27.78 | 2 | 0.05 | 0.85 | 11.5 |
| Phosphate (mg/l) | 22.23 | 6.88 | 4.45 | 3.98 | 3.5 | 3.5 | 0.0001 | 0.0001 |
| Copper (Cu)(mg/l) * | ND | 1.08 | ND | 0.16 | 0.01 | 0.001 | 0.013 | 0.009 |
| Iron (Fe)(mg/l) | 0.195 | 2.83 | 0.451 | 0.25 | 0.5 | 0.05 | 1 | 1 |
| Lead(Pb)(mg/l)# * | ND | 0.81 | ND | 0.079 | 0.1 | 0.01 | 0.065 | 0.0025 |
| Manganese (Mn)(mg/l) | 0.074 | 0.81 | 0.112 | 0.27 | 5 | ns | 0.1 | 0.1 |
| Mercury (Hg) (mg/l) #* | ND | 0.089 | ND | 0.004 | 0.0005 | 0.001 | 0.0014 | 0.0007 |
| Zinc (Zn)(mg/l)* | 0.169 | 18.25 | 0.32 | 10.2 | 0.2 | 0.01 | 0.12 | 0.12 |
| ND: Not Detected, na: Not Available, ns: Not Specified. *USEPA designated priority pollutant; #NESREA designated hazardous substance. 1: Criterion continuous concentration (CCC): to protect against chronic effects. 2: Criterion maximum concentration (CMC): to protect against acute and lethal effects. | ||||||||
Table 6:Physical and chemical quality of abattoir wastes and Ogun river.
Fish growth is considered a good indicator of fish and aquatic health because it manifests the influence of biotic and abiotic variables from the inhabited environment [14,23]. Bonga stated that growth rates reflect food availability, appetite and consumption, intestinal uptake and metabolic rates, all of which can be influenced by stressors [1].
Fish species from our study were apparently affected by the prevailing water condition as shown by reduction in growth indices (TL, SL and TBW) compared with reports on Ch. nigrodigitatus [24- 30]; T. guineensis [26,31]; C. gariepinus [32-36] and C. anguillaris [37]. Adebisi in his studies recorded a range 10.40 -58.20 cm in TL for fishes from Ogun river, although none of the species in our own study was included, this differ from a range of 10.00-41.00 in our study [6]. Further supporting our submission of steady reduction in sizes of fishes resident in Ogun river.
While it is important not to overlook gear selectivity as suggested by Fagade [26]. The possibility of gear selectivity as size factor simultaneously for species in this study is severely limited. Disturbance in body mechanisms possibly due to chronic organic pollution appeared a more viable determining factor.
Although, Fafioye and Oluajo [36] on Ch. nigrodigitatus (TL=21.75 ± 7.55 and TBW=85.5 ± 69.5) and C. gariepinus (TL=15.5 ± 8.5 and TBW=143.0 ± 132.0) from Epe lagoon and on C. anguillaris (TL=26.0 and SL=23.0) from Owena reservoir respectively reported values lower than our own observations reasons were not clearly stated for size observations.
Liver as a growth indicator is sensitive to food intake in fishes under variety of environment, especially stressed conditions and pollution impacts [14,37,38]. Differences in liver weight among sexes, as observed in this study, had been previously suggested [39-41] as indicative of allocation of resources to gonad development in females.
Comparable information on sex-related liver weight on Ch. nigrodigitatus, T. guineensis and C. anguillaris agreed largely with the conclusions of Htun-Han [39], Hussain et al. [40], Lambert and Dutil [41] and van der Oost et al. [38] on changes in liver weight due to environmental stress.
Reduction in reproductive potential was apparent in Ch. nigrodigitatus, T. guineensis and C. gariepinus compared values reported by Fagade [25,26,29,30,42]. Similar conclusions were difficult to draw for and C. anguillaris due lack of previous reports. However, when compared with post-oil spill reports of Ekwu [42], probability of reduction in reproductive potential is high because the values were not so incongruous. Effects of oil pollution are severe, immediate and obvious, unlike subtle and cumulative nature of organic pollution.
The use of GSI as index of the relationship between ovary weight and fecundity has been demonstrated [43]. The reported mean GSI for all species were very low compared with those from previous studies. Fagade and Adebisi [25] reported a range of 5-50 for Ch. nigrodigitatus; and Ekwu [42] 15.28 ± 2.22-18.6 ± 1.89 and 13.2 ± 3.73-14.9 ± 2.75 from pollution episode from Cross river for same species. Ofori- Danson [44] calculated a maximum GSI of 6.5 for Ch. nigrodigitatus from Volta lake in Ghana.
Tsadu and Adebisi [32] reported 0.225-0.594 and 4.732-11.511 respectively for male and female C. gariepinus. This was lower than 12.28 ± 9.85 -14.79 ± 2.75 later reported [42]. T. guineensis and C. anguillaris were not reported with GSI values from available literature. However with mean GSI of 0.85 ± 1.02, T. guineensis, recorded significantly lower values compared with cichlids from Ogun river [6], Sombreiro river [45] and Cross river [42].
GSI reported for C. anguillaris was low compared values reported by Ekwu [42] for C. gariepinus, a comparable fish species. In contrast to observations on cichlids having reduced GSI due frequency of spawning, as reported by Adebisi [6] and Ekwu [42], only C. anguillaris had higher GSI compared with T. guineensis.
Information on gonad weights in males from earlier reproductive studies involving species used in this study was not available.
Generally males recorded lower gonadal weights compared with females, similar to observations of Hussain et al. [40], Tsadu and Adebisi [32] and Buchtová et al. [46].
Reduced fecundity is regarded as robust indicator of toxicity, because agents of pollution interact with endocrine control mechanisms of reproduction [1,38].
T. guineensis recorded the highest K value in our study, comparable only to the minimum (K=3.77-5.30) reported by Fagade [26] for the species. Present conditions [14,37], past condition [37] nutritional status [37] and environmental stress [14,42,43] have been clearly linked to low or reduced K. This shows that fishes from our study were not in the desirable condition.
C. gariepinus was the only species with previous reports of LSI which Tsadu and Adebisi [32] as a measure of food availability. LSI of C. gariepinus from our study were clearly elevated. The sensitivity of liver to environmental changes [37,44-62] and pollution [14,38] greatly influence Lwt and LSI.
Toxicants had also been reported to cause both increase and decrease in liver depending on the type of pollutant [14,38]. The mean LSI ( < 1) for all species reported was much lower compared with 1.75 [40]; 1.1 ± 0.1 [14] and 1.4 ± 0.3 [17] as their least values. Fishes from our study were clearly experiencing some pathology.
The remaining derived indices of GI, VSI and PDO have not been reported for the species under study or used as indicator of aquatic health.
GI combines information on fecundity and length weight relationship, signifying the magnitude of alteration in resource allocation in fish, due to integration of pollutants. This was however extended in this study to include both sexes. The influence of sex is shown by difference of the GI value from gonad weight (Tables 2-5). Schulz and Martins-Junior [15] reported GI values for Astyanax fasciatus only females. Variations in GI in our observations probably reflected the toxic effects of pollutants on fecundity as suggested by Wandeerlar-Bonga [1].
The extent of lipid storage in the viscera of individual fish is described by VSI and PDO. The high values of PDO of above 50% in all cases indicated some consistency in energy allocation, with highest allocation by Ch. nigrodigitatus and least by T. guineensis.
The Length-Weight Relationship (LWR) reported clearly defined the diversed body configuration associated with fish species from Nigerian waters in agreement with King [28], Fafioye and Oluajo [36]. C. anguillaris for both sexes and pooled and, C. gariepinus females clearly approximated allometric growth. However, Ch. nigrodigitatus and T. guineensis in all cases have the tendency to become thinner with size. This was also the case with C. gariepinus males and pooled [12]. Tsadu and Adebisi [32] reported similar sexual differences growth configuration in C. gariepinus. In contrast Ch. nigrodigitatus (b=3.042 ± 0.003) and C. gariepinus (b=2.880 ± 0.15) populations from Epe lagoon were apparently allomeric in their growth configurations.
The observed reduction in size as with other body indices can be associated with immediate environment. It is therefore possible that reallocation of metabolic resources away from growth towards activities requiring intensification to restore homeostasis [1]. This continuous reallocation towards homeostatic balance, under prevailing conditions, could be responsible for the observed variability in relationships between length and derived indices.
The prevailing conditions in Ogun river has changed over time, as evident by differences of physico-chemical parameters compared with Adebisi [6], Odukoya [47], Asonye [10] and Jaji et al. [9]. The limited abiotic measurements previously reported on Ogun river were within allowable Nigerian limits with some exceptions from Asonye et al. [10]. Our study departed significantly, because more parameters were evaluated most of which exceeded USEPA [48] and NESREA [49] limits. NH3-N and PO4 3-, two of parameters for detecting organic pollution [50] were above allowable limits in both wastes and aquatic life for all locations. This was similar to observations of Osibanjo and Adie on Bodija abattoir Ibadan, Southwest Nigeria (NH3-N=62-159; PO4 3-=142-180). The presence of NH3-N at elevated concentration may reduce growth, fecundity and survival of fishes [51-53], although toxicity is influenced by actions of pH and presence of certain heavy metals [51,53].
The effects of excessive PO4 3-on fish species are indirect, either via enhanced production of blue-green algae which is of low nutritive value to most fishes [51,54] or plankton die-offs after a bloom [51]; Pearl and Tucker, associated with peak-fall cycle of nutrients [21,51,54]. The effect is reduced DO from high BOD requirement of decomposition with many physiological implications for fish species [51,52]. Similar observations were reported from Orogodo river, South-south Nigeria, due to inputs of abattoir wastes [56], while elevated conductivity in Delimmi river Jos Nigeria, was traced to organic pollution [57-73].
Conclusion
Indices from finfishes as environmental sentinel is a robust and premium tool with an advantage of being inexpensive. The results from this study indicated that Ogun river is under stress from organic loading associated with the abattoirs from Lafenwa and Isheri-Olofin. Ch. nigrodigitatus, T. guineensis, C. gariepinus and C. anguillaris manifested this aquatic stress differently for comparable parameters. However the paucity of information on this aspect of the species created some difficulties in establishing benchmarks. Further evaluations of the species used in our study, and other abundant but less economically important species as observed in the use of fishes as bioindicators in other part of the world is highly desirable.
References
- Bonga WSE (1999) The stress response in fish.Physiol Rev 77: 591-625.
- Sydenham DHJ (1977) The qualitative composition and longitudinal zonation of fish fauna of the River Ogun, Western Nigeria. Revue de ZoologieAfricaine 91: 974-997.
- AdebisiAA (1981) Thephysico-chemical hydrology of a tropical seasonal river-upper Ogun river. Hydrobiologia79: 157-165.
- Hugueny B (1989) West African rivers as biogeographic islands: species richness of fish communities. Oecologia 79: 236-243.
- AdebisiAA (1981) Analysis of stomach content of pscivorous fishes of the upper Ogun River in Nigeria.Hydrobiologia 79: 167-177.
- AdebisiAA (1987) Therelationship between fecundities, gonadsomatic indices and egg size of some fishes of Ogun river, Nigeria. ArchivfürHydrobiologie 111: 151-156.
- AdebisiAA (1988) Changes in structural components of fish community of seasonal river. ArchivfürHydrobiologie 113: 457-463
- AdeniyiAA, YusufKA,OkedeyiOO (2008) Assessment of the exposure of two fish species to metals pollution in the Ogun river catchments, Ketu, Lagos, Nigeria. Environmental Monitoring and Assessment 137: 451-458.
- Jaji MO, Bamgbose O, Odukoya OO, Arowolo TA (2007) Water quality assessment of Ogun River, South West Nigeria. Environmental Monitoring and Assessment 133: 473-482.
- Asonye CC, Okolie NP, Okenwa EE, Iwuanyawu UG (2007) Some physicochemical characteristics and heavy metal profiles of Nigerian rivers, streams and waterways.African Journal of Biotechnology 6: 617-624.
- Bagenal TB, Tesch FW (1978) Age and growth. In ed. Bagenal TB, In Methods for assessment of Fish production in freshwaters. Blackwell Science Publications Ltd. Oxford: 101-136.
- Le Cren ED (1951) The length-weight relationship and seasonal cycle in gonad weight and condition in the perch, (Percafluviatilis).Journal Animal Ecology 20: 201-219.
- Bagenal TB, Braum E (1978) Eggs and early history. In: Ricker WE(ed.) Methods for assessment of Fish production in freshwaters. International Biological Program Handbook. Blackwell Science Publications Ltd. Oxford: 165-201.
- Hoque MT, Yusoff FM, Law AT, Syed MA (1995) Effect of hydrogen sulphide on liver somatic index and Fulton’s condition factor in Mystusnemurus.Journal of Fish Biology 52: 23-30.
- Schulz UH, Junior MH (2001) Astyanaxfasciatus as bioindicator of water pollution of Rio Dos Sinos, RS, Brazil. Brazilian Journal of Biology 61: 615-622.
- Alberto A, Camargo AFM, Verani JR, Costa OFT, Fernandes MN (2005) Health variables and gill morphology in the tropical fish Astyanaxfasciatus from sewage-contaminated river. Ecotoxicology and Environmental Safety 61: 247-255.
- Torre DLFR, Ferrari L, Saliban A (2005) Biomarkers of a native fish species (Cnesterodondecemmaculatus) application to the water toxicity assessment of a peri-urban polluted river of Argentina. Chemosphere 59: 577-583.
- Freidrich G, Chapman D, Bein A (1992) The use of biological material. In:Chapman D (ed.)Water Quality Assessments: A guide to the use of biota, sediments and water in environmental monitoring. Pub.: Chapman and Hall for UNESCO, WHO and UNEP: 171-237.
- Graham JH, Freeman DC, Emleen, JM (1993) Developmental stability: A sensitive indicator of populations under stress. In: Environmental Toxicology and Risk Assessment: 136-158.
- CY, Sowunmi AA (2000) Algal Biomonitoring: a case study of River Owo and Ologe lagoon, Agbara South-west Nigeria. Journal of Science Engineering and Technology 7: 2404-2422.
- Ayoade AA, Sowunmi AA, Nwachukwu HI (2004) Gill asymmetry in Labeoogunensis from Ogunriver Southwest Nigeria. Revista de Biologia Tropical52: 171-175.
- Jeje CY, Winful AN, Sowunmi AA (2003) Nutrient characteristic of cowdung and composite of chicken manure of brewers’ wastes.Asian Journal Microbiology, Biotechnology and EnvironmentalSciences 5: 151-156.
- Anene A (2005) Condition factor of four cichlids species of a man-made lake in Imo state, Southeastern, Nigeria. Turkish Journal Fisheries AquaticSciences 5: 43-47.
- Ikusemiju K (1975) A comparative racial study of the catfish, Chrysicthysnigrodigitatus(LACÉPÈDE) from Lagos and Lekki Lagoons Nigeria. Bulletin de L’IinstitutFrançais d’ Africaine Noire 37A: 887-898.
- Fagade SO, Adebisi AA (1979) On the fecundity of chrysichthysnigrodigitatus (Lacépède) of the Asejire dam, Oyo State, Nigeria. Nigerian Journal Natural Sciences 1: 127-131.
- Fagade SO (1978) On the biology of tilapia guineensis (Dumeril) from the lekki lagoon, Lagos state, Nigeria. Nigerian Journal Science 12: 73-83.
- Obiekezie AI, Enyeheni UK (1988) Henneguyachrysicthyi sp. nov. (Protozoa: Myxozoa) from the gills of esturine catfish Chrysichthysnigrodigitatus (Lacépède) (Pisces: Bagridae) in Nigeria. Revue de ZoologieAfricaine 102: 33-42.
- King RP (1996) Length-Weight relationship of Nigerian Coastal water fishes. NAGA, The ICLARM Quarterly 19: 53-58.
- Ekanem SB (2000) Some reproductive aspects of Chrysichthysnigrodigitatus (Lacépède) from Cross River, Nigeria.NAGA, The ICLARM Quarterly 23: 24-28.
- Fapohunda OO, Godstates R (2007) Biometry and composition of fish species in owenareservoir, Ondo State, Nigeria.Journal of Central European Agriculture 8: 99-104.
- King RP (1997) Length-Fecundity relationship of Nigerian fish population. NAGA, The ICLARM Quarterly 20: 29-33.
- Tsadu SM, Adebisi AA (1997) Comparison of condition indices of pond raised and wild populations of African catfish, Clariasgariepinus (Burchell,1822) (Pisces-Clariidae) in Plateau and Niger states, Nigeria. Journal of Aquatic Sciences 12: 49-58.
- Eyo JE (2002) Conspecific discrimination in ratio morphometric character among members of the Pisces Genus: ClariasScopoli. The Zoologist 2: 23-34
- Sowunmi AA (2003) Heamatology of the African catfish Clariasgariepinus (Burchell, 1822) from Eleiyele reservoir Ibadan, Southwest Nigeria. The Zoologist 2: 85-91.
- Sowunmi AA (2005) Incidence of gill asymmetry in Oreochromisniloticus (L.) Clariasgariepinus (Burchell, 1822) from Asejire reservoir, Southwest Nigeria. West African Journal Applied Ecology 7: 135-140.
- Fafioye OO, Oluajo OO (2005) Length-weight relationships of five fish species in Epe lagoon, Nigeria. African Journal of Biotechnology 4: 749-751
- Lambert Y, Dutil JD (1997) Can simple condition indices be used to monitor and quantify seasonal changes in the energy reserves of Atlantic cod (Gadusmorhua). Canadian Journal of Aquatic Sciences54: 104-112.
- Oost VR, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environmental Toxicology and Pharmacology 13: 57-149.
- Htun-Han M (1978) The reproductive biology of the dab Limandalimanda (L.) in the North Sea: goandosomatic index, hepatosomatic index and condition factor. Journal of Fish Biology 13: 369-378.
- Hussain MG, Rao GPS, Humayun NM, Randall CF, Penman DJ, et al.(1995) Comparative performance of growth, biochemical composition and endocrine profile in diploids and triploids tilapia Oreochromisniloticus L. Aquacutlture 138: 87-97.
- Dutil JD, Castonguay M, Gilbert D, Gascon D (1999) Growth, condition and environmental relationships in Atlantic cod (Gadusmorhua) in the northern Gulf of St. Lawrence and implications for management strategies in the Northwest Atlantic. Canadian Jornal of Aquatic Sciences 56: 1818-1831.
- Ekwu AO (2006) Impact of oil spill on the fecundity and goandosomatic index of icthyo faunal speciesin the Cross river coastal waters. Pollution Research 25: 213-216.
- Ofori-Danson PK, de Graaf GJ, Vanderpruye CJ (2002) Population parameters estimates for Chrysichthysauratus and C. nigrodigitatus (Pisces: Claroteidae) in Lake Volta, Ghana. Fisheries Research 54: 267-277.
- Jons GD, Miranda LE (1997) Ovarian weight as an index of fecundity, maturity and spawning periodicity. Journal of Fish Biology 50: 150-156.
- Nwadiaro CS (1987) Fecundity of cichlid fishes of the Sombreiroriver in the lower Niger Delta.Revue de ZoologieAfricaine 101: 433-437.
- Buchtová H, Svobodová Z, Flajšhans M, Vorlová L (2003) Analysis of growth, weight and relevant indices of diploid and triploid population of tenchTincatinca (Linnaeus 1758). Aquaculture Research 34: 719-726.
- Odukoya OO (2000) Pollution trends in ogun river, abeokuta, nigeria. Nigerian Journal Science 34: 183-186.
- United States Environmental Protection Agency (USEPA) (2002) National Recommended Water Quality Criteria.
- National Environmental Standards and Regulations Enforcement Agency (NESREA) (2011).
- Saad MAH, Amuzu AT, Biney C, Calamari DI, Imevbore AM, et al (1994) Domestic and Industrial organic loads. InReview of pollution in the African aquatic environment. CIFA TechnicalPaper 25, 23-31.5.
- Boyd CE (1979) Water quality in warm water pond. Auburn University, Agricultural Experimental Stations: 353.
- Alabaster JS, Lloyd RS (1982) Water quality criteria for freshwater fish.Butterworth Scientific, London 1-382.
- Beaumont MW, Butler PJ, Taylor EW (1995) Plasma ammonia concentration in brown trout in soft acidic water and its relationship to decreased swimming performance. The Journal ExperimentalBiology 198: 2213-2220.
- Pearl HW, Tucker CS (1995) Ecology of blue-green algae in aquaculture ponds. Journal of the World Aquaculture Society 26: 109-131.
- Sowunmi AA, Jeje CY (2004) Response of food organisms to inorganic nitrogen availability. Global Journal Pure and Applied Sciences 10: 243-248.
- Arimoro FO, Ikomi RB, Iwegbue CMA (2007) Ecology and abundance of oligochaetes as indicators of organic pollution in an urban stream in Southern Nigeria. Pakistani Journal Biological Sciences 10:446-453.
- Kelly MG, Ali AD (1993) The effect of organic pollution on algal communities in a tropical pond. Tropical Freshwater Biology 3: 353-370.
- APHA (1996) Standard methods for the examination of wastes and wastewater. American Public Health Association, Washington DC
- AwaJ, AnyanwuP, EzenwaB (1988) Incidence of parasitic infection of pond raised Tilapia species and some cultivable fish species from three ecological areas of Lagos State.Nigerian Inst. for Oceanography and Marine Research: 1-24.
- ColeMB, ArnoldDE, WattenBJ,KriseWF (2001) Heamatological and physiological responses of brook charr, to untreated and limestone-neutralized acid mine drainage. Journal Fish Biology 59: 79-91.
- Curvin-AralarMLA,AralarEV (1993) Effects of long-term exposure to a mixture of Cadmium,Zinc and inorganic Mercury on two strains of Tilapia niloticus. Bulletin of Environmental Contamination and Toxicology 50: 891-897.
- LandisWG, HughesJS, Lewis MAeds. ASTM STP 1179, American Society for Testing and Materials, Philadelphia,pp: 136-157.
- HaylorGS (1993) Aspects of the biology and culture of the African catfish, Clariasgariepinus (Burchell, 1822) with particular reference to developing African countries. Recent AdvancesAquaculture 4: 235-289.
- HovatterPS, RossRH (1995)Acomparative analysis of the toxicity of Boron compound to freshwater and saltwater species.In: Hughes JS, BiddingerGR, Mones E (eds) Environmental Toxicology and Risk Assessment,American Society for Testing and Materials, Philadelphia 3: 288-301.
- KingRP (1998) Weight-Fecundity relationship of Nigerian Coastal water fishes. NAGA, The ICLARM Quarterly 21: 33-36.
- KotzeP, du PerezHH, van VurenJHJ (1999) Bioaccumulation of Copper and Zinc in Oreochromismossambicus and Clariasgariepinus, from the Olifants River, Mpumalanga, South Africa. Water South Africa25: 99-110
- LambertY, DutilJD (1997) Condition and energy reserves of Atlantic cod (Gadusmorhua) during the collapse of the northern Gulf of St. Lawrence stock. Canadian Journal of Aquatic Sciences54: 2388-2400.
- National Environmental (Surface and GroundWater Quality Control) Regulations.S.I. 22.
- WepenerV, VurenJHJ, Du PreezHH (1992)Uptake and distribution of a copper iron and zinc mixture in gill, liver and plama of freshwater teleost, Tilapia sparrmani. Water South Africa 27: 99-108.
- World Health Organization WHO (1993) Guidelines for Drinking water quality. International reference point for standard setting and Drinking water safety.Geneva
- Abrahim AT (2007) Distribution patterns of some heavy metals and pollution induced changes in some organs of three Nile fish species from Assiut, Egypt. AssiutUnivEgypt.
- Anwar SM, El Shafy AA, El Serafy SSM, Ibrahim II, Ali EA (2001) Accumulation of trace elements in fish at lake quaroun as a biomarker of environmental pollution. J Egypt GerSoc Comp PhysiolZool 36: 46-65.
- Mekkawy IAA, Mahmoud UM, Ibrahim ATA (2008) Heavy metal distribution and their corresponding damage effect in some organs of Bagrusbajad (Forsskal, 1775) from three localities at Assiut. EgyptJ Egypt GerSocZool54: 119-137
Relevant Topics
- Acoustic Survey
- Animal Husbandry
- Aquaculture Developement
- Bioacoustics
- Biological Diversity
- Dropline
- Fisheries
- Fisheries Management
- Fishing Vessel
- Gillnet
- Jigging
- Livestock Nutrition
- Livestock Production
- Marine
- Marine Fish
- Maritime Policy
- Pelagic Fish
- Poultry
- Sustainable fishery
- Sustainable Fishing
- Trawling
Recommended Journals
Article Tools
Article Usage
- Total views: 11201
- [From(publication date):
September-2016 - Mar 29, 2025] - Breakdown by view type
- HTML page views : 9947
- PDF downloads : 1254