Incidence and Outcomes of Invasive Pulmonary Aspergillosis in Critically Ill Patients with COVID-19: A Systematic Review and Meta-Analysis
Received: 16-Mar-2022 / Manuscript No. jidt-22-51743 / Editor assigned: 18-Mar-2022 / PreQC No. jidt-22-51743(PQ) / Reviewed: 04-Apr-2022 / QC No. jidt-22-51743 / Revised: 11-Apr-2022 / Manuscript No. jidt-22-51743(R) / Published Date: 18-Apr-2022
Abstract
Background: Recent reports of coronavirus disease 2019 (COVID-19)-associated pulmonary aspergillosis (CAPA) have raised concerns about fungal super-infections in critically ill patients with COVID-19. However, true incidence of CAPA is difficult to estimate given the challenges of diagnosing invasive pulmonary aspergillosis as well as the heterogeneity in case definitions.
Methods: We conducted a systematic review of literature (through 31st December 2021) for relevant studies reporting on the CAPA incidence in critically ill COVID-19 patients. Random effects meta-analyses were conducted. Risk of bias and certainty of evidence were assessed using the appropriate Joanna Briggs Institute checklists and the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) approach respectively. The primary outcome was the overall incidence of CAPA. Secondary outcomes included CAPA-associated mortality, ICU length of stay, duration of invasive mechanical ventilation, and the time between COVID-19 symptoms and CAPA diagnosis.
Results: Of 53 observational studies (11,013 adult COVID-19 patients), 1097 patients had CAPA; the pooled incidence of CAPA was 13.0% (95%-CI: 8.7%-18.0%), albeit with publication bias (pegger=0.002) and the pooled mortality was 63.4% (95%-CI: 56.2%-70.4%). Patients with CAPA were older (+3.1 years (95%-CI: 1.3-4.9, p=0.0006) with significantly increased mortality (Risk Ratio: 2.13 (95%- CI: 1.80-2.52, p<0.0001) compared to COVID-19 patients without CAPA. Meta-regression analysis found that corticosteroids and/or hypertension were potential risk factors for CAPA
Conclusion: Critically ill patients with COVID-19 are vulnerable to develop CAPA with considerably high mortality rates; patients with hypertension and those treated with corticosteroids as an adjuvant therapy appear to be at higher risk of CAPA.
Keywords: COVID-19; Associated pulmonary aspergillosis; Incidence; Mortality; IL-6 antagonists; Corticosteroids
Abbreviations
COVID-19: Coronavirus Disease-2019; CAPA: COVID-19 Associated Pulmonary Aspergillosis; IL-6: Interleukin-6; IPA: Invasive Pulmonary Aspergillosis; ICU: Intensive Care Unit; LOS: Length of Stay; IMV: Invasive Mechanical Ventilation; SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2 IAPA: Influenza- Associated Pulmonary Aspergillosis; EORTC-MSG: European Organization for Research and Treatment of Cancer Mycoses Study Grou; JBI: Joanna Briggs Institute BAL: Broncho Alveolar Lavage; CIs: Confidence Intervals; SMD: Standardised Mean Differences; GRADE: Grading of Recommendations, Assessments, Developments and Evaluations.
Introduction
With the increasing global burden of coronavirus disease 2019 (COVID-19) cases; there has been a concomitant rise in incidence of secondary bacterial and fungal co-infections [1]. Viral pneumonia, in general, is associated with an increased incidence of secondary co- infections, and this is estimated to be between 6%-16% in critically ill patients with COVID-19 [2]. COVID-19 associated pulmonary aspergillosis (CAPA) has being increasingly reported worldwide, which is associated with increased mortality and morbidity [3]. It has been postulated that these co-infections are secondary to dysregulated immune responses associated with COVID-19 itself or due to the use of adjunctive therapies such as corticosteroids or interleukin-6 (IL-6) antagonists [2,4,5,6]. Prior reviews reported that the CAPA incidence varies between 10-13% [6-8,9]. However, the true incidence of CAPA in critically ill hospitalized patients is still undetermined due to diagnostic challenges involved as well as the observational nature of the published studies. With new studies being published, we conducted this systematic review and meta-analysis to explore the incidence, risk factors and outcomes associated with invasive pulmonary aspergillosis (IPA) in critically ill patients with COVID-19 [4,6,10].
Methodology
Search strategy and selection criteria
This review was registered with PROSPERO (CRD42021227821) and was conducted in adherence with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement. We searched Medline, Embase, Cochrane, Scopus, medRxiv databases from 1st December 2019 through 31st December 2021 using the following keywords and their variations: “COVID-19” and “aspergillosis” (Supplementary Table 1) [11]. We assessed all the relevant studies, and their citation lists to identify articles for inclusion. All studies reporting on at least 10 critically ill adult patients (≥ 18 years) with COVID-19 were included. We excluded any non-human studies, and any case reports to avoid publication bias. In the case of overlapping patient data across two or more studies, we included the larger study
| Study | CAPA | Total | Incidence of CAPA (%) | Incidence (%) | 95% CI | Weight |
|---|---|---|---|---|---|---|
| Autier 2021 | 67 | 239 |  |
28.0 | [22.4; 34.2] | 2.0% |
| Bartoletti 2020 | 30 | 185 | 16.2 | [11.2; 22.3] | 2.0% | |
| Borman 2020 | 15 | 719 | 2.1 | [ 1.2; 3.4] | 2.0% | |
| Boyd 2021 | 0 | 55 | 0.0 | [ 0.0; 6.5] | 1.9% | |
| Bretange 2021 | 154 | 244 | 63.1 | [56.7; 69.2] | 2.0% | |
| Chauvet 2020 | 6 | 46 | 13.0 | [ 4.9; 26.3] | 1.8% | |
| Delliere 2020 | 21 | 366 | 5.7 | [ 3.6; 8.6] | 2.0% | |
| Dupont 2020 | 19 | 106 | 17.9 | [11.2; 26.6] | 1.9% | |
| Ergun 2021 | 58 | 219 | 26.5 | [20.8; 32.9] | 2.0% | |
| Fekkar 2021 | 7 | 145 | 4.8 | [ 2.0; 9.7] | 1.9% | |
| Ghazanfari 2021 | 22 | 105 | 21.0 | [13.6; 30.0] | 1.9% | |
| Gregoire 2021 | 9 | 141 | 6.4 | [ 3.0; 11.8] | 1.9% | |
| Hatzl 2021 | 10 | 132 | 7.6 | [ 3.7; 13.5] | 1.9% | |
| Hoenigl 2021 | 28 | 59 | 47.5 | [34.3; 60.9] | 1.9% | |
| Iqbal 2021 | 61 | 307 | 19.9 | [15.6; 24.8] | 2.0% | |
| Janssen 2021 cohort 1 | 42 | 519 | 8.1 | [ 5.9; 10.8] | 2.0% | |
| Janssen 2021 cohort 2 | 21 | 304 | 6.9 | [ 4.3; 10.4] | 2.0% | |
| Jasim 2021 | 11 | 102 | 10.8 | [ 5.5; 18.5] | 1.9% | |
| Kluzik 2021 | 7 | 19 | 36.8 | [16.3; 61.6] | 1.6% | |
| Koehler 2020 | 5 | 19 | 26.3 | [ 9.1; 51.2] | 1.6% | |
| Lahmer 2021 | 11 | 32 | 34.4 | [18.6; 53.2] | 1.8% | |
| Lamoth 2020 | 3 | 118 | 2.5 | [ 0.5; 7.3] | 1.9% | |
| Machado 2020 | 8 | 239 | 3.3 | [ 1.5; 6.5] | 2.0% | |
| Maes 2021 | 3 | 81 | 3.7 | [ 0.8; 10.4] | 1.9% | |
| Martin 2021 | 3 | 19 | 15.8 | [ 3.4; 39.6] | 1.6% | |
| Meijer 2021 | 13 | 66 | 19.7 | [10.9; 31.3] | 1.9% | |
| Nasir 2020 | 5 | 23 | 21.7 | [ 7.5; 43.7] | 1.7% | |
| Nebreda−Mayoral 2021 | 3 | 113 | 2.7 | [ 0.6; 7.6] | 1.9% | |
| Oliva 2021 | 0 | 55 | 0.0 | [ 0.0; 6.5] | 1.9% | |
| Paramythiotou 2021 | 6 | 179 | 3.4 | [ 1.2; 7.2] | 2.0% | |
| Prattes 2021 | 109 | 592 | 18.4 | [15.4; 21.8] | 2.0% | |
| Rabagliati 2021 | 13 | 146 | 8.9 | [ 4.8; 14.7] | 1.9% | |
| Ranhel 2021 | 10 | 248 | 4.0 | [ 2.0; 7.3] | 2.0% | |
| Razazi 2020 | 13 | 90 | 14.4 | [ 7.9; 23.4] | 1.9% | |
| Ripa 2020 | 11 | 731 | 1.5 | [ 0.8; 2.7] | 2.0% | |
| Roman-Montes 2020 | 14 | 144 | 9.7 | [ 5.4; 15.8] | 1.9% | |
| Rutsaert 2020 | 4 | 34 | 11.8 | [ 3.3; 27.5] | 1.8% | |
| Segrelles-Calvo 2020 | 7 | 215 | 3.3 | [ 1.3; 6.6] | 2.0% | |
| Sivasubramanian 2021 | 48 | 970 | 4.9 | [ 3.7; 6.5] | 2.0% | |
| Sogaard 2021 | 2 | 41 | 4.9 | [ 0.6; 16.5] | 1.8% | |
| Szabo 2021 | 20 | 20 | 100.0 | [83.2; 100.0] | 1.7% | |
| Takazono 2021 | 10 | 10 | 100.0 | [69.2; 100.0] | 1.4% | |
| Tio 2021 | 4 | 50 | 8.0 | [ 2.2; 19.2] | 1.8% | |
| van Arkel 2020 | 6 | 31 | 19.4 | [ 7.5; 37.5] | 1.8% | |
| van Grootveld 2021 | 11 | 63 | 17.5 | [ 9.1; 29.1] | 1.9% | |
| Velez 2021 | 16 | 83 | 19.3 | [11.4; 29.4] | 1.9% | |
| Versyck 2021 | 2 | 54 | 3.7 | [ 0.5; 12.7] | 1.9% | |
| Wang 2020 | 0 | 26 | 0.0 | [ 0.0; 13.2] | 1.7% | |
| Wasylyshyn 2021 | 3 | 256 | 1.2 | [ 0.2; 3.4] | 2.0% | |
| White 2021 | 25 | 135 | 18.5 | [12.4; 26.1] | 1.9% | |
| Xu 2021 | 78 | 393 | 19.8 | [16.0; 24.1] | 2.0% | |
| Yusuf 2021 | 10 | 92 | 10.9 | [ 5.3; 19.1] | 1.9% | |
| Zhang 2021 | 33 | 1633 | 2.0 | [ 1.4; 2.8] | 2.0% | |
|
Random effects model Heterogeneity: I2= 96.3% |
11013 | 13.0 | [8.7; 18.0] | 100.0% |
Table 1: Pooled incidence of COVID-19 associated pulmonary aspergillosis among critically ill patients with COVID-19.
Data collection
Data were collected using a prespecified data extraction form, and covered study characteristics (study design, study duration, name and country of study centre), patient demographics (sample size, number of male/female patients, age, comorbidities), Intensive Care Unit (ICU) characteristics (time to CAPA from onset of COVID-19 symptoms, classification system for CAPA, methods used to diagnose CAPA (bronchoscopic and non-bronchoscopic techniques), PaO2/FiO2 ratio, use of IL-6 antagonists and corticosteroids), patient outcomes (incidence of proven, probable, putative, and possible CAPA, incidence of Aspergillus colonisation, CAPA-associated mortality) and other relevant clinical outcomes (ICU length of stay [LOS] and duration of Invasive Mechanical Ventilation [IMV]).
CAPA is defined as IPA in temporal proximity to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in patients requiring ICU admission due to respiratory failure. We defined proven, probable, putative and possible CAPA for this review as per the: Influenza-Associated Pulmonary Aspergillosis (IAPA) case definition, 2) the European Organization for Research and Treatment of Cancer Mycoses Study Group (EORTC-MSG), 3) crude or modified Aspergillosis in ICU (crude/mAsp-ICU) criteria or 4) 2020 European Confederation for Medical Mycology and the International Society for Human and Animal Mycology (ECMM/ISHAM) consensus criteria.2,12-14 In cases where both the crude Asp-ICU and mAsp-ICU criteria were used, we chose the mAsp-ICU criteria to define CAPA [2,12-14].
Risk of bias assessment
We used the appropriate Joanna Briggs Institute (JBI) critical appraisal checklists (Supplementary Table 2) to assess the eligibility of studies. The possibility of publication bias was assessed using Egger’s test. Two sensitivity analyses were performed for our meta-analysis: One where we excluded studies which had comparatively higher risks of bias (JBI score<8). The screening of articles, data collection, and risk of bias assessment were conducted independently by at least two reviewers (RRL, SAM, MRL, and SM), any conflicts were resolved by KR.
| Meta-analysis | Number of studies | Incidence of CAPA (%) | CAPA | 95%-CI |
|---|---|---|---|---|
|
Colonisation Heterogeneity: I2 =89%, τ2 =0.0158, p<0.01 |
11 | 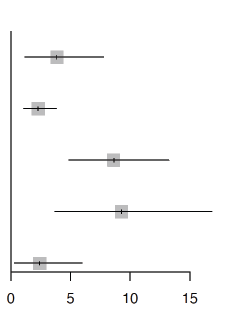 |
3.9 | [1.2; 7.8] |
|
Possible Heterogeneity: I2 =88%, τ2 =0.0061, p<0.01 |
16 | 2.3 | [1.1; 3.8] | |
|
Probable Heterogeneity: I2 =97%,τ2 =0.0376, p<0.01 |
29 | 8.6 | [4.9; 13.2] | |
|
Putative Heterogeneity: I2 =91%, τ2 =0.0506, p<0.01 |
18 | 9.3 | [3.7; 16.8] | |
|
Proven Heterogeneity: I2 =91%, τ2 =0.0188, p<0.01 |
11 | 2.4 | [0.3; 6.0] |
Table 2: Pooled incidence of COVID-19 associated pulmonary aspergillosis among critically ill patients with COVID-19 stratified by definition.
Statistical analysis
Statistical analyses were performed on R4.0.2. For continuous variables, we pooled the means and standard deviations from the aggregate data presented in each study as per Wan, et al. [15] The primary outcome was the overall incidence of CAPA. Secondary outcomes included CAPA-associated mortality, ICU LOS, duration of IMV, and the time between the onset of COVID-19 symptoms and diagnosis of CAPA. We anticipated significant inter-study heterogeneity given variations in the demographics of patients with CAPA and its subsequent management in different countries. As such, random-effects meta-analyses (DerSimonian and Laird) were conducted, and 95% confidence intervals (CIs) were computed using the Clopper-Pearson method. Survival outcomes are presented as pooled proportions, dichotomous outcomes as pooled risk ratios (RR), and continuous outcomes as pooled means or mean differences (MD), each with their respective 95%-CIs [16,17]. Planned subgroup analyses were conducted with continuity correction to include studies with zero events, and included: The definition of CAPA (proven, probable, putative, and possible), study type (cohort studies, case control studies, case series), geographical region (Europe, Asia-Pacific, South America, North America, or International), the consensus definitions that were used in each study (EORTC-MSG, Asp-ICU [crude and/or modified], IAPA, 2020 ECMM/ISHAM, or mixed), and the methods of diagnosis {bronchoalveolar lavage (BAL), or no BAL}. Post-hoc subgroup analyses include timing of patient enrolment (before and after the publication of the Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial final report in February 2021. Univariable study-level meta-regression was conducted when at least 6 data points were collected to explore potential sources of heterogeneity or prognostically-relevant study-level covariates [18]. As inter-study heterogeneity can be misleadingly large when assessed using I2 statistics for observational studies, we used the Grading of Recommendations [19,20], Assessments, Developments and Evaluations (GRADE) approach to assess the inter-study heterogeneity [21,22].
Results
Of the 832 references screened; our search yielded 127 potentially relevant studies across the four databases (Supplementary Figure 1: PRISMA chart). 53 retrospective observational studies reporting on 11,013 adult patients with COVID-19 admitted to the ICU were included [23-74]. There were 24 cohort studies, 2 case control studies and 24 case series; 38 studies were from Europe, 7 from Asia, 5 from North America, 1 from South America, and 2 were conducted internationally. 44 studies reported the criteria that they used to diagnose CAPA: 7 studies diagnosed patients with CAPA using the EORTC-MSG criteria, 8 studies used either the crude Asp-ICU or mAsp-ICU criteria, 3 study used the IAPA case definition, 16 studies used the ECMM/ISHAM criteria, and 9 studies reported CAPA using two or more of the above criteria. All studies except for 6 employed BAL to diagnose CAPA. Only one study employed histopathological examination to diagnose CAPA.
Baseline demographics
The pooled age of patients with COVID-19 patients in the ICU was 62.5 years (95%-CI: 60.8-64.3). They were predominantly male (63.2% (95%-CI: 56.0-70.1%, 30 studies) with moderate-to-severe ARDS (PaO2/FiO2 ratio: 130.9, 95%-CI: 86.1-175.7, 8 studies). Patients with CAPA were older (+3.1 years, 95%-CI: 1.3-4.9, p=0.0006, 14 studies) and predominantly comprising males (RR: 1.14, 95%-CI: 1.0- 1.21, p<0.0001, 14 studies) when compared to COVID-19 patients without CAPA. The pooled prevalence of hypertension (HTN, 30 studies), obesity (17 studies), diabetes mellitus (DM, 39 studies) and chronic obstructive pulmonary disease (COPD, 15 studies) were 27.2% (95%-CI: 17.2%-38.5%), 19.1% (95%-CI: 9.6%-30.7%), 18.4% (95%- CI: 13.8%-23.4%) and 9.7% (95%-CI: 4.8%-16.1%) respectively. The prevalence of IL-6 antagonists and corticosteroid therapy were 8.2% (95%-CI: 3.6%-14.3%, 21 studies) and 26.3% (95%-CI: 15.7%-38.4%, 29 studies) respectively. All the included patients were admitted in intensive care unit (ICU).
Primary outcomes
Of 53 observational studies (11,013 critically ill patients with COVID-19), 1097 patients had CAPA; the pooled overall CAPA incidence was 13.0% (95%-CI: 8.7%-18.0%, moderate certainty, Table 1), with significant publication bias (pegger=0.002, Supplementary Figure 2). After excluding studies with a JBI score of <8, the pooled overall incidence of CAPA was 12.7% (95%-CI: 8.0% to 18.3%). The incidence of CAPA changed significantly {4.1% (95%-CI: 1.1% to 8.4%)} after correcting for small study effects using the random-effects (R0 estimator) trim-and-fill procedure.
Subgroup analysis
The pooled incidence of colonisers (11 studies), possible (16 studies), probable (29 studies), putative (18 studies) and proven CAPA (11 studies), was 3.9% (95%-CI: 1.2%-7.8%), 2.3% (95%-CI: 1.1%- 3.8%),8.6% (95%-CI: 4.9%-13.2%), 9.3% (95%-CI: 3.7%-16.8%) and 2.4% (95%-CI: 0.3%-6.0%) respectively (Table 2). The pooled incidence of CAPA among studies from Europe (38 studies), Asia (7 studies), North America (5 studies), International (2 studies) and South America (1 study) was 12.2% (95%-CI: 7.5%-17.9%), 22.0% (95%-CI: 3.0%- 50.2%), 5.8% (95%-CI:1.4%-12.6%), 31.4% (95%-CI:7.6%-62.1%) and 8.9% (95%-CI:4.8% to 14.1%) respectively. The CAPA incidence based on ECMM/ISHAM (16 studies), Asp-ICU (8 studies), EORTC-MSG (7 studies), IAPA (3 studies) and mixed (9 studies) consensus criteria was 12.2% (95%-CI: 8.3%-16.8%), 9.1% (95%-CI:2.4%-18.9%), 7.9% (95%-CI:4.3% to 12.4%), 6.8% (95%-CI: 0.9%-16.8%) and 19.6% (95%- CI: 4.3%-41.8%) respectively found no significant difference (p=0.16). CAPA incidence was estimated to be 15.5% (95%-CI:9.5%-22.5%) when BAL was used for diagnosis compared to 9.9% (95%-CI:4.4%-17.1%) when non-bronchoscopic techniques were advocated for diagnosis (p>0.05). No significant difference ( p=0.44) in CAPA incidence was noted between pre-RECOVERY (43 studies, 14.1%, 95%-CI: 8.8-20.3%) and post RECOVERY trials (9 studies, 10.7%, 95%-CI: 5.1%-17.8%). There was no significant difference in the pooled incidence reported between study types (p=0.81). The details of the subgroup analysis are summarised in Table 3.
| Subgroup analysis | Pooled estimate (95%-CI) | |
|---|---|---|
| Definition of CAPA | Colonisers | 3.9% (1.2% to 7.8%) |
| Possible | 2.3% (1.1% to 3.8%) | |
| Probable | 8.6% (4.9% to 13.2%) | |
| Putative | 9.3% (3.7% to 16.8%) | |
| Proven | 2.4% (0.3% to 6.0%) | |
| Consensus criteria | AspICU | 9.1% (2.4% to 18.9%) |
| ECMM/ISHAM | 12.2% (8.3% to 16.8%) | |
| EORTC-MSG | 7.9% (4.3% to 12.4%) | |
| IAPA | 6.8% (0.9% to 16.8%) | |
| Mixed | 19.6% (4.3% to 41.8%) | |
| Geographical region | Europe | 12.2% (7.5% to 17.9%) |
| International | 31.4% (7.6% to 62.1%) | |
| Asia-Pacific | 22.0% (3.0% to 50.2%) | |
| South America | 8.9% (4.8% to 14.1%) | |
| North America | 5.8% (1.4% to 12.6^) | |
| Diagnostic methods | Bronchoalveolar lavage | 15.5% (9.5% to 22.5%) |
| No bronchoalveolar lavage | 9.9% (4.4% to 17.1%) | |
Table 3: Pooled incidence of COVID-19 associated pulmonary aspergillosis among critically ill patients with COVID-19.
Meta-regression analyses
Univariable meta-regression found that the prevalence of HTN (Regression co-efficient [B]: 0.559, 95%-CI: 0.212-0.905, p=0.002, Supplementary Figure 3) and corticosteroids (B:0.506, 95%-CI: 0.097-0.915, p=0.015, Supplementary Figure 4) use were significantly associated with increased CAPA incidence, while overall sample size was negatively associated with the incidence of CAPA (B:-0.002, 95%-CI:-0.005 to-0.000, p=0.037, Supplementary Figure 5). Other demographic factors such as age, prevalence of male patients, use of IL-6 antagonists and other comorbidities were not associated with the incidence of CAPA. The details of the meta-regression analyses are summarised in Table 4.The availability of simplified analytic modeling tools that can help decision makers determines when and how to reopen certain congregate settings, like schools, is an absolute necessity. In this research, we offer a strategic analytic tool for utilization of low-cost antigen tests in a comprehensive, targeted testing strategy, which in our perspective as academics specializing in business and biotechnology management is critical and allows for effective use of the various planning and execution protocols. Furthermore, strategic deployments have the potential to improve dramatically the production, procurement, and distribution of test kits, and can be of critical help to control and mitigate the spread of the SARS-CoV-2 virus in the U.S., and around the globe.
| Covariate | Studies | B | LCI | UCI | P-value |
|---|---|---|---|---|---|
| Sample size | 53 | -0.0024 | -0.0047 | -0.0001 | 0.037 |
| Age | 34 | 0.0091 | -0.0072 | 0.0255 | 0.27 |
| PaO2-to-FiO2 ratio | 8 | -0.0007 | -0.0015 | 0.0002 | 0.15 |
| Proportion of male patients | 30 | -0.0562 | -0.5464 | 0.4341 | 0.82 |
| Proportion of patients receiving IL-6A | 22 | 0.6738 | -0.2636 | 1.6112 | 0.16 |
| Proportion of patients with HTN | 30 | 0.5587 | 0.2124 | 0.905 | 0.0016 |
| Proportion of patients with DM | 39 | 0.3742 | -0.1999 | 0.9484 | 0.2 |
| Proportion of patients receiving steroids | 29 | 0.5059 | 0.0967 | 0.915 | 0.015 |
| Proportion of patients receiving RRT | 17 | 0.5818 | -0.6568 | 1.8203 | 0.36 |
Note: B: regression coefficient, LCI: lower 95% confidence interval, UCI: upper 95% confidence interval, IL-6A: interleukin 6 antagonists, DM: Type 2 diabetes mellitus, HTN: Hypertension., RRT: Renal replacement therapy,
*Values in bold indicate p<0.05.
Table 4: Pooled incidence of COVID-19 associated pulmonary aspergillosis among critically ill patients with COVID-19 stratified by definition.
Secondary outcomes
Overall CAPA-associated mortality (42 studies, 814 patients with CAPA) was 63.4% (95%-CI: 56.2%-70.4%, moderate certainty, Table 5). CAPA was associated with increased risk of mortality in critically ill patients (18 studies, RR: 2.13 (95%-CI: 1.80-2.52, p<0.0001) compared to critically ill patients without CAPA. The pooled mean time for diagnosis of CAPA (19 studies, 353 patients with CAPA) was made 12.8 days (95%-CI: 10.5-15.1, moderate certainty) after the onset of COVID-19 symptoms. The pooled ICU LOS among patients with CAPA (13 studies, 287 patients) was 18.4 days (95%-CI: 13.7-23.1, moderate certainty), but there was no significant mean difference (MD) in ICU LOS between patients with and without CAPA (8 studies, 0.22 days, 95%-CI:-4.23 to 4.68, p=0.92). The mean duration of IMV among patients with CAPA was 17.2 days (5 studies, 95%-CI:15.5-19.0, moderate certainty).
| Study | Mortality | CAPA | Mortality (%) | Mortality (%) | 95% CI | Weight |
|---|---|---|---|---|---|---|
| Maes 2021 | 1 | 3 |  |
33.3 | [ 0.8; 90.6] | 1.1% |
| Martin 2021 | 1 | 3 | 33.3 | [ 0.8; 90.6] | 1.1% | |
| Lamoth 2020 | 1 | 3 | 33.3 | [ 0.8; 90.6] | 1.1% | |
| Wasylyshyn 2021 | 1 | 3 | 33.3 | [ 0.8; 90.6] | 1.1% | |
| Nebreda-Mayoral 2021 | 2 | 3 | 66.7 | [ 9.4; 99.2] | 1.1% | |
| Versyck 2021 | 2 | 2 | 100.0 | [15.8; 100.0] | 0.9% | |
| Koehler 2020 | 3 | 5 | 60.0 | [14.7; 94.7] | 1.6% | |
| Ranhel 2021 | 3 | 10 | 30.0 | [ 6.7; 65.2] | 2.3% | |
| Nasir 2020 | 3 | 5 | 60.0 | [14.7; 94.7] | 1.6% | |
| Tio 2021 | 3 | 4 | 75.0 | [19.4; 99.4] | 1.4% | |
| Lahmer 2021 | 4 | 11 | 36.4 | [10.9; 69.2] | 2.4% | |
| Rutsaert 2020 | 4 | 4 | 100.0 | [39.8; 100.0] | 1.4% | |
| Paramythiotou 2021 | 4 | 6 | 66.7 | [22.3; 95.7] | 1.7% | |
| Fekkar 2021 | 4 | 7 | 57.1 | [18.4; 90.1] | 1.9% | |
| Chauvet 2020 | 4 | 6 | 66.7 | [22.3; 95.7] | 1.7% | |
| Rabagliati 2021 | 4 | 13 | 30.8 | [ 9.1; 61.4] | 2.5% | |
| van Arkel 2020 | 4 | 6 | 66.7 | [22.3; 95.7] | 1.7% | |
| Gregoire 2021 | 5 | 9 | 55.6 | [21.2; 86.3] | 2.1% | |
| Segrelles-Calvo 2020 | 5 | 7 | 71.4 | [29.0; 96.3] | 1.9% | |
| Takazono 2021 | 5 | 10 | 50.0 | [18.7; 81.3] | 2.3% | |
| Velez 2021 | 5 | 16 | 31.2 | [11.0; 58.7] | 2.7% | |
| Meijer 2021 | 6 | 13 | 46.2 | [19.2; 74.9] | 2.5% | |
| Kluzik 2021 | 7 | 7 | 100.0 | [59.0; 100.0] | 1.9% | |
| Dupont 2020 | 7 | 19 | 36.8 | [16.3; 61.6] | 2.9% | |
| Hatzl 2021 | 8 | 10 | 80.0 | [44.4; 97.5] | 2.3% | |
| Machado 2020 | 8 | 8 | 100.0 | [63.1; 100.0] | 2.0% | |
| Roman-Montes 2020 | 8 | 14 | 57.1 | [28.9; 82.3] | 2.6% | |
| Janssen 2021 cohort 2 | 9 | 21 | 42.9 | [21.8; 66.0] | 3.0% | |
| van Grootveld 2021 | 10 | 11 | 90.9 | [58.7; 99.8] | 2.4% | |
| Szabo 2021 | 12 | 20 | 60.0 | [36.1; 80.9] | 3.0% | |
| White 2021 | 13 | 25 | 52.0 | [31.3; 72.2] | 3.2% | |
| Delliere 2020 | 15 | 21 | 71.4 | [47.8; 88.7] | 3.0% | |
| Bartoletti 2020 | 16 | 30 | 53.3 | [34.3; 71.7] | 3.3% | |
| Hoenigl 2021 | 18 | 28 | 64.3 | [44.1; 81.4] | 3.2% | |
| Ghazanfari 2021 | 20 | 22 | 90.9 | [70.8; 98.9] | 3.0% | |
| Janssen 2021 cohort 1 | 22 | 42 | 52.4 | [36.4; 68.0] | 3.5% | |
| Zhang 2021 | 22 | 33 | 66.7 | [48.2; 82.0] | 3.4% | |
| Ergun 2021 | 29 | 58 | 50.0 | [36.6; 63.4] | 3.7% | |
| Sivasubramanian 2021 | 40 | 48 | 83.3 | [69.8; 92.5] | 3.6% | |
| Xu 2021 | 51 | 78 | 65.4 | [53.8; 75.8] | 3.9% | |
| Iqbal 2021 | 56 | 61 | 91.8 | [81.9; 97.3] | 3.8% | |
| Prattes 2021 | 62 | 109 | 56.9 | [47.0; 66.3] | 4.0% | |
| Random effects model Heterogeneity: I2 = 65.7% |
814 | 63.4 | [56.2; 70.4] | 100.0% |
Table 5: Pooled proportion of non-survivors among critically ill patients with COVID-19 associated pulmonary aspergillosis.
Assessment of study quality
Based on the JBI critical appraisal checklists for cohort studies, case control studies, and case series, the majority studies included for this review were of high quality according to the appropriate checklist (Supplementary Table 2). There was moderate certainty on the level of evidence for the primary outcome. A summary of the assessment of certainty using the GRADE approach is presented in Table 6.
| № of studies | Certainty assessment | Effect | Certainty | Importance | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | No of events | No of individuals | Rate (95% CI) |
|||||||||||
| Incidence of COVID-19 associated pulmonary aspergillosis (CAPA) | |||||||||||||||||||
| 53 | observational studies | not serious | not seriousa | not serious | not seriousb | publication bias strongly suspected | - | 11013 | mean 13.0 % (8.7 to 18) | ⨁⨁⨁◯ Moderate |
Critical | ||||||||
| Mortality among patients with CAPA | |||||||||||||||||||
| 42 | observational studies | not serious | not seriousc | not serious | seriousd | none | - | 814 | mean 63.4 % (56.2 to 70.4) | ⨁⨁⨁◯ Moderate |
Critical | ||||||||
| Intensive care unit length of stay for patients with CAPA | |||||||||||||||||||
| 13 | observational studies | not serious | not seriousc | not serious | seriousd | none | - | 287 | mean 18.4 days (13.7 to 23.1) | ⨁⨁⨁◯ Moderate |
Important | ||||||||
| Duration of invasive mechanical ventilation for all patients with COVID-19 | |||||||||||||||||||
| 5 | observational studies | not serious | not serious | not serious | seriousd | none | - | 178 | mean 17.2 days (15.5 to 19) | ⨁⨁⨁◯ Moderate |
Important | ||||||||
| Time from onset of COVID-19 symptoms to diagnosis of CAPA | |||||||||||||||||||
| 19 | observational studies | not serious | not seriousc | not serious | seriousd | none | - | 353 | mean 12.8 days (10.5 to 15.1) | ⨁⨁⨁◯ Moderate |
Important | ||||||||
Note: *High certainty: We are very confident that the true incidence of CAPA lies close to that of the estimate.⨁⨁⨁◯ Moderate certainty: We are moderately confident that the true incidence of CAPA is likely to be close to the estimate, but there is a possibility that it is substantially different. Low certainty: Our confidence in the estimate is limited: The true incidence of CAPA may be substantially different from the estimate. Very low: We have very little confidence in the estimate: the true incidence of CAPA is likely to be substantially different from the estimate.
Table 6: Pooled proportion of non-survivors among critically ill patients with COVID-19 associated pulmonary aspergillosis.
Discussion
This review highlighted the incidence, characteristics and outcomes of critically ill patients with CAPA in the first two years of the COVID-19 pandemic. Around 13% of critically ill older and mostly male patients with COVID-19 in ICU developed CAPA, with a significant publication bias in the incidence of CAPA. Our meta- analysis found a high mortality rate (63.4%) in patients with CAPA. We noted that patients with hypertension and those received
corticosteroids were at higher risk of contracting CAPA. However, we failed to note any significant difference in CAPA incidence between pre-and post-RECOVERY trial.
Immune dysregulation is commonly observed in critically ill patients with COVID-19, predisposing them to potentially fatal invasive fungal infections. Also, COVID-19 results in impaired ciliary function and decreased lymphocytes count that predispose these patients to IPA [75]. We observed the pooled incidence of CAPA in our critically ill cohort was 13%. Similarly, Mitaka, et al. [6,76]. and Chong, et al. reported overall CAPA incidence of 10.2% and 13.5% respectively. Our analysis demonstrated that observational studies with a large sample size had a lower incidence of CAPA, thereby pointing towards reporting bias in studies with smaller sample size [6,9]. We noted that corticosteroids usage was significantly associated with an increased incidence of CAPA in ICU patients; this could be potentially attributable to their immunomodulatory properties which increase host susceptibility to invasive Aspergillus infection. Our subgroup analysis failed to demonstrate any such increase in CAPA incidence between pre-and post-RECOVERY trials. We were somewhat surprised that IL-6 antagonists were not found to be a risk factor for CAPA in this meta-analysis, but there were only 8% of patients in the published studies who were on IL-6 antagonists [19,20].
There are multiple challenges in diagnosing the true incidence of CAPA in critically ill patients. Rather than a standardised definition to classify CAPA, [2] various criteria like IAPA case definition, EORTC- MSG, crude/mAsp-ICU or ECMM/ISHAM consensus criteria were used to individually define CAPA. In addition to this, typical host factors or radiological features described in these classification systems might not apply to patients with CAPA [2,12-14]. However, while mycological evidence is pivotal in diagnosing CAPA, [2,10] it is hampered by the difficulties in distinguishing colonisation from angio-invasive disease [2]. Interestingly, oursubgroup analysis demonstrated that the various case definitions were not significantly different in estimating the CAPA incidence in this cohort [2]. Another reason for heterogeneity in estimating the incidence may be due to differences in CAPA screening in intensive care unit. Galactomannan testing from BAL fluid is the most sensitive test for diagnosing IPA in ICU patients; [77,78] however, BAL is infrequently carried out in critically ill patients with COVID-19 due to aerosol generation and risk of disease transmission. Up to 80% of positive respiratory samples without biopsies from critically ill immune competent hosts are colonised samples and not IPA, [79-82] it may be plausible that colonised samples were falsely reported as CAPA because consensus criteria was not clearly defined until recently. Along with the abovementioned points, using diagnostic bronchoscopy without employing histological or cytological examinations to diagnose CAPA routinely could potentially lead to over-diagnosis of CAPA among critically ill patients [2]. Our subgroup analysis failed to note any significant difference in estimating CAPA incidence based on various methods of diagnosis.
Well known studies on IPA and recent literature on CAPA both suggest that Aspergillus co-infection may contribute to increased mortality. High mortality rates (35% to 70%) have been reported among critically ill patients with IPA [2,6]. Prior reviews noted pooled mortality from CAPA was 48% to 55% [14,83]. In contrast to these findings, the pooled mortality among patients with CAPA in our review was 63.4% [6,9,84]. The increased mortality among patients with CAPA can potentially be explained by the severity of COVID-19. Previous reviews have shown that age is a risk factor for disease severity and mortality. In line with previous single-centre observational studies, our review found that patients with CAPA were older than patients without CAPA, and possibly experience more severe disease, leading to higher mortality rates [85]. From this perspective, we believe that there is reasonable justification to routinely screen critically ill patients with COVID-19 for CAPA if they remain without improvement, or rapidly deteriorate after initial improvement from COVID-19, and especially if the patients have received immunomodulatory treatments such as corticosteroids either alone or with IL-6 antagonists. Consideration can be given for clinical trials to determine whether antifungal prophylaxis early screening and preemptive treatment will safely reduce the incidence and overall mortality in critically ill COVID-19 patients.
The strengths of this review include robust inclusion and exclusion criteria. Our review included more than 50 studies encompassing 11,000 critically ill patients with COVID-19 across the globe. In contrast to the previous reviews’ which reported CAPA incidence in the general population, this is the first study to highlight incidence of CAPA in critically ill patients with COVID-19. We reduced confounding and accounted for heterogeneity by elucidating factors correlating with CAPA incidence via subgroup analysis and meta regression. Nonetheless, we recognize several limitations of this study. The significant publication bias, inverse relationship with sample size along and lack of granularity in diagnosis criteria make the true estimation of CAPA incidence difficult. This could also be attributed to the inability to collect respiratory specimens from all eligible patients in the context of overstretched resources due to the pandemic.
The results should be interpreted with caution as this could potentially point to a significant overestimation of CAPA among critically ill patients with COVID-19. Searches for unpublished manuscripts and pre-print texts to reduce publication bias did not yield any articles for inclusion. Data related to the duration of IMV was scarce, so it is difficult to assess whether this is also a risk factor for CAPA. Also, the patients were not stratified based on age, which could be a plausible risk factor for CAPA. Owing to the paucity of data, it was difficult to determine whether patients received corticosteroids specifically for COVID-19 or were on long-term therapy for any underlying illness. Finally, our analysis is based mainly on retrospective studies with significant heterogeneity, which can introduce confounding factors given the lack of risk-adjustment or propensity-score techniques. However, JBI critical appraisal of the included studies suggests that most of the articles are of high quality, and GRADE assessment suggested moderate certainty of the evidence in our review.
Conclusion
Clinicians should be cognizant that critically ill patients with COVID-19 may acquire CAPA, especially in those who are receiving corticosteroids. The outcomes for patients with CAPA are poor; an earlier diagnosis with multimodal diagnostic workup and management with pre-emptive antifungal agents should be considered on a case- by-case basis. With numerous diagnostic challenges involved, future studies should use a standardized definition to stratify patients at risk of or with CAPA, increase suspicion and early diagnosis of CAPA, and ideally
improve overall patient outcomes. This may be even more necessary in the post-RECOVERY trial era, where corticosteroids often with IL-6 inhibitors are being routinely used to treat hypoxaemic patients with COVID-19
Funding
None
Competing Interests
None
Consents for Publication
All authors agreed for publication of this study
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- Segrelles-Calvo G, de S Araújo GR, Frases S (2020) Systemic mycoses: A potential alert for complications in COVID-19 patients. Future Microbiol 15:1405-1413.
[Crossref] [Google Scholar] [PubMed]
- Koehler P, Bassetti M, Chakrabarti A, Chen SC, Colombo AL (2021) Defining and managing COVID-19 associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis 21:149-162.
[Crossref] [Google Scholar] [PubMed]
- Chong WH, Saha BK, Ramani A, Chopra A (2021) State-of-the-art review of secondary pulmonary infections in patients with COVID-19 pneumonia. Infect.
[Crossref] [Google Scholar] [PubMed]
- Gangneux JP, Bougnoux ME, Dannaoui E, Cornet M, Zahar JR (2020) Invasive fungal diseases during COVID-19: We should be prepared. J Mycol Med 30:100971.
[Crossref] [Google Scholar] [PubMed]
- Verweij PE, Gangneux JP, Bassetti M, Brüggemann RJ, Cornely OA (2020) Diagnosing COVID-19-associated pulmonary aspergillosis. The Lancet Microbe 1:53-55.
[Crossref] [Google Scholar] [PubMed]
- Mitaka H, Kuno T, Takagi H, Patrawalla P (2021) Incidence and mortality of COVID‐19 associated pulmonary aspergillosis: A systematic review and meta‐analysis. Mycoses.
[Crossref] [Google Scholar] [PubMed]
- Bassetti M, Kollef MH, Timsit JF (2020) Bacterial and fungal superinfections in critically ill patients with COVID-19. Intensive Care Med 46:2071-2074.
[Crossref] [Google Scholar] [PubMed]
- Lai CC, Yu WL (2021) COVID-19 associated with pulmonary aspergillosis: A literature review. J Microbiol Immunol Infect 54:46-53.
[Crossref] [Google Scholar] [PubMed]
- Chong WH, Neu KP (2021) The incidence, diagnosis, and outcomes of COVID-19-associated pulmonary aspergillosis (CAPA): A systematic review. J Hosp Infect.
[Crossref] [Google Scholar] [PubMed]
- Fekkar A, Poignon C, Blaize M, Lampros A (2020) Fungal infection during COVID-19: does Aspergillus mean secondary invasive aspergillosis? Am J Respir Crit Care Med 202:902-903.
[Crossref] [Google Scholar] [PubMed]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma Group (2009) Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med 6:1000097.
[Crossref] [Google Scholar] [PubMed]
- Blot SI, Taccone FS, Van den Abeele AM, Bulpa P, Meersseman W, et al. (2012) A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am J Respir Crit Care Med 186:56-64.
[Crossref] [Google Scholar] [PubMed]
- Schauwvlieghe AF, Rijnders BJ, Philips N, Verwijs R, Vanderbeke L, et al. (2018) Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: A retrospective cohort study. Lancet Respir Med 6:782-792.
[Crossref] [Google Scholar] [PubMed]
- Verweij PE, Rijnders BJ, Brüggemann RJ, Azoulay E, Bassetti M, et al. (2020) Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: An expert opinion. Intensive Care Med 46:1524-1535.
[Crossref] [Google Scholar] [PubMed]
- Wan X, Wang W, Liu J, Tong T (2014) Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res 14:1-3.
[Crossref] [Google Scholar] [PubMed]
- Clopper CJ, Pearson ES (1934) The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 404-413.
- DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177-188.
- Kalil AC, Stebbing J (2021) Baricitinib: The first immunomodulatory treatment to reduce COVID-19 mortality in a placebo-controlled trial. Lancet Respir Med 9:1349-1351.
[Crossref] [Google Scholar] [PubMed]
- Jia D, Yang IX, Ling RR, Syn N, Poon WH, et al. (2020) Vascular complications of extracorporeal membrane oxygenation: A systematic review and meta-regression analysis. Crit Care Med 48:1269-1277.
[Crossref] [Google Scholar] [PubMed]
- Mitra S, Ling RR, Tan CS, Shekar K, MacLaren G, et al. (2021) Concurrent use of renal replacement therapy during extracorporeal membrane oxygenation support: A systematic review and meta-analysis. J Clin Med 10:241.
[Crossref] [Google Scholar] [PubMed]
- Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello-Petersen R, et al. (2014) A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. Bmj 24:349.
[Crossref] [Google Scholar] [PubMed]
- Iorio A, Spencer FA, Falavigna M, Alba C, Lang E, et al. (2015) Use of GRADE for assessment of evidence about prognosis: Rating confidence in estimates of event rates in broad categories of patients. Bmj 16:350.
[Crossref] [Google Scholar] [PubMed]
- Autier B, Prattes J, White PL, Valerio M, Machado M, et al. (2021) Aspergillus lateral flow assay with digital reader for the diagnosis of COVID-19 associated pulmonary aspergillosis (CAPA): A multicenter study. J Clin Microbiol JCM.
[Crossref] [Google Scholar] [PubMed]
- Bartoletti M, Pascale R, Cricca M, Rinaldi M, Maccaro A, et al. (2021) Epidemiology of invasive pulmonary aspergillosis among intubated patients with COVID-19: A prospective study. Clin Infect Dis 73:3606-3614.
[Crossref] [Google Scholar] [PubMed]
- Borman AM, Palmer MD, Fraser M, Patterson Z, Mann C, et al. (2020) COVID-19 associated invasive Aspergillosis: Data from the UK National Mycology Reference Laboratory. J Clin Microbiol 59:e02136.
[Crossref] [Google Scholar] [PubMed]
- Boyd S, Martin-Loeches I (2021) Rates of Aspergillus Co-infection in COVID patients in ICU not as high as previously reported. Clin Infect Dis.
[Crossref] [Google Scholar] [PubMed]
- Bretagne S, Sitbon K, Botterel F, Dellière S, Letscher-Bru V, et al. (2021) COVID-19-associated pulmonary aspergillosis, fungemia, and pneumocystosis in the intensive care unit: A retrospective multicenter observational cohort during the first french pandemic wave. Microbiol Spectr 9:e01138.
[Crossref] [Google Scholar] [PubMed]
- Chauvet P, Mallat J, Arumadura C, Vangrunderbeek N, Dupre C, et al. Risk factors for invasive pulmonary aspergillosis in critically Ill patients with coronavirus disease 2019-induced acute respiratory distress syndrome. Crit care explor 2020.
[Crossref] [Google Scholar] [PubMed]
- Dellière S, Dudoignon E, Fodil S, Voicu S, Collet M, et al. (2021) Risk factors associated with COVID-19-associated pulmonary aspergillosis in ICU patients: A French multicentric retrospective cohort. Clin Microbiol Infect 27:790.
[Crossref] [Google Scholar] [PubMed]
- Salmanton-Garcia J, Sprute R, Stemler J, Bartoletti M, Dupont D, et al. COVID-19 associated pulmonary aspergillosis, March-August 2020. Emerg Infect Dis 2021 27:1077.
- Ergün M, Brüggemann RJ, Alanio A, Dellière S, van Arkel A, et al. (2021) Aspergillus Test Profiles and Mortality in Critically Ill COVID-19 Patients. J Clin Microbiol. 59:e01229. [Crossref] [Google Scholar] [PubMed]
- Fekkar A, Lampros A, Mayaux J, Poignon C, Demeret S, et al. (2021) Occurrence of invasive pulmonary fungal infections in patients with severe COVID-19 admitted to the ICU. Am J Respir Crit Care Med 203:307-317. [Crossref]
- Ghazanfari M, Arastehfar A, Davoodi L, Charati JY, Moazeni M, et al. (2021) Pervasive but Neglected: A Perspective on COVID-19-Associated Pulmonary Mold Infections Among Mechanically Ventilated COVID-19 Patients. Front Med 8.
[Crossref] [Google Scholar] [PubMed]
- Gregoire E, Pirotte BF, Moerman F, Altdorfer A, Gaspard L, et al. (2021) Incidence and Risk Factors of COVID-19-Associated Pulmonary Aspergillosis in Intensive Care Unit-A Monocentric Retrospective Observational Study. Pathogens10:1370.
[Crossref] [Google Scholar] [PubMed]
- Hatzl S, Reisinger AC, Posch F, Prattes J, Stradner M, et al. (2021) Antifungal prophylaxis for prevention of COVID-19-associated pulmonary aspergillosis in critically ill patients: An observational study. Crit Care. 25:1.
[Crossref] [Google Scholar] [PubMed]
- Hoenigl M, Egger M, Boyer J, Schulz E, Prattes J, et al. (2021) Serum Lateral Flow assay with digital reader for the diagnosis of invasive pulmonary aspergillosis: A two‐centre mixed cohort study. Mycoses. 64:1197-202.
[Crossref] [Google Scholar] [PubMed]
- Iqbal A, Ramzan M, Akhtar A, Ahtesham A, Aslam S, et al. (2021) COVID-associated pulmonary aspergillosis and its related outcomes: A single-center prospective observational study. Cureus 13(8).
[Crossref] [Google Scholar] [PubMed]
- Rouzé A, Lemaitre E, Martin-Loeches I, Povoa P, Diaz E, et al. (2022) Invasive pulmonary aspergillosis among intubated patients with SARS-CoV-2 or influenza pneumonia: A European multicenter comparative cohort study. Criti Care 26:1-4.
[Crossref] [Google Scholar] [PubMed]
- Jasim NO, Klaaf M (2021) Pulmonary aspergillosis associated with COVID-19. International J Drug Deliv Sci Technol 11:892-895.
- Kluzik A, Szrama J, Hampelska K (2021) Prevalence of invasive aspergillosis among patients with COVID-19 hospitalized in intensive care units: Not a rare problem. Pol Arch Intern Med 131:875-877.
[Crossref] [Google Scholar] [PubMed]
- Salmanton-Garcia J, Sprute R, Stemler J, Bartoletti M, Dupont D, et al. (2021) COVID-19-associated pulmonary aspergillosis, March-August 2020. Emerg Infect Dis 27:1077.
[Crossref] [Google Scholar] [PubMed]
- Lahmer T, Kriescher S, Herner A, Rothe K, Spinner CD, et al. (2021) Invasive pulmonary aspergillosis in critically ill patients with severe COVID-19 pneumonia: Results from the prospective AspCOVID-19 study. PloS one 16(3):e0238825.
[Crossref] [Google Scholar] [PubMed]
- Lamoth F, Glampedakis E, Boillat-Blanco N (2020) Incidence of invasive pulmonary aspergillosis among critically ill COVID-19 patients. Clin Microbiol Infect 26:1706-1708.
[Crossref] [Google Scholar] [PubMed]
- Machado M, Valerio M, Álvarez‐Uría A, Olmedo M, Veintimilla C, et al. (2021) Invasive pulmonary aspergillosis in the COVID‐19 era: An expected new entity. Mycoses 64:132-143.
- Maes M, Higginson E, Pereira-Dias J, Curran MD, Parmar S, et al. (2021) Ventilator-associated pneumonia in critically ill patients with COVID-19. Crit Care 25:1.
[Crossref] [Google Scholar] [PubMed]
- Meijer EF, Dofferhoff AS, Hoiting O, Meis JF (2021) COVID‐19-associated pulmonary aspergillosis: a prospective single‐center dual case series. Mycoses 64:457-464.
- Nasir N, Farooqi J, Mahmood SF, Jabeen K (2021) COVID-19 associated mucormycosis: A life-threatening complication in patients admitted with severe to critical COVID-19 from Pakistan. Clin Microbiol Infect 27:1704-1707.
[Crossref] [Google Scholar] [PubMed]
- Nebreda-Mayoral T, Miguel-Gómez MA, March-Rosselló GA, Puente-Fuertes L, Cantón-Benito E, et al. (2021) Bacterial/fungal infection in hospitalized patients with COVID-19 in a tertiary hospital in the Community of Castilla y León, Spain. Enferm Infecc Microbiol Clin 2020.
[Crossref] [Google Scholar] [PubMed]
- Oliva A, Ceccarelli G, Borrazzo C. (2021) Comparison of clinical features and outcomes in COVID-19 and influenza pneumonia patients requiring intensive care unit admission. Infection 2021;49:965-75.
- Paramythiotou E, Dimopoulos G, Koliakos N, Siopi M, Vourli S, et al. (2021) Epidemiology and incidence of COVID-19-associated pulmonary aspergillosis (CAPA) in a Greek tertiary care academic reference hospital. Infect Dis Ther 10(3):1779-1792.
[Crossref] [Google Scholar] [PubMed]
- Prattes J, Wauters J, Giacobbe DR, Salmanton-García J, Maertens J, et al. (2021) Risk factors and outcome of pulmonary aspergillosis in critically ill coronavirus disease 2019 patients- a multinational observational study by the European Confederation of Medical Mycology. Clin Microbiol Infect.
[Crossref] [Google Scholar] [PubMed]
- Rabagliati R, Rodríguez N, Núñez C, Huete A, Bravo S, et al. (2021) COVID-19-Associated Mold Infection in Critically Ill Patients, Chile. Emerg Infect Dis 27:1454.
[Crossref] [Google Scholar] [PubMed]
- Ranhel D, Ribeiro A, Batista J, Pessanha M, Cristovam E, et al. (2021) COVID-19-Associated Invasive Pulmonary Aspergillosis in the Intensive Care Unit: A Case Series in a Portuguese Hospital. J Fungi 7:881.
[Crossref] [Google Scholar] [PubMed]
- Razazi K, Arrestier R, Haudebourg AF, Benelli B, Carteaux G, et al. (2020) Risks of ventilator-associated pneumonia and invasive pulmonary aspergillosis in patients with viral acute respiratory distress syndrome related or not to Coronavirus 19 disease. Crit Care 24:1. [Crossref]
- Ripa M, Galli L, Poli A, Oltolini C, Spagnuolo V, et al. (2021) Secondary infections in patients hospitalized with COVID-19: Incidence and predictive factors. Clin Microbiol Infect 27:451-457. [Crossref] [Google Scholar] [PubMed]
- Roman‐Montes CM, Martinez‐Gamboa A, Diaz‐Lomelí P, Cervantes‐Sanchez A, Rangel‐Cordero A, et al. (2021) Accuracy of galactomannan testing on tracheal aspirates in COVID‐19‐associated pulmonary aspergillosis. Mycoses 64:364-371. [Crossref] [Google Scholar] [PubMed]
- Rutsaert L, Steinfort N, Van Hunsel T, Bomans P, Naesens R, et al. (2020) COVID-19-associated invasive pulmonary aspergillosis. Ann Intensive Care Dec 10:1-4. [Crossref] [Google Scholar] [PubMed]
- Feys S, Almyroudi MP, Braspenning R, Lagrou K, Spriet I, et al. (2021) A Visual and Comprehensive Review on COVID-19-Associated Pulmonary Aspergillosis (CAPA). J Fungi 7:1067. [Crossref] [Google Scholar] [PubMed]
- Segrelles-Calvo G, Araújo GRS, Llopis-Pastor E, et al. (2021) Prevalence of opportunistic invasive aspergillosis in COVID-19 patients with severe pneumonia. Mycoses 64:144-151. [Crossref] [Google Scholar] [PubMed]
- Sivasubramanian G, Ghanem H, Maison-Fomotar M, Jain R, Libke R (2021) COVID-19- Associated Pulmonary Aspergillosis: A Single-Center Experience in Central Valley, California, January 2020-March 2021. J Fungi (Basel) 7:948. [Crossref] [Google Scholar] [PubMed]
- Søgaard KK, Baettig V, Osthoff M, Marsch S, Leuzinger K, et al. (2021) Community-acquired and hospital-acquired respiratory tract infection and bloodstream infection in patients hospitalized with COVID-19 pneumonia. J Intensive Care 9:1. [Crossref] [Google Scholar] [PubMed]
- Szabo BG, Lakatos B, Bobek I, Szabo E, Szlavik J, et al. (2021) Invasive fungal infections among critically ill adult COVID-19 patients: First experiences from the national centre in Hungary. Med Mycol J 31:101198. [Crossref] [Google Scholar] [PubMed]
- Takazono T, Mukae H, Izumikawa K, Hasegawa N, Yokoyama A (2021) COVID-19 associated pulmonary aspergillosis: A nationwide survey by the Japanese Respiratory Society. ERJ Open Res 7. [Crossref] [Google Scholar] [PubMed]
- Tio SY, Williams E, Worth LJ, Deane AM, Bond K, et al. (2021) Invasive pulmonary aspergillosis in critically ill patients with COVID‐19 in Australia: Implications for screening and treatment. Intern Med J 51:2129-2132. [Crossref] [Google Scholar] [PubMed]
- Van Arkel AL, Rijpstra TA, Belderbos HN, Van Wijngaarden P, Verweij PE, et al. (2020) COVID-19-associated pulmonary aspergillosis. Am J Respir Crit Care Med 202:132-135. [Crossref] [Google Scholar] [PubMed]
- Van Grootveld R, Van Paassen J, De Boer MG, Claas EC, Kuijper EJ, et al. (2021) LUMC‐COVID‐19 Research Group. Systematic screening for COVID‐19 associated invasive aspergillosis in ICU patients by culture and PCR on tracheal aspirate. Mycoses.64:641-650. [Crossref] [Google Scholar] [PubMed]
- Vélez Pintado M, Camiro-Zúñiga A, Aguilar Soto M, Cuenca D, Mercado M, et al. (2021) ARMII study gruop. COVID-19-associated invasive pulmonary aspergillosis in a tertiary care center in Mexico City. Med Mycol J 59:828-833. [Crossref] [Google Scholar] [PubMed]
- Versyck M, Zarrougui W, Lambiotte F, Elbeki N, Saint-Leger P (2021) Invasive pulmonary aspergillosis in COVID-19 critically ill patients: Results of a French monocentric cohort. Med Mycol J 31:101122. [Crossref] [Google Scholar] [PubMed]
- Wang J, Yang Q, Zhang P, Sheng J, Zhou J, et al. (2020) Clinical characteristics of invasive pulmonary aspergillosis in patients with COVID-19 in Zhejiang, China: A retrospective case series. Crit Care 24:1-4. [Crossref] [Google Scholar] [PubMed]
- Wasylyshyn AI, Wasylyshyn GR, Linder KA, Miceli MH (2021) COVID-19-Associated Pulmonary Aspergillosis at an Academic Medical Center in the Midwestern United States. Mycopathologia 186:499-505. [Crossref] [Google Scholar] [PubMed]
- White PL, Dhillon R, Cordey A, Hughes H, Faggian F, et al. (2021) A national strategy to diagnose coronavirus disease 2019-associated invasive fungal disease in the intensive care unit. Clin Infect Dis 73:e1634-1644. [Crossref] [Google Scholar] [PubMed]
- Xu J, Yang X, Lv Z, Zhou T, Liu H, et al. (2021) Risk Factors for Invasive Aspergillosis in Patients Admitted to the Intensive Care Unit With Coronavirus Disease 2019: A Multicenter Retrospective Study. Front Med 8. [Crossref] [Google Scholar] [PubMed]
- Yusuf E, Vonk A, van den Akker JP, Bode L, Sips GJ, et al. (2021) Frequency of positive aspergillus tests in COVID-19 patients in comparison to other patients with pulmonary infections admitted to the intensive care unit. J Clin Microbiol 59:e02278. [Crossref] [Google Scholar] [PubMed]
- Zhang SX, Balada-Llasat JM, Pancholi P, Sullivan KV, Riedel S (2021) COVID-Associated Pulmonary Aspergillosis in the United States: Is it Rare or Have We Missed the Diagnosis? J Clin Microbiol 59:e01135-e01121. [Crossref] [Google Scholar] [PubMed]
- Pemán J, Ruiz-Gaitán A, García-Vidal C, Salavert M, Ramírez P, et al. (2020) Fungal co-infection in COVID-19 patients: Should we be concerned?. Iberoamerican J Mycol. 37:41-46. [Crossref] [Google Scholar] [PubMed]
- Thompson Iii GR, Cornely OA, Pappas PG, Patterson TF, Hoenigl M, et al. (2020) Invasive aspergillosis as an under-recognized superinfection in COVID-19. In Open forum infectious diseases. [Crossref] [Google Scholar] [PubMed]
- Apostolopoulou A, Esquer Garrigos Z, Vijayvargiya P, Lerner AH, Farmakiotis D (2020) Invasive pulmonary aspergillosis in patients with SARS-CoV-2 infection: A systematic review of the literature. Diagnostics 10:807. [Crossref] [Google Scholar] [PubMed]
- Schauwvlieghe AF, Rijnders BJ, Philips N, Verwijs R, Vanderbeke L, et al. (2018) Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: A retrospective cohort study. Lancet Respir Med 6:782-792. [Crossref] [Google Scholar] [PubMed]
- Koehler P, Cornely OA, Böttiger BW, Dusse F, Eichenauer DA, et al. (2020) COVID‐19 associated pulmonary aspergillosis. Mycoses 63:528-534. [Crossref] [Google Scholar] [PubMed]
- Azoulay É, Afessa B (2012) Diagnostic criteria for invasive pulmonary aspergillosis in critically ill patients. Am J Respir Crit Care Med 186:8-10.
[Crossref] [Google Scholar] [PubMed]
- Guinea J, Torres-Narbona M, Gijón P, Muñoz P, Pozo F, et al. (2010) Pulmonary aspergillosis in patients with chronic obstructive pulmonary disease: Incidence, risk factors, and outcome. Clin Microbiol Infect 16:870-877.
[Crossref] [Google Scholar] [PubMed]
- Vandewoude KH, Blot SI, Benoit DO, Colardyn F, Vogelaers D (2004) Invasive aspergillosis in critically ill patients: Attributable mortality and excesses in length of ICU stay and ventilator dependence. J Hosp Infect 56:269-276.
[Crossref] [Google Scholar] [PubMed]
- Sun KS, Tsai CF, Chen SC, Huang WC (2017) Clinical outcome and prognostic factors associated with invasive pulmonary aspergillosis: An 11-year follow-up report from Taiwan. PLoS One 12:0186422.
[Crossref] [Google Scholar] [PubMed]
- Permpalung N, Chiang TP, Massie AB, Zhang SX, Avery RK, et al. (2022) Coronavirus Disease 2019-Associated Pulmonary Aspergillosis in Mechanically Ventilated Patients. Clin Infect Dis 74:83-91.
[Crossref] [Google Scholar] [PubMed]
- Liu Y, Mao B, Liang S, Yang JW, Lu HW (2020) Association between age and clinical characteristics and outcomes of COVID-19. Eur Res J 1;55(5).
[Crossref] [Google Scholar] [PubMed]
Citation: Mitra S, Ling RR, Mahendran SA , Ling RM, Subramaniam A, et al. (2022) Incidence and Outcomes of Invasive Pulmonary Aspergillosis in Critically Ill Patients with COVID-19: A Systematic Review and Meta-Analysis. J Infect Dis Ther S2:001.
Copyright: © 2022 Mitra S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 2390
- [From(publication date): 0-2022 - Nov 14, 2025]
- Breakdown by view type
- HTML page views: 1805
- PDF downloads: 585
