Increased Peripheral Platelet Count is Unrelated to Decreased Liver Fibrosis Score Following lamivudine Treatment of Patients with Chronic Hepatitis B
Received: 15-Jan-2018 / Accepted Date: 19-Jan-2018 / Published Date: 26-Jan-2018 DOI: 10.4172/2161-069X.1000549
Abstract
Background and study aims: Liver fibrosis score is associated with peripheral platelet count in patients with chronic hepatitis B (CHB). Lamivudine therapy decreases the liver fibrosis score in these patients. The aim of this study was to evaluate the relationship between increasing peripheral platelet count and decreasing liver fibrosis scores in patients with CHB on lamivudine therapy.
Patients and methods: One hundred and fifty-three patients with CHB who received at least 2-years of lamivudine therapy in a tertiary care center were enrolled in the study. Patients with splenomegaly and/or bi- or pancytopenia were excluded. Liver biopsy had been performed prior to and at the end of the second year of the lamivudine therapy. Lamivudine-induced alterations of aspartate aminotransferase, alanine aminotransferase levels, fibrosis score, and peripheral platelet count were compared with Wilcoxon test. A p-value of less than 0.05 was regarded as significant.
Results: Of 153 patients, 99 (64.7%) were male and mean age (± SD) was 39.05 ± 11.8 years. HBeAg was found to be positive in 71 (46.4%) patients. Lamivudine decreased aspartate aminotransferase, alanine aminotransferase levels, fibrosis score, and increased peripheral platelet count. Increased peripheral platelet count showed a significant correlation with decreased alanine aminotransferase levels (p<0.05). Increased peripheral platelet count showed no correlation with decreased aspartate aminotransferase levels or fibrosis score (p>0.05).
Conclusions: Increased peripheral platelet count during the lamivudine therapy is not related decreased liver fibrosis score in patients with CHB.
Keywords: Lamivudine; Hepatitis B; Fibrosis; Platelet count
Introduction
The pathogenesis of thrombocytopenia in liver diseases is not well established. In addition to splenic congestion, increased destruction of platelets, decreased platelet production, anti-platelet antibodies, disseminated intravascular coagulation, translocated toxins or other gut-derived substances, and portosystemic shunting may also play a role in thrombocytopenia related to liver disease [1-8].
Moreover, infections, drugs, toxins, and nutritional deficiencies may also cause thrombocytopenia through bone marrow depression. An important observation in this field was the identification of thrombopoietin and its role as a primary physiological regulator of platelet production. It is produced primarily in the liver, although the kidney, spleen, and bone marrow also contribute to its production [9]. Serum levels of thrombopoietin decrease in patients with cirrhosis [10,11].
The expression of thrombopoietin mRNA in the liver tissue decreases with progressive liver disease in both humans and rats [12]. These findings suggest that thrombocytopenia in liver disease is caused not only by splenic sequestration but also by decreased platelet production due to reduced synthesis of thrombopoietin and/or other humoral factor(s) by the liver.
Lamivudine inhibits hepatitis B virus replication and reduces viral load, leading to clinical, biochemical, serological, and histological improvement in patients with chronic hepatitis B (CHB) [13,14]. Results of a histological study showed that 3 years’ lamivudine therapy reversed fibrosis in most patients [15]. Liver biopsy is still the gold standard for the evaluation of the fibrosis score in these patients; however, it is an invasive method.
Thus, recent studies have focused on searching for noninvasive and repeatable methods for assessing the alterations of the fibrosis score in these patients. Peripheral platelet count (PPC) is a component of several noninvasive indexes that have been used to assess liver fibrosis in patients with CHB; these indexes include APRI index [includes aspartate aminotransferase (AST) and PPC] [16], AP index (includes age and PPC) [17] and FIB4 index [includes age, alanine aminotransferase (ALT), AST, and PPC] [18,19]. Lamivudine reduces liver fibrosis in patients with CHB. The aim of this study was to evaluate the relationship between increasing PPC and decreasing liver fibrosis in patients with CHB on lamivudine therapy.
Materials and Methods
Patients
Patients were retrieved from a study performed between 2000 and 2005 in which the stopping rule(s) of lamivudine therapy were investigated. There were no guidelines or criteria for stopping lamivudine therapy during this period.
In this unpublished and abandoned study, 196 treatment-naive patients were followed up for at least 2 years and pre-treatment and 2- year liver biopsies were performed. To exclude the impact of hypersplenism, patients whose ultrasonography indicated splenomegaly and/or those with bi or pan-cytopenia were not included in the study.
Other exclusion criteria were the presence of insufficient data, lamivudine resistance, co-infection with hepatitis C or D virus, coexisting positivity for one of the autoimmune hepatitis markers (antinuclear antibody, anti-mitochondrial antibody, anti-smooth muscle antibody, or anti-liver-kidney-microsomal antibody), ethanol consumption more than 40 g/day, and patients on interferon or adefovir dipivoxil therapy.
Data of AST, ALT levels, fibrosis score, and PPC prior to and in the second year of lamivudine therapy were collected from the patients’ medical reports. The associations between lamivudine-induced alterations of AST, ALT, fibrosis score, and PPC were analyzed. Determination of PPC was made by a routine complete blood count auto-analyzer (Coulter Gen-S, USA), which was routinely performed within 7 days prior to percutaneous liver biopsies for all patients.
Histopathological assessment of the liver was performed by 2 experienced pathologists according to the Knodell system [20]. The final evaluation was reported after the consensus of both pathologists. Informed consent in writing was obtained from all patients, and the study protocol conformed to the ethical guidelines of the 1964 Declaration of Helsinski.
Statistical analysis
Wilcoxon test was used to evaluate the association between the alterations in fibrosis score, PPC, ALT, and AST levels after a 2-year therapy with lamivudine.
Data are presented as the mean ± SD. A p-value of less than 0.05 was regarded as significant. All statistical analyses were performed using SPSS 11.0.
Results
A total of 153 patients with CHB were found to be eligible. The mean age of 153 patients (99 males, 54 females) was 39.05 ± 11.8 years (range: 14-67).
HBeAg was positive in 71 (46.4%) patients. Mean AST, ALT levels, fibrosis score, and PPC of the patients before lamivudine therapy were 75 ± 84.4 IU/L, 133 ± 281.0 IU/L, 2.06 ± 1.3 (Knodell score) and 194 ± 57.7 cells × 109⁄L, respectively.
Mean AST, ALT levels, fibrosis score, and PPC of the patients at the end of the second year of lamivudine therapy were 39 ± 34.0 IU/L, 52 ± 61.3 IU/L, 1.43 ± 1.4 (Knodell score), and 212 ± 63.9 cells × 109⁄L, respectively. Lamivudine decreased AST, ALT levels, and fibrosis score, and increased PPC (Table 1).
| Parameter | Before therapy | Second year of therapy |
|---|---|---|
| Fibrosis score (mean ± s.d., Knodell) | 2.06 ± 1.3 | 1.43 ± 1.4 |
| ALT (mean ± s.d., IU/L) | 133 ± 281.0 | 52 ± 61.3 |
| AST (mean ± s.d., IU/L) | 75 ± 84.4 | 39 ± 34.0 |
| PPC (mean ± s.d., cells x 109⁄L) | 194 ± 57.7 | 212 ± 63.9 |
| ALP (mean ± s.d., IU/L) | 204 ± 111.7 | 174 ± 69.9 |
| Albumin (mean ± s.d., g/dL) | 4.50 ± 0.46 | 4.45 ± 0.42 |
| Hemoglobin (mean ± s.d., g/dL) | 14.6 ± 1.5 | 14.5 ± 1.4 |
| WBC (mean ± s.d., cells x 109⁄L) | 6695 ± 2019 | 6641 ± 2142 |
Table 1: Fibrosis score, hematological parameters, and liver function tests of 153 patients with chronic hepatitis B before and at the second year of the lamivudine therapy; [ALT: Alanine Aminotransferase, AST: Aspartate Aminotransferase, PPC: Peripheral Platelet Count, ALP: Alkaline Phosphatase, WBC: White Blood Cell Count].
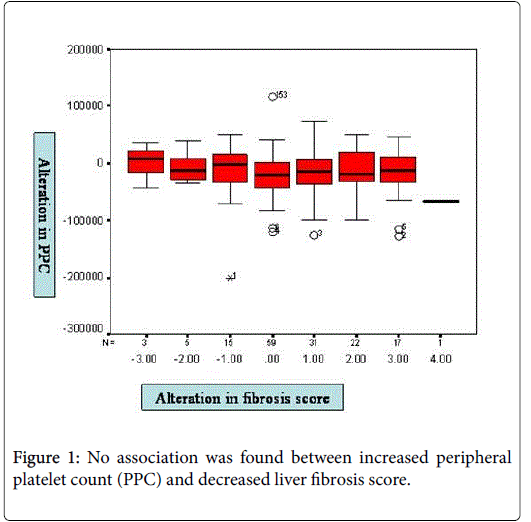
HBV-DNA was negative at the end of the second year of lamivudine therapy in all patients. There was no association between increased PPC and decreased liver fibrosis score (p>0.05) (Figure 1).
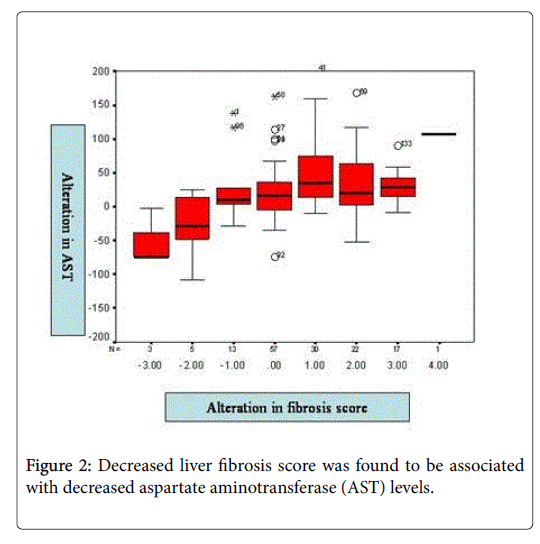
Increased PPC was found to be associated with decreased ALT levels (p<0.05). Decreased liver fibrosis score was found to be associated with decreased AST levels (p<0.05) (Figure 2). No association was found between decreased liver fibrosis score and increased PPC or decreased ALT levels (p>0.05).
Discussion
Liver fibrosis score is important for the prognosis and management of patients with CHB. An invasive method, liver biopsy, is still the gold standard for detecting the extent of liver fibrosis. Thus, recent studies focused on searching for noninvasive and repeatable methods for assessing severity of liver diseases, with results that suggest that the aminotransferases and PPC are the most accurate noninvasive markers of hepatitis B virus-related fibrosis [16,18,19,21-23]. Lamivudine is an established drug used in the reversal of fibrosis in most patients with CHB [15]. However, to the best of our knowledge, no previous study investigated the correlation between alterations of noninvasive markers and fibrosis score during lamivudine therapy.
In this study, a 2-year therapy of lamivudine resulted in a decrease in liver fibrosis score, ALT, and AST levels, and an increase in PPC. Although we previously demonstrated that PPC has a negative correlation with liver fibrosis score in patients with CHB [21], this study did not reveal this association after a 2-year lamivudine therapy. Although PPC increased and liver fibrosis score decreased, no association was found between the alterations of these two parameters after 2-year lamivudine therapy. This finding might suggest the absence of direct interaction between these two parameters. We also found that a decreased liver fibrosis score is associated with decreased AST levels. It is well-known that with progressing hepatic fibrosis, reduction of AST clearance and increased releasing of AST from mitochondrial injury lead to increased serum AST levels rather than ALT levels [24,25].
Our study showed that increased PPC was associated with decreased ALT levels. ALT is known to be a marker of hepatic injury/ inflammation. Thus, alteration in hepatic inflammation during the lamivudine therapy might result in an alteration in PPC. Several studies have shown that nucleoside analogs have immunomodulating capabilities in patients with CHB [26-30]. Factors such as the release of inflammatory cytokines during lamivudine therapy might be responsible for these alterations in PPC and liver fibrosis score.
Conclusion
In conclusion, the present study showed that lamivudine therapy increased PPC independently from decreased fibrosis score. Increased PPC was correlated only with decreased ALT levels. Further studies investigating the potential interaction between the alterations of PPC and inflammatory status during therapy with nucleoside analogs are needed.
References
- Landolfi R, Leone G, Fedeli G, Storti S, Laghi F, et al. (1980) Platelet-associated IgG in acute and chronic hepatic diseases. Scand J Haematol 25: 417-422.
- de Noronha R, Taylor BA, Wild G, Triger DR, Greaves M (1991) Interrelationships between platelet count, platelet IgG, serum IgG, immun-complexes and severity of liver disease. Clin Lab Haematol 13: 127-135.
- Skootsky SA, Rosove MH, Langley MB (1986) Immune thrombocytopenia and response to splenectomy in chronic liver disease. Arch Intern Med 146: 555-557.
- Paramo JA, Rocha E (1993) Hemostasis in advanced liver disease. Semin Thromb Hemost 19: 184-190.
- Wehmeier A, Scharf RE, Schneider W (1990) Influence of splenectomy on platelet morphometry and function. Klin Wochenschr 68: 847-852.
- Thomas HC, McSween RN, White RG (1973) Role of the liver in controlling the immunogenicity of commensal bacteria in the gut. Lancet 1: 1288-1291.
- Triger DR, Wright R (1973) Hyperglobulinaemia in liver disease. Lancet 1: 1494-1496.
- Kew MC (1996) Hepatitis B and C viruses and hepatocellular carcinoma. Clin Lab Med 16: 395-406.
- Sungaran R, Markovic B, Chong BH (1997) Localization and regulation of thrombopoietin mRNA expression in human kidney, liver, bone marrow and spleen using in situ hybridization. Blood 89: 101-107.
- Martin TG 3rd, Somberg KA, Meng YG, Cohen RL, Heid CA, et al. (1997) Thrombopoietin levels in patients with cirrhosis before and after orthotopic liver transplantation. Ann Intern Med 127: 285-288.
- Peck-Radosavljevic M, Zacherl J, Meng YG, Pidlich J, Lipinski E, et al. (1997) Is inadequate thrombopoietin production a major cause of thrombocytopenia in cirrhosis of the liver? J Hepatol 27: 127-131.
- Ishikawa T, Ichida T, Matsuda Y, Sugitani S, Sugiyama M, et al. (1998) Reduced expression of thrombopoietin is involved in thrombocytopenia in human and rat liver cirrhosis. J Gastroenterol Hepatol 13: 907-913.
- Lai CL, Chien RN, Leung NW, Chang TT, Guan R, et al. (1998) A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med 339: 61-68.
- Jarvis B, Faulds D (1999) Lamivudine. A review of its therapeutic potential in chronic hepatitis B. Drugs 58: 101-141.
- Dienstag JL, Goldin RD, Heathcote EJ, Hann HW, Woessner M, et al. (2003) Histological outcome during long-term lamivudine therapy. Gastroenterology 124: 105-117.
- Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, et al. (2003) A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 38: 518-526.
- Poynard T, Bedossa P (1997) Age and platelet count: A simple index for predicting the presence of histological lesions in patients with antibodies to hepatitis C virus. METAVIR and CLINIR Cooperative Study Groups. J Viral Hepatol 4: 199-208.
- Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, et al. (2006) Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 43: 1317-1325.
- Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, et al. (2007) FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology 46: 32-36.
- Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, et al. (1981) Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 1: 431-435.Â
- Karasu Z, Tekin F, Ersoz G, Gunsar F, Batur Y, et al. (2007) Liver fibrosis is associated with decreased peripheral platelet count in patients with chronic hepatitis B and C. Dig Dis Sci 52: 1535-1539.
- Shin WG, Park SH, Jang MK, Hahn TH, Kim JB, et al. (2008) Aspartate aminotransferase to platelet ratio index (APRI) can predict liver fibrosis in chronic hepatitis B. Dig Liver Dis 40: 267-274.
- Kim BK, Kim SA, Park YN, Cheong JY (2007) Noninvasive models to predict liver cirrhosis in patients with chronic hepatitis B. Liver Int 27: 969-976.
- Nalpas B, Vassault A, Le Guillou A, Lesgourgues B, Ferry N, et al. (1984) Serum activity of mitochondrial aspartate aminotransferase: a sensitive marker of alcoholism with or without alcoholic hepatitis. Hepatology 4: 893-896.
- Kamimoto Y, Horiuchi S, Tanase S, Morino Y (1985) Plasma clearance of intravenously injected aspartate aminotransferase isozymes: Evidence for preferential uptake by sinusoidal liver cells. Hepatology 5: 367-375.
- Boni C, Penna A, Ogg GS, Bertoletti A, Pilli M, et al. (2001) Lamivudine treatment can overcome cytotoxic T-cell hyporesponsiveness in chronic hepatitis B: New perspectives for immune therapy. Hepatology 33: 963-971.
- You J, Sriplung H, Geater A, Chongsuvivatwong V, Zhuang L, et al. (2008) Impact of viral replication inhibition by entecavir on peripheral T lymphocyte subpopulations in chronic hepatitis B patients. BMC Infect Dis 8: 123.
- Evans A, Riva A, Cooksley H, Phillips S, Puranik S, et al. (2008) Programmed death 1 expression during antiviral treatment of chronic hepatitis B: Impact of hepatitis B e-antigen seroconversion. Hepatology 48: 759-769.
- Wu ZG, Yan WM, Guo W, Chen T, Zou Y, et al. (2010) Telbivudine preserves T-helper 1 cytokine production and downregulates programmed death ligand 1 in a mouse model of viral hepatitis. J Viral Hepat 17(Suppl 1): 24-33.
- Shi TD, Zhang JM, Wang XF, Chen M, Sun H, et al. (2012) Effects of antiviral therapy with Telbivudine on peripheral iNKT cells in HBeAg(+) chronic hepatitis B patients. Clin Exp Med 12: 105-113.
Citation: Tekin F, Karasu Z, Gunsar F, Nart D, Yilmaz F, et al. (2018) Increased Peripheral Platelet Count is Unrelated to Decreased Liver Fibrosis Score Following lamivudine Treatment of Patients with Chronic Hepatitis B . J Gastrointest Dig Syst 8: 549. DOI: 10.4172/2161-069X.1000549
Copyright: © 2018 Tekin F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4939
- [From(publication date): 0-2018 - Apr 19, 2025]
- Breakdown by view type
- HTML page views: 4100
- PDF downloads: 839