Review Article Open Access
Increasing Intracerebral Infections Caused by Free-Living Amebae in the United States and Worldwide
James H. Diaz*
School of Public Health, Louisiana State University, Health Sciences Center New Orleans, 1615 Poydras St., Suite 1400, New Orleans, LA 70112, USA
- *Corresponding Author:
- James H. Diaz
School of Public Health, Louisiana State University
Health Sciences Center New Orleans, 1615
Poydras St., Suite 1400, New Orleans, LA 70112, USA
E-mail: jdiaz@lsuhsc.edu
Received date: 27 July 2010; Accepted date: 23 September 2010
Visit for more related articles at Journal of Neuroinfectious Diseases
Abstract
Free-living amebae of the genera Acanthamoeba, Balamuthia, Naegleria, and Sappinia are rare causes of infectious diseases in humans with the exception of Acanthamoeba keratitis (AK) which is reported in millions of soft contact lens wearers worldwide each year. Unlike several Acanthamoeba species, only one species of Naegleria, N. fowleri, is known to infect humans by causing an acute, fulminant, usually lethal, central nervous system (CNS) infection, known as primary amebic meningoencephalitis (PAM). Balamuthia mandrillaris, another opportunistic, free-living ameba, is, like Acanthamoeba spp., capable of causing skin lesions and granulomatous amebic encephalitis (GAE) in individuals with compromised or competent immune systems, who inhale infective cysts or develop indolent, granulomatous skin lesions in soil-contaminated wounds. Lastly, Sappinia pedata, a recently identified free-living ameba that lives in soil and animal and reptile feces, has caused a single case of nongranulomatous amebic encephalitis in an immunocompetent Texas farmer. CNS infections caused by these ubiquitous organisms remain rare, but are, nevertheless, increasing today in the US and worldwide due to a combination of environmental and host susceptibility factors. The purpose of this review will be to describe the current epidemiology, pathophysiology, clinical manifestations, diagnosis, management, and prevention of free-living amebic infections of the CNS.
Keywords
free-living amebae; free-living amebic infec-tions; primary amebic meningoencephalitis (PAM); gran-ulomatous amebic encephalitis (GAE); Acanthamoeba species; Naegleria species; Naegleria fowleri; Balamuthia mandrillaris; balamuthiasis; Sappinia species; Sappinia diploidea; Sappinia pedata.
Introduction
Free-living amebae of the genera Acanthamoeba, Bal-amuthia, Naegleria, and Sappinia are rare causes of infectious diseases in humans, with the exception of Acanthamoeba keratitis (AK) which is reported in over 1-2cases per million contact lens wearers in the US annually (Table 1) [2, 3, 9, 13, 25, 37]. Unlike several Acanthamoeba species, only one species of Naegleria, N. fowleri, is known to infect humans by causing an acute, fulminant, usually lethal, central nervous system (CNS) infection, known as primary amebic meningoencephalitis (PAM) [2, 3, 9, 13, 25]. Both Acanthamoeba species and N. fowleri are distributed worldwide, found commonly in freshwater, and have even been isolated from tap and well water, air conditioning systems, sewers, and improperly maintained swimming pools [2, 3, 9, 13, 25].
| Infections | Primary amebic meningoencephalitis (PAM) | Granulomatous amebic encephalitis (GAE-2ΓΆΒ?Β¦ Acanthamoeba or Balamuthia mandrillaris) | Sappinia amebic encephalitis (SAE) | Acanthamoeba keratitis (AK) | |
|---|---|---|---|---|---|
| Pathogens | Naegleria fowleri | Acanthamoeba spp. | Balamuthia mandrillaris | Sappinia pedata | Acanthamoeba spp. |
| Distribution | Worldwide in warm freshwater, bottom sediment, & soil | Worldwide in freshwater & soil | Worldwide in freshwater & soil; more common in US South & South America | Demonstrated in soil & tree bark in the US only | Worldwide in freshwater & soil |
| Cases reported worldwide | 180–200 | ≤ 200 | Approximately 150 | Only 1 case reported | 1-2 cases per 1 million contact lens users (US) per year |
| Seasonal occurrence | Summertime or warmest seasons | Year-round | Year-round | Year-round | Year-round |
| High-risk groups | Immunocompetent children & young adults, especially males with histories of freshwater exposures (skiing, wakeboarding) within 2 weeks | Immunocompromised children & adults (AIDS, cancer or chemo, organ or bone marrow transplant, liver or renal failure); rarely in the immunocompetent | Immunocompetent children & adults, most often males with soil exposures (dirtbiking, agriculture) and/or of Hispanic origin; less commonly in immunocompromised with AIDS or iv drug use | Immunocompetent farmer with pre-existing sinus infection exposed to aerosols of domestic animal feces | Immunocompetent soft contact lens users, more often females; use of contaminated contact lens cleaning solutions; swimming or showering with soft contact lenses; post corneal trauma |
| Pathology | Trophozoites penetrate nasal mucosa & cribriform plate & migrate via olfactory nerves to olfactory bulbs & tracts along basilar brain to cerebellum | Hematogenous dissemination from granulomatous skin ulcers or lung granulomas, across blood- brain barrier to CNS | Hematogenous dissemination from granulomatous skin ulcers, often facial, or lung granulomas across bloodbrain barrier to CNS | Aerosolized cysts and/or trophozoites enter nasopharynx & directly invade CNS | Soil or stagnant waterdwelling infective cysts or trophozoites directly invade corneal epithelium predisposed by prolonged soft contact use, contaminated cleaning solutions, corneal foreign bodies or trauma |
| Incubation period | Mean 5–7 days (Range 1–16 days) | Weeks to months following indolent draining skin ulcers, sinusitis, or pneumonia | Mean 8.5 days (Range 1– 30 days) following indolent pneumonia or draining granulomas on the face or upper arms | Unknown | Unknown & often misdiagnosed & treated as bacterial or herpetic keratitis or keratoconjunctivitis |
| Clinical features | Fever, headache, stiff neck (meningismus), nausea, vomiting, specific cranial nerve dysfunction (altered senses of smell & taste, anisocoria), seizures, disorientation, coma | Same as PAM, early mental status changes, visual loss, photophobia | Same as PAM & GAE with early confusion-disorientation, nonspecific CN dysfunction | Same as PAM, GAE, BAE, with sinusitis, early blurred vision, diplopia, photophobia | Eye pain & foreign body sensation, conjunctival injection, blurred vision, photophobia, excessive tearing |
| Laboratory studies | Trophozoites in CSF wet mounts, stained CSF sediment or brain tissues enhanced by IIF or IFA; N. fowleri DNA by PCR on CSF or unfixed brain | Both cysts & trophozoites in fixed, stained brain tissue enhanced by IIF or IFA; Acanthamoeba DNA by PCR on CSF or unfixed brain | IFA staining of fixed brain tissue; PCR for Balmuthia DNA in CSF or brain tissue | Distinctive trophozoites (double nucleus connected by filament, large contractile vacuole) in stained, fixed brain tissue | Acanthamoeba Cysts and/or trophozoites in corneal smears; fixed, stained corneal scrapings; Acanthamoeba DNA by PCR; confocal microscopy for pathognomonic dendriform epitheliopathy |
| Imaging studies by CT and/or MRI | Nonspecific: basilar leptomeningeal enhancement, intraparenchymal lesions and/or hemorrhagic necrosis; evidence of ICP-cerebral edema, midline shift, cisternal & ventricular compression | Nonspecific: Multiple space-occupying lesions, with or without ringenhancing effects | Nonspecific: cerebral edema, hydrocephalus, multiple space-occupying & ringenhancing in cortex & cerebellum | Single large solitary mass lesion with slight ring-enhancing effect-fronto-parietal or temporo-parietal | Not applicable |
| Treatment | Intravenous (IV) & Intrathecal (IT): amphotericin B, azolesfluconazole, itraconazole, miconazole synergistic antibiotics: azithromycin po, rifampin experimental: chlorpromazine or other phenothiazines | IV & IT: azoles IV: azoles, flucytosine, pentamidine, rifampin, trimethoprim/ sulfamethoxazole experimental: miltefosine | IV: azoles-albendazole, fluconazole, itraconazole, pentamidine, flucytosine, sulfadiazine synergistic macrolides: azithromycin, clarithromycin experimental: phenothiazinesthioridazine, trifluoperazine | IV: pentamidine, flucytosine, itraconazole synergistic antibiotic: azithromycin po | Topical: 0.02% chlorhexidine, 0.02% polyhexamehtylene biguanide, 1% imidazole; PO: azoles: itraconazole, ketoconazole, voriconazole |
| Outcomes (CFRs) | Death within 3–7 days (> 95%) | Usually fatal in immunocompromised (90–94%); immunocompetent children most likely to survive | Usually fatal (≥ 90%) | 1 survivor in the US | Treatment successes, 75–85% vs. failures, 15–25%, which will require corneal transplantation or enucleation |
CFR: Case fatality rate; CSF: Cerebrospinal fluid; ICP: Intracranial pressure; IFA: Immunofluorescent assay; IIF: Indirect immunofluorescence; PCR: Polymerase chain reaction nucleic acid assay; AIDS: Acquired immunodeficiency syndrome; CN: cranial nerve; CNS: central nervous system.
Table 1: Human free-living amebic infections.
Balamuthia mandrillaris, another opportunistic, free-living ameba, is, like Acanthamoeba spp., capable of causing skin lesions and granulomatous amebic encephalitis (GAE) in individuals with compromised or competent immune systems, who inhale infective cysts or develop indolent, granulomatous skin lesions in soil-contaminated wounds [8]. Lastly, Sappinia pedata, a recently identified free-living ameba that lives in soil and animal and reptile feces, has caused a single case of nongranulomatous amebic encephalitis in an immunocompetent Texas farmer.
CNS infections caused by these ubiquitous organisms remain rare despite expanding world populations but are, nevertheless, increasing today in the US and worldwide due to a combination of factors including increased freshwater recreational activities during heat waves for PAM, more immunocompromised individuals susceptible to GAE, and more soft contact lens wearers at risk of AK [26, 28]. The purpose of this review is to describe the epidemiology, pathophysiology, clinical manifestations, diagnosis, management, and prevention of free-living amebic infections of the CNS.
Materials and Methods
Initially, Medline, PubMed, Google R , and Google Scholar R search engines were queried for references using the following key MESH words: free-living amebae, free-living amebic infections, primary amebic menin-goencephalitis, granulomatous amebic encephalitis, Acan-thamoeba species, acanthamoebiasis, Naegleria species, Naegleria fowleri, Balamuthia mandrillaris, Balamuthia amebic encephalitis, balamuthiasis, Leptomyxed ameba, Sappinia species, Sappinea diploidea, and Sappinia pedata.
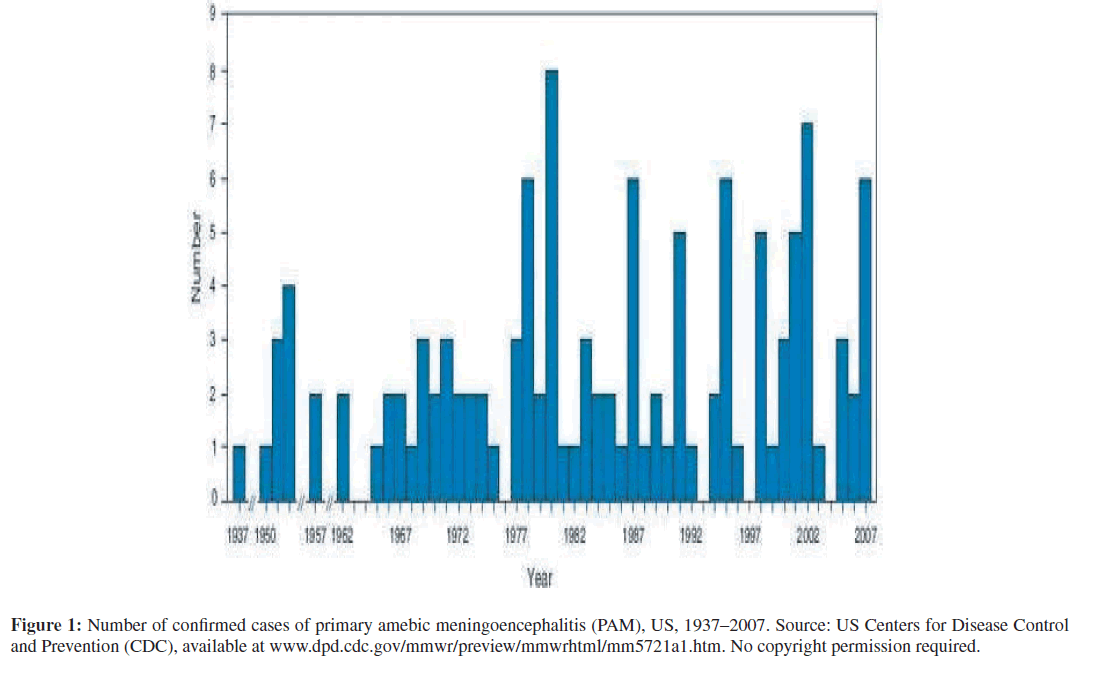
The only cases of PAM included in the review were cases with laboratory-confirmed detection of N. fowleri, Acanthamoeba spp., or Balamuthia mandrillaris life formsor DNA as detected by polymerase chain reaction (PCR) in cerebrospinal fluid (CSF), brain biopsy, or fixed brain tissue at autopsy. Sources of US cases of PAM came from the reg-istry of the CDC’s Naegleria Workgroup, which ultimately confirmed 121 cases of PAM in the US during the period 1937–2007 (Figure 1) [9]. Similar analyses were conducted for all CDC laboratory-confirmed cases of balamuthiasis (N = 15) in the US during the period, 1999–2007. Sources of US cases of Balmuthia GAE came from state departments of public health and the California Encephalitis Project (CEP), a joint project launched in 1998 by the California Department of Public Health and the CDC [8]. International cases of free-living amebic infections of the brain required the same confirmatory diagnostics as the US cases, many of which were also confirmed by the US CDC.
Figure 1: Number of confirmed cases of primary amebic meningoencephalitis (PAM), US, 1937–2007. Source: US Centers for Disease Control and Prevention (CDC), available at www.dpd.cdc.gov/mmwr/preview/mmwrhtml/mm5721a1.htm. No copyright permission required.
Results
Primary amebic meningoencephalitis (PAM)
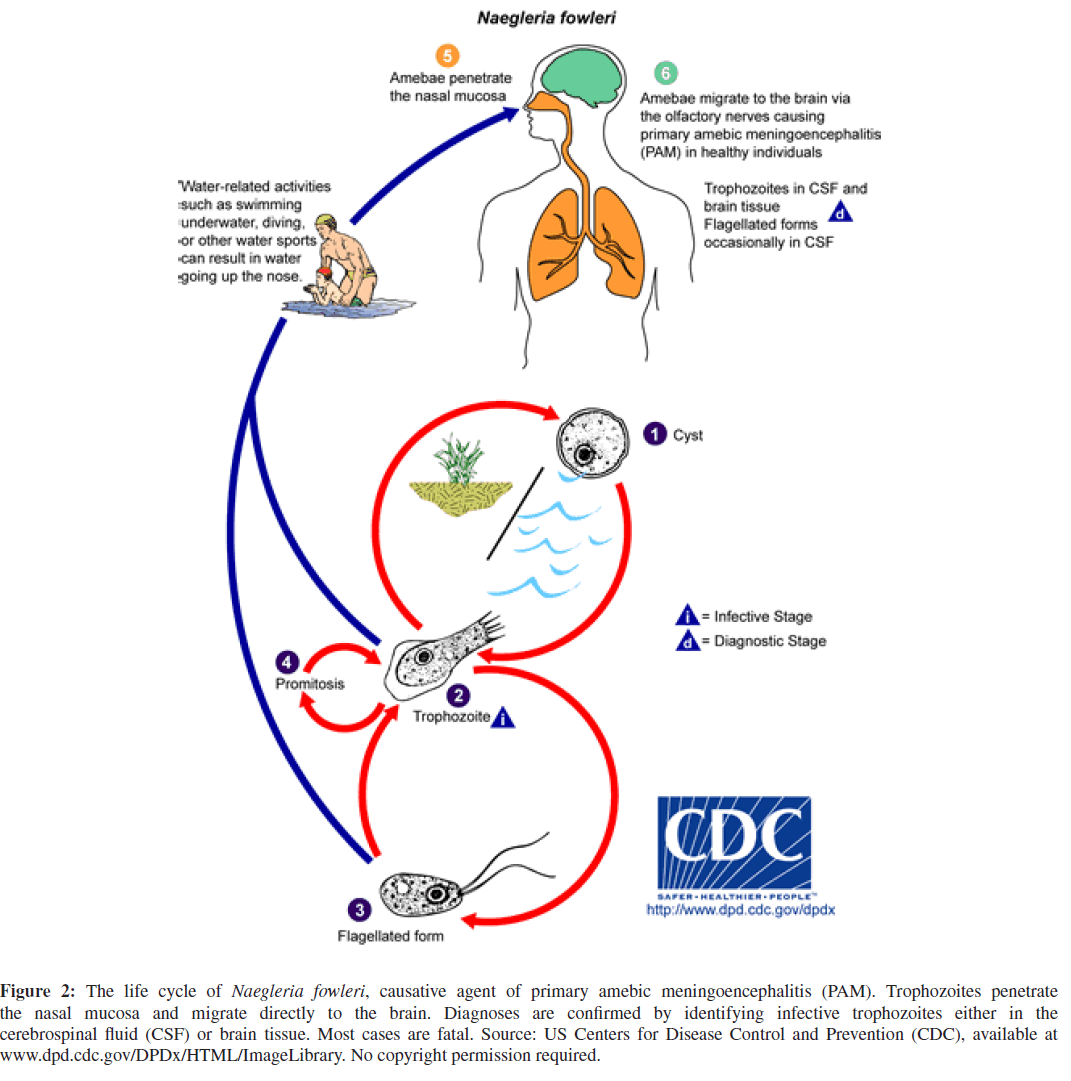
N. fowleri, the single causative agent of PAM, is a free-livingameboflagellate, that has no animal or human reservoirs [2, 3, 9, 13]. N. fowleri thrives in most types of hot freshwater including geothermal springs and warm water discharges from electrical power plants [2, 3, 9, 13, 25]. The free-living ameba feeds on bacteria and organic debris in freshwater, and exists in 3 life forms, 2 of which are infective: the environmentally stable cyst form and the motile amoeboid-form, or trophozoite [6, 22, 24, 27, 36]. Although cases of PAM have resulted from inhalation of cyst-contaminated dust, infective trophozoites typically invade humans via intact or disrupted nasal mucosa; cross the cribriform plate; migrate along the basilar brain from the olfactory bulbs and tracts to the cerebellum; deeply penetrate the cortex to the periventricular system; and incite a purulent meningoencephalitis with rapid cerebral edema, resulting in increased intracranial pressure with early, usually fatal uncal and cerebellar herniation [6, 9, 10, 13, 17, 22, 24, 27, 36, 40, 44, 46, 49]. The life cycle of N. fowleri is depicted in Figure 2.
Figure 2: The life cycle of Naegleria fowleri, causative agent of primary amebic meningoencephalitis (PAM). Trophozoites penetrate the nasal mucosa and migrate directly to the brain. Diagnoses are confirmed by identifying infective trophozoites either in the cerebrospinal fluid (CSF) or brain tissue. Most cases are fatal. Source: US Centers for Disease Control and Prevention (CDC), available at
www.dpd.cdc.gov/DPDx/HTML/ImageLibrary. No copyright permission required.
PAM cases typically occur when it is hot and dry for prolonged seasonal periods causing both higher freshwa-ter temperatures and lower freshwater body levels [9]. The incubation period from freshwater exposure and infection to meningoencephalitis may range from 1–16 days, but is usually 5–7 days [9]. Significant risk factors for PAM in the US include male sex and warm recreational freshwater exposures in a seasonal summer pattern (July-August) in a southern tier state (Table 1) [9,10].
The presenting clinical manifestations of PAM mimic acute bacterial meningitis and include presenting symptoms of headache, anorexia, nausea, vomiting, rhinitis, lethargy, fever, and stiff neck. Disorientation, ataxia, cranial nerve dysfunction (anisocoria, altered senses of smell and taste), mental status changes, seizure activity, and loss of consciousness may follow shortly and within hours of initial assessment.
Initial screening laboratory studies are nonspecific and often demonstrate peripheral leukocytosis, hyperglycemia, and glycosuria. Blood cultures and peripheral blood Gram stains will be negative for bacteria and other microorganisms. The laboratory diagnosis of PAM may be confirmed by one or more of the following laboratory techniques: (1) microscopic visualization of actively moving N. fowleri trophozoites in wet mount preparations of freshlycentrifuged CSF, not previously frozen or refrigerated; (2) microscopic visualization of N. fowleri trophozoites in slide smears of centrifuged CSF sediments or stained, fixed brain biopsy or autopsy specimens; (3) microscopic visualization under ultraviolet light of N. fowleri trophozoites by immunofluorescent techniques using indirect fluorescent antibodies in slide sections of either hematoxylin and eosin (H&E)-stained unfixed/frozen brain tissue or H&E-stained fixed brain tissue; (4) demonstration of N. fowleri DNA by PCR from either CSF or brain tissue samples; or (5) microbiological culture of N. fowleri on agar media [9, 10, 27, 44].
Neuroimaging studies in PAM are also nonspecific and may be normal on initial cranial axial computerized tomography (CT) and magnetic resonance imaging (MRI) scans [10, 44]. Subsequent neuroimaging findings may include basilar leptomeningeal enhancement, massive cerebral edema, evidence of elevated intracranial pressure (midline shift, compressed ventricles, compressed brainstem and basilar cisterns, absence of subarachnoid spaces), and multifocal parenchymal lesions, often with evidence of hemorrhagic infarction or necrosis [10, 44].
Although usually futile, successful treatment strategies for PAM have included combinations of aggressive cerebral edema-reducing therapies (corticosteroids, moderate hyperventilation, diuresis, hypertonic saline) and spe-cific pharmacotherapy with antifungals (amphotericin B, miconazole) and synergistic antibiotics (rifampin, azithromycin) [17, 40, 46, 49]. The optimal duration of therapy is unknown. In 2004, Schuster noted that of the approximately 150 PAM cases reported by then, most patients had died and less than 10 patients had survived [46].
Today, PAM is best prevented by a combination of educational and behavioral modification strategies including the following [9, 10]. (1) Avoid water-related activities, such as swimming, diving, water skiing, jet skiing, and wakeboarding in bodies of warm freshwater, hot springs, and thermally polluted water, such as around coal-burning and nuclear electrical power plants. (2) Avoid similar water-related activities in warm freshwater during prolonged periods of high water temperatures and low water levels. (3) Hold the nose shut or use nose clips to avoid any traumatic disruptions in the nasal mucosal linings during vigorous water-related activities in warm freshwater, such as lakes, rivers, ponds, bayous, and hot springs. (4) Avoid similar water-related activities in drainage ditches, retention or oxidation ponds, and irrigation canals. (5) Avoid digging in or stirring up the sediment during all water-related activities in shallow, warm freshwater areas [9, 10].
Granulomatous amebic encephalitis (GAE)
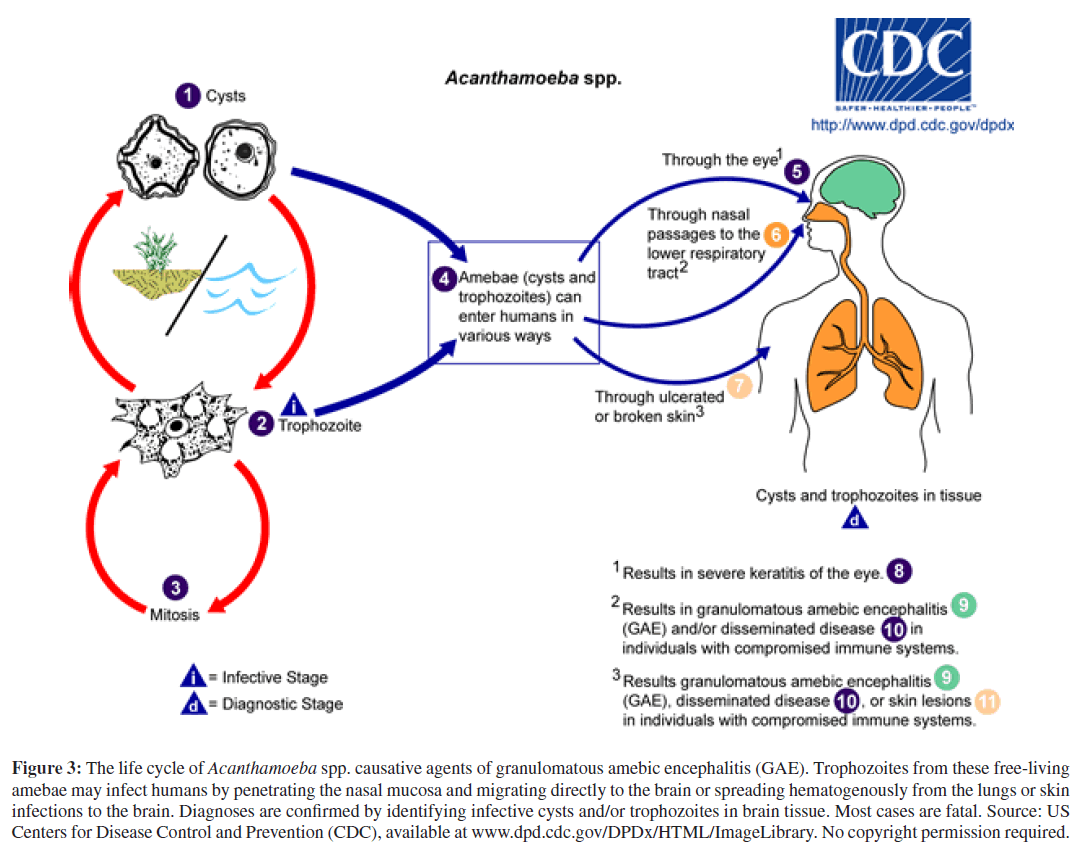
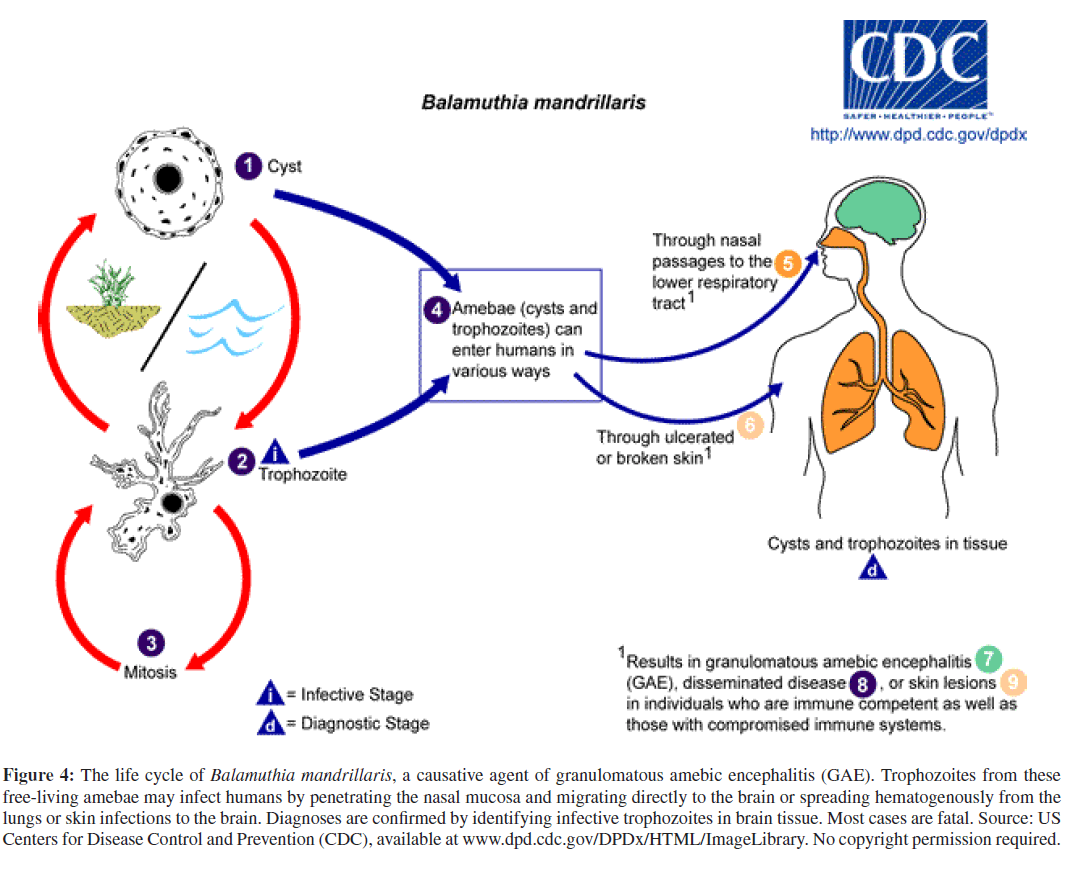
Unlike acute PAM, granulomatous amebic encephalitis (GAE) is a chronic infection of the brain that may disseminate to other organs hematogenously and usually occurs in immunosuppressed patients with AIDS or organ transplants, or in patients receiving chemotherapy for cancer or tuberculosis [1, 5, 29, 37, 47]. GAE may be caused by several species of Acanthamoeba or by another, phylogenet-ically related, free-living ameba, Balamuthia mandrillaris. Acanthamoeba species and Balamuthia mandrillaris aredistributed worldwide in freshwater and soil, and can cause GAE year round [19, 29]. The portal of entry for these opportunistic pathogens is through the respiratory tract or via ulcerating skin wounds with hematogenous spread to the CNS and, less commonly, with dissemination to other organs in the severely immunocompromised (Figures 3 and 4) [19]. To date, approximately 200 cases of Acanthamoeba GAE and 150 cases of Balamuthia GAE have been reported with acanthamoebiasis still confined mostly to the immunocompromised; and balamuthiasis, affecting both immunocompromised and immunocompetent individuals [1, 4, 18, 20, 35]. Besides immunocompromise, other potential risk factors for balamuthiasis may include contact with stagnant freshwater or with contaminated soil, often through agricultural work, desert motorcycling, dirt-biking, or even gardening [18].
Figure 3: The life cycle of Acanthamoeba spp. causative agents of granulomatous amebic encephalitis (GAE). Trophozoites from these free-living amebae may infect humans by penetrating the nasal mucosa and migrating directly to the brain or spreading hematogenously from the lungs or skin infections to the brain. Diagnoses are confirmed by identifying infective cysts and/or trophozoites in brain tissue. Most cases are fatal. Source: US Centers for Disease Control and Prevention (CDC), available at www.dpd.cdc.gov/DPDx/HTML/ImageLibrary. No copyright permission required.
Figure 4: The life cycle of Balamuthia mandrillaris, a causative agent of granulomatous amebic encephalitis (GAE). Trophozoites from these free-living amebae may infect humans by penetrating the nasal mucosa and migrating directly to the brain or spreading hematogenously from the lungs or skin infections to the brain. Diagnoses are confirmed by identifying infective trophozoites in brain tissue. Most cases are fatal. Source: US Centers for Disease Control and Prevention (CDC), available at www.dpd.cdc.gov/DPDx/HTML/ImageLibrary. No copyright permission required.
The incubation period for Acanthamoeba GAE could extend for weeks or months after primary inoculation in the skin, sinuses, or lungs, with subsequent draining ulcers, chronic sinusitis, or pneumonia [18]. Although primary inoculation with Balamuthia mandrillaris is also via the skin or lungs, the incubation period is shorter than in Acanthamoeba GAE with a mean of 8.5 days and a range of 1–30 days [19]. The clinical presentation of GAE from either causative pathogen is the same with early behavioral and personality changes, fever, depressed mental status, seizures, photophobia, visual loss, and nonspecific cranial nerve dysfunction, followed by signs of increased intracranial pressure, including headache, nausea, vomiting, and loss of consciousness [8, 34].
The laboratory diagnosis of GAE from either pathogen is also similar with cysts and trophozoites rarely identified in the CSF, but more often identified in fixed and stained skin ulcer biopsies, brain biopsies, and post-mortem brain tissues. Recently, several immunodiagnostic tests have been developed for diagnostic specimens in cases of suspected GAE including indirect immunofluorescent ultraviolet microscopy, indirect immunofluorescent antibody ultravio-let microscopy with specific anti-pathogen antibodies, and PCR assays for identification of specific pathogen DNA [33].
In 2006, Qvarnstrom and coinvestigators at the CDC described a new multiplex real-time PCR assay for the simultaneous detection of Acanthamoeba spp., Balamuthia mandrillaris, and Naegleria fowleri, which will permitrapid and specific detection of a single free-living ameba in clinical specimens within 5 hours [33]. This nucleic acid test will offer an important adjunct in the differential diagnosis of free-living amebic infections of the CNS, especially when immunodiagnostic tests are equivocal or unavailable, and cysts and trophozoites cannot be identified microscopically in CSF or brain specimens [33].
Neuroimaging studies by CT and/or MRI in GAE are nonspecific and often include single to multiple space-occupying lesions in the brain from the frontal cortex to the cerebellum with ring enhancing effects slightly more common in balamuthiasis than in acanthamoebiasis. Evidence of increased intracranial pressure will be present or will occur including midline shifts, cisternal and ventricular compression, and hydrocephalus.
Treatment strategies for GAE will include combinations of critical care techniques to reduce increased intracranial pressure, craniotomy for biopsy or excision of mass lesions, and combination pharmacotherapy with antifungals, anti-protozoal agents, synergistic antibiotics, and several experimental drug therapies that have shown promise in vitro, such as phenothiazines. Although case fatality ratesin GAE are very high (90–94% in acanthamoebiasis and ≥ 90% in balamuthiasis), successful drug treatment com-binations in acanthamoebiasis have included intravenous pentamidine isethionate; flucytosine (5-flurocytosine); the antifungals, amphotericin B, itraconazole or fluconazole; several synergistic antibiotics, including rifampin and trimethroprim/sulfamethoxazole (TMP/SMX), or amikacin, or oral sulfadiazine, and topical ketoconazole or miltefosine for skin ulcers [12, 19, 31, 39, 45].
In 2008, Aichelburg and colleagues in Vienna reported treating a patient successfully with disseminated tuberculo-sis and acanthamoebiasis with topical and oral miltefosine, a phosphocholine analog used to treat visceral leishmaniasis, and a combination of intravenous fluconazole, TMP/SMX, amikacin, and 4 tuberculostatic drugs [1]. Successful intra-venous drug treatment combinations in balamuthiasis have included the antifungal azoles (albendazole, fluconazole, or itraconazole), flucytosine, pentamidine, sulfadiazine, the synergistic macrolide antibiotics (azithromycin or clarithromycin), and phenothiazines (thioridazine or trifluoperazine) [8]. In 2004, Schuster and Visvesvara demonstrated that the phenothiazines demonstrated in vitro efficacy against Balamuthia mandrillaris in clinical specimens, but the exact mechanism of action remains unexplained [39].
Prevention and control strategies for GAE should include (1) consideration of GAE in organ transplant and immunocompromised patients with encephalitis and skin ulcers not improving with standard therapies; (2) recognition of genetic risk factors for acanthamoebiasis and balamuthiasis in Hispanics less able to produce antibodies against causative free-living amebae; and (3) recognition of other soil or stagnant water risk factors in immunocompetent patients with skin ulcers and unexplained meningoencephalitis [8, 23, 38]. factors in immunocompetent patients with skin ulcers and unexplained meningoencephalitis [8, 23, 38].
Sappinia amebic encephalitis (SAE)
The genus Sappinia contains two species of free-living amebae found in soil, tree bark, and animal feces, S. diploidea and S. pedata [7, 32]. Only one case of SAE hasbeen reported in a 38-year-old immunocompetent male farmer in Texas who had contact with grazing animals and fecal-contaminated aerosols and soil; and presented with emesis, blurred vision, photophobia, headache, and loss of consciousness following a sinus infection [14, 15]. MRI demonstrated a 2 cm mass in the left temporal lobe with slight ring enhancement [14, 15]. The lesion was excised and cryosections of brain tissue showed trophozoites of free-living amebae with distinctive double nuclei, initially identified as S. diploidea, and later confirmed by RT-PCR at the CDC as S. pedata [7, 14, 15, 32]. The patient was successfully treated with intravenous azithromycin, flucytosine, itraconazole, and pentamidine, and made a full recovery [14, 15].
International reports of initial cases of free-living amebic infections
In keeping with the increases in reported and confirmed cases of free-living amebic infections of the CNS in the US, new initial cases and case-clusters of free-living amebic infections of the CNS are now being reported throughout the temperate world. In 1993, Lares-Villa and coauthors reported the first 5 case-cluster of PAM in Mexico and isolated Naegleria from an irrigation canal [21]. In 1999, Sugita and coinvestigators reported the first case of PAM due to N. fowleri in Japan following an autopsy on a 25-year-old female, which demonstrated suppurative meningoencephalitis with amebic trophozoites in brain tissue [48]. In 2002, Shenoy and coauthors reported the first fatal case of PAM caused by N. fowleri in South India in a 5-month-old infant without a history of recreational freshwater exposure, other than being bathed in an artificial well, which later revealed infective N. fowleri trophozoites [42]. In 2004, Cogo and coauthors reported the first fatal case of PAM in Italy in a 9-year-old boy with a history of swimming in the Po River during the European heat wave of summer 2003, 10 days before the onset of symptoms [11]. As in the US experience with PAM, all international decedents were immunologically competent infants, children, and young adults [11, 21, 42, 43, 48].
In 2002, Shirabe and co-investigators reported the first case of Balamuthia mandrillaris GAE in Japan following an autopsy on a 78-year-old woman with Sjorgren’s syndrome and no known environmental exposures, although potted plant exposures could not be excluded [43]. In 2004, Intalapaporn and coauthors reported the first case of Balamuthia mandrillaris GAE in Thailand following anautopsy on a 23-year-old healthy man who ran into a swamp during a motorcycle accident and sustained a nonhealing granulomatous wound on his nose 6 months earlier [16]. Although no microorganisms were detected in a biopsy of the nasal lesion, Balamuthia cysts and trophozoites were detected in fixed and stained brain tissue at autopsy [16]. In 2006, Oddo and coinvestigators reported the first case of Balamuthia mandrillaris GAE in Chile following an autopsy with identification of Balamuthia cysts and trophozoites in brain tissue in a 7-month-old, healthy male infant with a several week history of fever, seizures, and personality changes [30]. In 2009, Sheng and co-authors reported the first case of Acanthamoeba sp. GAE in Taiwan in a 63-year-old, previously healthy farmer who had fallen into a ditch of muddy water 2 weeks earlier; and survived after treatment with amphotericin B, rifampin, and corticosteroids [41]. As in the US cases of balamuthiasis, most international cases of Balamuthia GAE were fatal and several were associated with either soil or stagnant water exposures and indolent, draining, granulomatous skin lesions [16, 30, 41, 43].
Conclusions
Once considered nonpathogenic, the free-living amebae have emerged over recent decades as significant pathogenic threats to human health for several reasons including the following. (1) Free-living amebae are widely distributed in soil and freshwater throughout the temperate and tropical world, have environmentally stable cyst forms for over-wintering, and have taken advantage of longer warm seasons to parasitize humans in their outdoor pursuits [8]. (2) Some free-living are frequently opportunistic, but can also evade host responses in immunocompetent individuals, such as Acanthamoeba spp., Balamuthia mandrillaris, and Sappinia pedata. (3) Free-living amebae are resistant toantimicrobial monotherapy and require combined therapy with a variety of antimicrobials and synergistic drugs. (4) Free-living amebic infections are difficult to diagnose unless suspected; the laboratory is alerted to the possibility of amebic forms in diagnostic specimens; and confirmatory immunological and molecular tests are available, usually at distant reference labs, such as the CDC. (5) Lastly, some ethnic groups, such as Hispanics, may be genetically predisposed to GAE because they cannot muster effective protective antibody responses to phylogenetically related Acanthamoeba spp. and Balamuthia mandrillaris [38].
Clinicians should suspect free-living amebic infections of the CNS in refractory cases of meningoencephalitis ini-tially managed as aseptic or bacterial infections, especially in patients predisposed to such infections by regions vis-ited, warm freshwater exposures, behavioral practices, eth-nicity, or immunosuppression. Future investigations will be required to determine the significance of freshwater wake-boarding, popular among adolescents, as a significant risk factor for PAM and to determine any dose-response effects of global warming on rising freshwater temperatures and lower surface freshwater volumes on the multiplication and infectivity of aquatic free-living amebae.
References
- A. C. Aichelburg, J. Walochnik, O. Assadian, H. Prosch, A. Steuer, G. Perneczky, et al., Successful treatment of disseminated Acan-thamoeba sp. infection with miltefosine, Emerg Infect Dis, 14(2008), pp. 1743–1746.
- K. Anderson and A. Jamieson, Primary amoebic meningoen-cephalitis, Lancet, 2 (1972), p. 379.
- J. Apley, S. K. Clarke, A. P. Roome, S. A. Sandry, G. Saygi, B. Silk, et al., Primary amoebic meningoencephalitis in Britain, Br Med J, 1 (1970), pp. 596–599.
- A. Bakardjiev, P. H. Azimi, N. Ashouri, D. P. Ascher, D. Janner, L. Schuster, G. S. Visvesvara, and G. C., Amebic encephalitis caused by Balamuthia mandrillaris: report of four cases, PediatrInfect Dis J, 22 (2003), pp. 447–453.
- S. Barete, A. Combes, J. F. de Jonckheere, A. Datry, S. Varnous, V. Martinez, et al., Fatal disseminated Acanthamoeba lenticulata infection in a heart transplant patient, Emerg Infect Dis, 13 (2007),pp. 736–738.
- N. D. Barnett, A. M. Kaplan, R. J. Hopkin, M. A. Saubolle, and M. F. Rudinsky, Primary amoebic meningoencephalitis with Naegleria fowleri: clinical review, Pediatr Neurol, 15 (1996),pp. 230–234.
- M. W. Brown, F. W. Spiegel, and J. D. Silberman, Amoeba at attention: phylogenetic affinity of Sappinia pedata, J EukaryotMicrobiol, 54 (2007), pp. 511–519.
- Centers for Disease Control and Prevention (CDC), Balamuthia amebic encephalitis—California, 1999–2007, MMWR Morb Mor-tal Wkly Rep, 57 (2008), pp. 768–771.
- Centers for Disease Control and Prevention (CDC), Primary amebic meningoencephalitis—Arizona, Florida, and Texas, 2007,MMWR Morb Mortal Wkly Rep, 57 (2008), pp. 573–577.
- Centers for Disease Control (CDC), Primary amebic meningoencephalitis—North Carolina, 1991, MMWR MorbMortal Wkly Rep, 41 (1992), pp. 437–440.
- P. E. Cogo, M. Scagli, S. Gatti, F. Rossetti, R. Alaggio, A. M. Laverda, et al., Fatal Naegleria fowleri meningoencephalitis, Italy, Emerg Infect Dis, 10 (2004), pp. 1835–1837.
- T. R. Deetz, M. H. Sawyer, G. Billman, F. L. Schuster, and G.S. Visvesvara, Successful treatment of Balamuthia amoebic encephalitis: presentation of 2 cases, Clin Infect Dis, 37 (2003),pp. 1304–1312.
- M. Fowler and R. F. Carter, Acute pyogenic meningitis probably due to Acanthamoeba sp.: a preliminary report, Br Med J, 2(1965), pp. 740–742.
- B. B. Gelman, V. Popov, G. Chaljub, R. Nader, S. J. Rauf, H. W. Nauta, et al., Neuropathological and ultrastructural features of amebic encephalitis caused by Sappinia diploidea, J NeuropatholExp Neurol, 62 (2003), pp. 990–998.
- B. B. Gelman, S. J. Rauf, R. Nader, V. Popov, J. Borkowski, G. Chaljub, et al., Amoebic encephalitis due to Sappinia diploidea, JAMA, 285 (2001), pp. 2450–2451.
- P. Intalapaporn, C. Suankratay, S. Shuangshoti, K. Phantumchinda,S. Keelawat, and H. Wilde, Balamuthia mandrillaris meningoen-cephalitis: the first case in southeast Asia, Am J Trop Med Hyg,70 (2004), pp. 666–669.
- R. Jain, S. Prabhakar, M. Modi, R. Bhatia, and R. Sehgal, Naegleria meningitis: a rare survival, Neurol India, 50 (2002),pp. 470–472.
- S. Jung, R. L. Schelper, G. S. Visvesvara, and H. T. Chang, Bala-muthia mandrillaris meningoencephalitis in an immunocompetent patient: an unusual clinical course and a favorable outcome, ArchPath Lab Med, 128 (2004), pp. 466–468.
- A. A. Koshy, B. G. Blackburn, and U. Singh, Free-living amebas, in Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, G. L. Mandell, J. E. Bennett, and R. Dolin, eds., Elsevier, Philadelphia, 7th ed., 2009, pp. 3427–3436.
- P. Lackner, R. Beer, G. Broessner, R. Helbok, B. Pfausler, C. Bren-neis, et al., Acute granulomatous acanthamoeba encephalitis in an immunocompetent patient, Neurocrit Care, 12 (2010), pp. 91–94.
- F. Lares-Villa, J. F. De Jonckheere, H. De Moura, A. Rechi-Iruretagoyena, E. Ferreira-Guerrero, G. Fernandez-Quintanilla, et al., Five cases of primary amebic meningoencephalitis in Mexicali, Mexico: study of the isolates, J Clin Microbiol, 31(1993), pp. 685–688.
- P. Ma, G. S. Visvesvara, A. J. Martinez, F. H. Theodore, P. M. Daggett, and T. K. Sawyer, Naegleria and Acanthamoeba infections: review, Rev Infect Dis, 12 (1990), pp. 90–513.
- S. K. Maciver, The threat from Balamuthia mandrillaris, J Med Microbiol, 56 (2007), pp. 1–3.
- F. Marciano-Cabral, M. L. Cline, and S. G. Bradley, Specificity of antibodies from human sera for Naegleria species, J ClinMicrobiol, 25 (1987), pp. 692–697.
- F. Marciano-Cabral, R. MacLean, A. Mensah, and L. LaPat-Polasko, Identification of Naegleria fowleri in domestic water sources by nested PCR, Appl Environ Microbiol, 69 (2003), pp. 5864–5869.
- D. J. Marcogliese, The impact of climate change on the parasites and infectious diseases of aquatic animals, Rev Sci Tech, 27(2008), pp. 467–484.
- A. J. Martinez and G. S. Visvesvara, Laboratory diagnosis of pathogenic free-living amoebas: Naegleria, Acanthamoeba, and Leptomyxid, Clin Lab Med, 11 (1991), pp. 861–872.
- , Free-living, amphizoic and opportunistic amebas, Brain Pathol, 7 (1997), pp. 583–598.
- D. Mutreja, Y. Jalpota, R. Madan, and V. Tewari, Disseminated acanthamoeba infection in a renal transplant recipient: a case report, Indian J Pathol Microbiol, 50 (2007), pp. 346–348.
- B. D. Oddo, A. S. Ciani, and C. P. Vial, [granulomatous amebic encephalitis caused by Balamuthia mandrillaris. First case diagnosed in Chile], Rev Chilena Infectol, 23 (2006), pp. 232–236.
- S. Oliva, M. Jantz, R. Tiernan, D. L. Cook, and M. A. Judson, Successful treatment of widely disseminated acanthamoebiasis,South Med J, 92 (1999), pp. 55–57.
- Y. Qvarnstrom, A. J. da Silva, F. L. Schuster, B. B. Gelman, and G. S. Visvesvara, Molecular confirmation of Sappinia pedata as a causative agent of amoebic encephalitis, J Infect Dis, 199 (2009), pp. 1139–1142.
- Y. Qvarnstrom, G. S. Visvesvara, R. Sriram, and A. J. da Silva, Multiplex real-time PCR assay for simultaneous detection of Acanthamoeba spp., Balamuthia mandrillaris, and Naegleria fowleri, J Clin Microbiol, 44 (2006), pp. 3589–3595.
- V. Radhakrishnan, R. Bhatia, G. S. Panda, and S. Bakhshi, Acan-thamebic meningoencephalitis presenting as personality change,Pediatr Infect Dis J, 28 (2009), p. 555.
- R. Ranjan, A. Handa, A. Choudhary, and S. Kumar, Acanthamoeba infection in an interhemispheric ependymal cyst: a case report,Surg Neurol, 72 (2009), pp. 185–189.
- M. F. Reilly, F. Marciano-Cabral, D. W. Bradley, and S. G. Bradley, Agglutination of Naegleria fowleri and Naegleria gruberi by antibodies in human serum, J Clin Microbiol, 17 (1983), pp. 576–581.
- D. A. Schaumberg, K. K. Snow, and M. R. Dana, The epidemic of Acanthamoeba keratitis: where do we stand?, Cornea, 17 (1998), pp.3–10.
- F. L. Schuster, C. Glaser, S. Honarmand, J. H. Maguire, and G. S. Visvesvara, Balamuthia amebic encephalitis risk, Hispanic Americans, Emerg Infect Dis, 10 (2004), pp. 1510–1512.
- F. L. Schuster and G. S. Visvesvara, Opportunistic amoebae: challenges in prophylaxis and treatment, Drug Resist Updat, 7(2004), pp. 41–51.
- J. S. Seidel, P. Harmatz, G. S. Visvesvara, A. Cohen, J. Edwards, and J. Turner, Successful treatment of primary amebic meningoen-cephalitis, N Engl J Med, 306 (1982), pp. 346–348.
- W. H. Sheng, C. C. Hung, H. H. Huang, S. Y. Liang, Y. J. Cheng, D. D. Ji, et al., First case of granulomatous amebic encephalitis caused by Acanthamoeba castellanii in Taiwan, Am J Trop MedHyg, 81 (2009), pp. 277–279.
- S. Shenoy, G. Wilson, H. V. Prashanth, K. Vidyalakshmi, B. Dhanashree, and R. Bharath, Primary meningoencephalitis by Naegleria fowleri: first reported case from Mangalore, South India,J Clin Microbiol, 40 (2002), pp. 309–310.
- T. Shirabe, Y. Monobe, and G. S. Visvesvara, An autopsy case of amebic meningoencephalitis. The first Japanese case caused by Balamuthia mandrillaris, Neuropathology, 22 (2002), pp. 213–217.
- P. Singh, R. Kochhar, R. K. Vashishta, N. Khandelwal, S. Prab-hakar, S. Mohindra, et al., Amebic meningoencephalitis: spectrum of imaging findings, AJNR Am J Neuroradiol, 27 (2006), pp. 1217–1221.
- T. Singhal, A. Bajpai, V. Kalra, S. K. Kabra, J. C. Samantaray, G. Satpathy, et al., Successful treatment of Acanthamoeba menin-gitis with combination oral antimicrobials, Pediatr Infect Dis J, 20(2001), pp. 623–627.
- S. M. Soltow and G. M. Brenner, Synergistic activities of azithromycin and amphotericin B against Naegleria fowleri in vitro and in a mouse model of primary amebic meningoencephalitis,Antimicrob Agents Chemother, 51 (2007), pp. 23–27.
- J. P. Steinberg, R. L. Galindo, E. S. Kraus, and K. G. Ghanem, Disseminated acanthamebiasis in a renal transplant recipient with osteomyelitis and cutaneous lesions: case report and literature review, Clin Infect Dis, 35 (2002), pp. e43–e49.
- Y. Sugita, T. Fujii, I. Hayashi, T. Aoki, T. Yokoyama, M. Mori-matsu, et al., Primary amebic meningoencephalitis due to Nae-gleria fowleri: an autopsy case in Japan, Pathol Int, 49 (1999),pp. 468–470.
- A. Wang, R. Kay, W. S. Poon, and H. K. Ng, Successful treatment of amoebic meningoencephalitis in a Chinese living in Hong Kong,Clin Neurol Neurosurg, 95 (1993), pp. 249–252.
Relevant Topics
- Bacteria Induced Neuropathies
- Blood-brain barrier
- Brain Infection
- Cerebral Spinal Fluid
- Encephalitis
- Fungal Infection
- Infectious Disease in Children
- Neuro-HIV and Bacterial Infection
- Neuro-Infections Induced Autoimmune Disorders
- Neurocystercercosis
- Neurocysticercosis
- Neuroepidemiology
- Neuroinfectious Agents
- Neuroinflammation
- Neurosyphilis
- Neurotropic viruses
- Neurovirology
- Rare Infectious Disease
- Toxoplasmosis
- Viral Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 14238
- [From(publication date):
December-2010 - Nov 24, 2024] - Breakdown by view type
- HTML page views : 9887
- PDF downloads : 4351