In vivo Toxicological Evolution of Protein Extract from Ulva lactuca L. in Marine Seaweed using Male Wistar Rats
Received: 11-Oct-2021 / Accepted Date: 25-Oct-2021 / Published Date: 01-Nov-2021
Abstract
This study was aimed to assess the toxicological profile of Ulva lactuca L . through acute and sub-acute toxicity tests based on the OECD guideline 407. Toxicity assessment of protein extracted from Ulva lactuca L . seaweed was performed. An acute toxicity study was carried out using a single dose of 2000 mg/kg for 28 days and multi dose sub-acute toxicity study, was carried out by administering the doses of 250, 500 and 1000 mg/kg/day for 28 days. Rats were observed weekly for toxicity symptoms and compared with the control group, each group three rats for these studies. At the end of study, the animals were killed and their body weight, haematology, serum chemistry, and histopathology evaluation were done. Compared to the control group in acute and sub-acute toxicity studies similar changes in body weight gain, feed consumption, clinical pathology evaluation, and organ weight were observed. The LD50 did not produce any significant treatment related changes in clinical observations and hence, haematological, histopathological and serum biochemical parameters were within the normal limits. The toxicity study did not show any toxicological mortality and no behavioural changes were observed in rats treated with acute and sub-acute studies.
Keywords: Ulva; Toxicity; Histopathology; Mortality
Introduction
Seaweeds are being explored for various commercial food, agricultural-horticultural, pharmaceutical, cosmetic, and bioenergy applications [1]. They are potential reservoirs of novel biologically active components; more specifically, they are rich sources of proteins and amino acids and thus seaweeds can be used as potential starting materials in the production of biologically active components [2].
Seaweeds naturally originated, protein-derived peptides can be used as persuasive alternatives in the pharmaceutical and biotechnological industries [3], Findings of the bio regulatory role of different endogenic peptides in the marine organism, and the appreciation of the molecular mechanism of actions of bioactive molecules on specific cellular targets were increased [4]. Bioactive peptides have a wide range of bioactivities, depending on their structure, composition, and their amino acids sequence [5,6]. Protein is one of the main nutrients that will be in short supply in the future. Alternative protein sources and production methods are required to fulfill the demand of consumers and to meet the predicted global protein requirements [7]. Seaweed and microalgae are considered a viable source of protein. Some species of seaweed and microalgae are known to contain protein levels similar to those of traditional protein sources such as, meat, egg, soybean, and milk [8,9]. These biological obstacles commonly edge the aggregate of intact proteins that arrive into the body. The efficacy of these fences has been well well-known by failed endeavors to orally administer proteins for therapeutic use [10-13].
Proteins found in the living organisms are isolated, characterized, and allocated to different functional classes having associated structures and functions. Even though there are a large number of known proteins, only a small number of them are known to stimulate adverse effects in all vertebrata following oral intake [14].
Toxicology analysis of proteins is acclaimed in cases where the weight of evidence regarding safety following Tier 1 assessment is considered to be incomplete or indecisive. The following testing should be assumption based to resolve any specific safety queries. Because proteins identified to be toxic to mammals and further organisms mostly work through particular mechanisms to cause contrary acute effects, testing can frequently performed using acute harmfulness tests [14,15].
To evaluate the potential exposures of proteins that obligate an acute mode of action, the US Environmental Protection Agency (US EPA, 2000) necessitates the routine of high (exposure) acute dosages gram/kg body weight (BW) wherever possible. In line with limited solubility of some proteins in dosing vehicles, it is not always possible to achieve gram/kg dosage levels. Candidate proteins intended for use in new algal crops are chosen to avoid potentially adverse consequences. In the unlikely scenario that an introduced protein has a biochemical function similar to a known anti-nutrient proteins found in marine seaweeds, such as lectins or protease inhibitors, an acute toxicity study may be insufficient to assess potential the toxicity.
These anti-nutrients generally spread their toxicity within a few weeks of dietary acquaintance by intrusive with protein digestion or by damaging cells lining the gastrointestinal tract typically the colon [14,16,17].
They can be tested for possible toxicity by using the study design enhanced from the Organization for Economic Cooperation and Development (OECD) test guideline 407 for 28-day toxicity studies with chemicals (OECD, 1995). The dosage administered perhaps based on a bounds dose of 1000 mg/kg as is done for chemicals [18,19], even though this is not systematically relevant to proteins in algal crops because the exposure levels are many orders of greatness lower than the limit dose attainable by chemicals. Consequently, it is desirable to employ dosages that provide at least a 100-1000-fold boundary of safety [20-22].
Supplementary toxicological studies may be indicated if the 28- day study novelties evidence of toxicity. The European Food Safety Authority (EFSA) has need for testing of presented proteins without a history of safe use (HOSU) in a 28-day study. They do not usually require acute tests on introduced proteins.
Materials and Methods
Chemical and apparatus
chemicals used were analytical grade: Ethanol, methanol, sulphuric acid, Whattman paper(No.1), ammonium hydroxide, diethyl ether, acetone, ammonium persulphate, acrylamide mix, Tris, silver straining, acetic acid, sodium dodecyl sulfate (SDS), glycine, acetonitrile and HPLC Grade H2O. All kinds of chemicals and kits were purchased from Sisco Research Laboratories Pvt. Ltd. (SRL), India. HPLC System (Shimadzu, Kyoto, Japan) and Mini-PROTEAN 3 Cell SDS PAGE set up.
Sample collection and identification
Sample was collected from Ramanathapuram Mandapam region and was authenticated by Dr. M. Palanisamy, Scientist, Southern Regional Centre Botanical Survey of India, Coimbatore-641 003 Tamil Nadu, India. The collected U lactuca L. sample was washed with seawater for 4 and 5 times and further washed one time with distilled water to remove epiphytes, salts, sands and contamination from other algae. The sample was air dried in shade condition at room temperature and then dried sample was homogenated and the placed in plastic bags for further usage.
Extraction of the sample
Homogenized algal biomass 5 g of ULP extraction and add distilled water (DW, 10 mL), 95% ethanol (10 mL) and concentrated sulphuric acid (0.72 mL) and kept in uniform shaking for 30 min. Again, 15 mL of DW and 95% ethanol (50 mL) were added and the pH was adjusted to 1.7 using normality sulphuric acid. The suspension was then filtered using Whattman paper (No. 1) and again the pH was adjusted to 3.0 using concentrated ammonium hydroxide few drops. To this suspension, 150 mL of 95% ethanol and 200 mL of diethyl ether were added and kept for 12 h at 4°C. After centrifugation at 7,000 × g for 20 min, the sediment was washed with acetone and diethyl ether before dissolving in 25% ethanol and the pH was adjusted to 8.5. The precipitate was collected by centrifugation after 18 h. at modified methods given by [23,24].
SDS PAGE profiling
A known quantity (100 μg) of protein samples was loaded on 18% gel and electrophoresed at a constant voltage. Resolving gel composition (18%), stacking gel composition (5%), 1X gel loading buffer, 25 mM Tris base, 250 mM glycine, 10% SDS, were used for electrophoresis and pH adjusted to 8.3. Run conditions were pH 6.8, and 0.01% bromophenol blue and manually shaken at regular intervals (10 min) for 2 h at 4°C. The prepared protein extracts (50 μL) and novex Tris glycine standard (10 μg, mixed in sample buffer in a ratio of 1:4) were loaded in wells [25]. Electrophoresis was done at 80 V for 1 hour and at 100 V for 1-2 hours at 4°C. Protein bands present are visualized by silver staining method.
Reverse Phase HPLC analysis
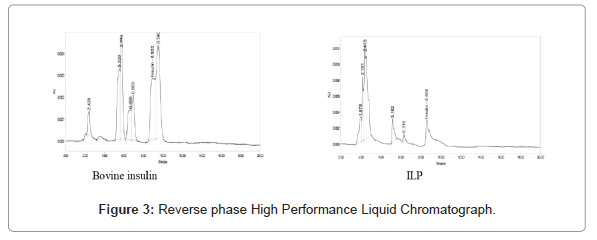
Proteins were separated and purified by RP-HPLC using a C-18 Phenomenex column (250 × 4.6 mm, 5 μm) with 50-50% acetonitrile in a 0.1% HPLC grade water gradient. The fraction obtained extract precipitation was dissolved in 500 μL of 0.1% HPLC grade water and applied to the column. The column was operated at 1 mL min-1 and the proteins detected by absorbance at 216 nm. Bovine insulin (50 μg of protein in 500 μL) was used as a standard to compare the retention times [26].
Animals
Male Wistar rats (aged 8-12 weeks, weight 100-200 g) were used for acute and sub-acute toxicity studies. Four rats were housed in polycarbonate cages with free access to normal diet and water. The rats were kept in a temperature controlled room at 25°C ± 30°C with 12 hours of light and dark cycle and with a relative humidity of <65%. The animals were acclimated to the laboratory condition for one week prior to the experiments. All procedures were performed according to the ethical principles in animal research adopted by Animal Science Department, Bharathidasan University, and approved by the institutional animal ethics committee (IAEC) (Reg. No. 418/GO/ Re/S/01/CPCSEA, Dt.04.06.2001). Animals were purchased from the Indian Institute of Science, Bangalore (Reg. no. 48/99/CPCSEA).
Acute toxicity
The acute oral toxicity study was accompanied in compliance with OECD guideline 423, which insist on the uses of only three animals (OECD 423). Three of the test animals were fasted overnight (~12 h) and weighed. Test doses of Ulva lactuca protein extract (ULP) were calculated in relation to the body weight of every fasted animal; and administered via oral feeding at 2000 mg/kg. The animals were daily and individually observed for behavioural changes and common toxicity signs after dosing for the first 24 h. Thereafter, observation was continued daily for a total of 28 days [27].
Sub-acute toxicity
The oral sub-acute toxicity study was carried out following the OECD guideline 407. Adult Wistar rats were divided into 4 groups of 3 animals each and were placed under dark and light conditions. Group I was considered as control and the other two groups which were considered as test groups which received the ULP extracts at a dose of 250, 500 and 1000 mg/kg body weight, respectively for 28 successive days.
Statistical analysis
Experimental data were expressed as means ± standard error. Statistical analysis was performed using SPSS 19.0 Software and data variance was analyzed by Turkey test, one-way ANOVA was conducted in order to compare any significant differences between the control group and the therapeutic groups. The differences between the groups were considered to be significant when p<3.
Results And Discussion
SDS PAGE Profile and RP-HPLC
After running gel in electrophoresis apparatus, it was fixed in fixative solution (50% methanol, 10% acetic acid) for one hour to overnight. The gel was washed with 20% ethanol for three times (10 minutes each) and was treated for precisely one minute with hypo solution (sodium thiosulfate solution 20 mg/mL). Then, it was washed with water for three times (20 seconds each) and treated with silver nitrate solution (200 mg/100 mL) with formaldehyde (70 μL/100 mL) for 30 minutes. Again the gel washed with water for three times (20 seconds each) and was developed in 100 mL developing solution (3 g sodium carbonate, 2 mL hypo solution, 25 μL formaldehyde). This can take from one minute to 30 minutes, depending on protein concentration. Once developed, it was stopped with 5% acetic acid and the gel was stored in fixative solution results on previous paper [24].
Confirmation of the insulin like protein from Ulva lactuca L. was performed by diethyl ether and the alcoholic extraction was precipitated followed by RP-HPLC analysis. Throughout the purification procedure, identification of ILP was archived using Tris glycine 4%-20% marker. This has been previously used for studying some plants with medicinal properties, marine seaweeds and some animals [28-33].
Toxicity study
In accordance with the assimilated results of LD50, the doses of ULP acute treatment (2000 mg/kg for 28) from the first day to 28 days did not produce any significant change in food, water intake, body weight and body temperature during the period of study. ULP acute treatment did not produce any significant changes in urinary parameter of male wistar rats as compared to control. ULP sub-acute treatment (250, 500 and 1000 mg/kg/day) did not cause any significant change in the blood hematological and serum biochemical markers as compared with control rats.
Changes in body and organ weights are clear symptoms of the damage caused by the substance test [34], while the hippocratic screening provides a general estimate of pharmacological and toxicological nature [35]. After the acute toxicity test, the dose of 2000 mg/kg (limit test-OECD, 2008a) of OPAC did not cause the death of any animal. The male rats exposed presented no behavioural changes during the treatment period, as well as no changes were observed in water and food consumption and significant evolution, in relation to the control group. No irregularity was found in the organs at autopsy.
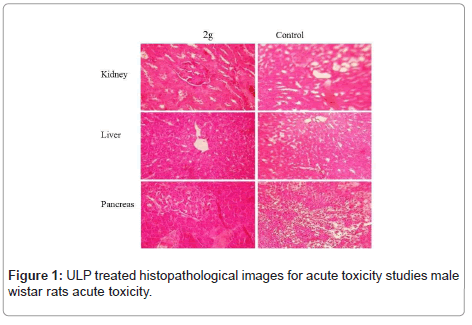
Therefore ULP tested cascades in a substance with oral dose (LD50) higher than 2000 mg/kg, hence considered of low toxicity (OECD, 2008a). At the end of the 28-day observation period was found that the OPAC showed no late toxic effects in the test animals (Figure 1). Similar results were observed in ULP gained from U Lactuca. In this study, the oral toxicity of ULP was assessed to be higher than 2000 mg/ kg, according to OECD Guidelines 423, indicating a certain safety edge associated with the use of ULP as beneficial to societies.
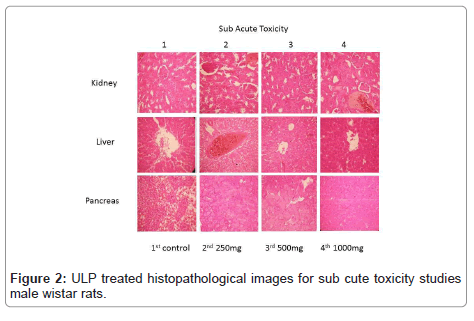
Toxicological evaluations after constant exposures are required by regulatory agencies to characterize the toxicological profile of any material (OECD, 2008b). In the study, following sub-acute exposure (Figure 2), the animals were active and responsive to inducements, with no clinical signs related to local or systemic toxic effects. There were no deaths and the behaviour of animals remained normal for the species. However, the consumption of water and food for the male treated groups with ULP, at all doses, reduced when compared to the control group. While the mean intake differed statistically, no biological importance was assigned to this, since the weight gain values (Table 1) did not vary among groups. Likewise, in the present study, the relative weights of all organs examined did not vary significantly among groups (Table 2), corroborating the hypothesis of low toxicity of the oil after sub-acute exposure. Clinical biochemistry resolves to investigate major toxic effects on tissues and, specifically, effects on kidney, liver and pancreas, which should be performed and under certain situations may provide useful information.
|
|
Sub-acute | Acute | |||
|---|---|---|---|---|---|
| Control | 250 mg | 500 mg | 1000 mg | 2000 mg | |
| Initial weight | 153.75 ± 10.82 | 148.75 ± 7.39 | 157.5 ± 5.59 | 163.75 ± 7.39 | 156 ± 15.57 |
| Final weight | 248 ± 12.18 | 337.5 ± 27.95 | 363.75 ± 13.40 | 389 ± 13.98 | 263.75 ± 15.83 |
| Food intake | 106.16 ± 8.57 | 100.8 ± 17.52 | 95 ± 11.89 | 85 ± 07.01 | 100 ± 17.88 |
| Water intake | 118.25 ± 24.51 | 112.50 ± 10.30 | 76.5 ± 15.33 | 60.25 ± 14 | 65.25 ± 9.36 |
Table 1: Animals body weight gain, food and water intake of rats observed for acute and sub-acute toxicity assay on extracted ULP at the study periods.
| Sub-acute | Acute | ||||
|---|---|---|---|---|---|
| Control | 250 mg | 500 mg | 1000 mg | 2000 mg | |
| Liver | 39.8 ± 0.58 | 51.65 ± 1.01 | 51.05 ± 0.36 | 46.55 ± 0.20 | 26.59 ± 1.17 |
| Kidney | 9.3 ± 0.27 | 57 ± 0.17 | 9.2 ± 1.42 | 10.15 ± 0.15 | 10.6 ± 0.77 |
| Spleen | 5.02 ± 0.08 | 5.72 ± 0.19 | 5.82 ± 0.83 | 6.67 ± 0.544 | 6.87 ± 0.44 |
| Heart | 4.22 ± 0.22 | 4.22 ± 0.08 | 4.175 ± 0.286 | 6.05 ± 0.111 | 5.47 ± 0.129 |
| Pancreas | 1.975 ± 0.82 | 2.0 ± 0.393 | 2.7 ± 0.158 | 4.675 ± 0.741 | 4.4 ± 0.25 |
Table 2: Organ weight of rat observed in toxicity assays and treated orally ULP at the study periods.
Some enzymes and proteins can be used as revealing of hepatocellular effects (such as AST, ALT, bilirubin, gamma-glutamyl transferas) [36,37], In this study, there was no statistical difference in liver or renal parameters (ALT, AST, bilirubin levels, creatinine and blood urea nitrogen) between the treated and control groups (Table 3). Some parameters (total protein, albumin, calcium and cholesterol) were statistically different when compared to the control group. However, this increase has no clinical significance. According to Giknis and Clifford [38], the values found in this study are within the normal range for healthy rats at this age, indicating the absence of liver or renal toxicity. The hematopoietic system is one of the most susceptible targets to toxic substances and is an important parameter for assessing the concentration level of protein.
| Parameters | Control | Sub-acute | Acute | ||
|---|---|---|---|---|---|
| 250 mg | 500 mg | 1000 mg | 2000 mg | ||
| Hemoglobin (Gms/dl) | 13.16 ± 0.405 | 14.86 ± 0.841 | 15.53 ± 0.375 | 13.83 ± 0.548 | 16.7 ± 0.264 |
| Total count (cells/cu.mm) |
6566 ± 409.6 | 3600 ± 251.66 | 6600 ± 416.33 | 3275 ± 3262 | 3435 ± 1726 |
| Polymorphs | 17 ± 0.57 | 15.33 ± 0.88 | 21.33 ± 0.88 | 18 ± 1.73 | 25.33 ± 0.88 |
| Lymphocytes (%) | 64 ± 5.03 | 60 ± 3.64 | 64.68 ± 4.84 | 58 ± 2 | 70.66 ± 5.36 |
| Eosinophils (%) | 7.66 ± 0.88 | 11 ± 0.577 | 7.66 ± 0.88 | 7.66 ± 0.88 | 5.33 ± 0.88 |
| Monocytes (%) | 4.66 ± 1.45 | 3.66 ± 0.88 | 5.66 ± 0.66 | 7.33 ± 1.45 | 4 ± 1 |
| Basophils (%) | 0 | 0 | 0 | 0 | 0 |
| Platelet count (Laksh/cu.mm) | 5.3 ± 0.568 | 6.6 ± 0.750 | 3.8 ± 0.208 | 6.13 ± 1.172 | 5.56 ± 0.635 |
| RBC count (mills/cu.mm) | 7.2 ± 0.351 | 8.3 ± 0.115 | 7.76 ± 0.338 | 6.2 ± 0.818 | 6.43 ± 0.688 |
| PCV (%) | 34 ± 4.163 | 45 ± 7.810 | 44.53 ± 3.219 | 29.66 ± 5.454 | 33.53 ± 3.159 |
| MCV (fl) | 61.33 ± 18.66 | 72 ± 2.081 | 75 ± 5.56 | 42.33 ± 6.691 | 74.66 ± 10.72 |
| MCH (pg) | 19.66 ± 0.705 | 21.13 ± 1.017 | 19.133 ± 1.88 | 17.23 ± 2.282 | 22.16 ± 1.647 |
| MCHC (%) | 33.66 ± 2.905 | 31.366 ± 0.272 | 30.76 ± 1.395 | 30.63 ± 2.152 | 31.633 ± 1.732 |
Table 3: Hematological parameters of orally treated rats in toxicity studies with ULP.
Physiological and pathological status in animals
Although some parameters observed in this study (Hematology total count and differential count) showed a statistical difference in the treated groups with ULP, The other hematological parameters were similar among groups (Table 4). Therefore, as in the biochemical analysis, the observed differences are not biologically meaningful, since the values are in the normal range for the species signifying that the ULP provided no opposing effects on circulating blood cells or on their production. The valuation of pathological changes in the organs of treated animals, both macro and microscopically, is the basis of a safety assessment. In the present study, the ULP, at all doses tested, produced no changes in the treated animals’ vigorous and reproductive organs in the qualitative analysis. In the same way, in the histopathological analyses there remained no findings suggestive of toxic effects (Figure 3). These results showed to be consistent with biochemical analyses, authorizing the safety of using the ULP.
| Parameter | Group of animals | ||||
|---|---|---|---|---|---|
| Sub-acute | Acute | ||||
| Control | 250 mg | 500 mg | 1000 mg | 2000 mg | |
| Total cholesterol (mg/dl) | 42.66 ± 3.71 | 36.66 ± 4.17 | 31.33 ± 0.66 | 44 ± 2.64 | 41 ± 0.57 |
| Triglycerides (mg/dl) | 80.66 ± 15.40 | 141.33 ± 28.04 | 124 ± 9.291 | 95.33 ± 3.48 | 91.66 ± 6.35 |
| LDL (mg/dl) | -34.8 ± 4.168 | -40 ± 3.17 | -40.83 ± 0.76 | -30.9 ± 1.06 | -30.7 ± 0.173 |
| HDL (mg/dl) | 53.33 ± 1.76 | 43 ± 1.52 | 49 ± 1.54 | 57.66 ± 1.76 | 56.66 ± 2.33 |
| VLDL (mg/dl) | 16.46 ± 2.482 | 39.33 ± 1.45 | 31.13 ± 4.627 | 22.1 ± 3.66 | 26.53 ± 3.61 |
| Random blood sugar (mg/dl) | 159 ± 32.188 | 119.66 ± 18.123 | 173.66 ± 17.52 | 100.66 ± 2.848 | 123 ± 28.04 |
| BUN (mg/dl) | 13.8 ± 2.007 | 24.66 ± 2.33 | 25.66 ± 2.728 | 20.33 ± 6.359 | 16.33 ± 2.1858 |
| Urea (mg/dl) | 38.86 ± 1.444 | 52.43 ± 4.481 | 52.43 ± 7.509 | 46.56 ± 3.73 | 37.76 ± 1.377 |
| Creatinine (mg/dl) | 0.94 ± 0.203 | 0.88 ± 0.193 | 0.74 ± 0.238 | 0.866 ± 0.240 | 0.76 ± 0.115 |
| Uric acid (mg/dl) | 3.93 ± 0.578 | 2 ± 0.608 | 3.2 ± 0.115 | 3.43 ± 0.260 | 3.53 ± 0.328 |
| Calcium (mg/dl) | 9.06 ± 1.00 | 9.33 ± 0.648 | 10.6 ± 0.866 | 8.3 ± 0.692 | 10.233 ± 0.466 |
| Phosphorus (mg/dl) | 14.06 ± 0.66 | 7.9 ± 1.637 | 11.23 ± 0.676 | 12.06 ± 1.299 | 10.4 ± 0.458 |
| Total bilirubin (mg/dl) | 0.233 ± 0.088 | 0.23 ± 0.068 | 0.203 ± 0.057 | 0.11 ± 0.049 | 0.046 ± 0.272 |
| Direct bilirubin (mg/dl) | 0.203 ± 0.057 | 0.11 ± 0.049 | 0.203 ± 0.057 | 0.2 ± 0.0577 | 0.203 ± 0.057 |
| SGOT/AST (mg/dl) | 310.33 ± 42.46 | 361 ± 27.06 | 522 ± 96.03 | 357 ± 55.32 | 399.33 ± 23.15 |
| SGPT/ALT (mg/dl) | 131.33 ± 47.68 | 86.33 ± 28.34 | 269.66 ± 100.40 | 193.33 ± 64.198 | 202.66 ± 39.218 |
| Alkaline phosphatase (mg/dl) | 73 ± 14.57 | 97.66 ± 14.52 | 146.33 ± 22.45 | 116 ± 50.61 | 157.33 ± 38.77 |
| Total protein (mg/dl) | 6.96 ± 0.49 | 6.76 ± 0.545 | 7.33 ± 0.392 | 6.8 ± 0.378 | 6.2 ± 0.550 |
| Albumin (mg/dl) | 1.2 ± 0.057 | 0.666 ± 0.284 | 1.06 ± 0.088 | 0.93 ± 0.120 | 1.03 ± 0.066 |
| Globulin (mg/dl) | 5.96 ± 0.466 | 5.53 ± 0.545 | 5.96 ± 0.088 | 6.433 ± 0.352 | 5.63 ± 0.290 |
| GGT (IU/l) | 6.06 ± 1.538 | 9.63 ± 4.937 | 19.33 ± 0.881 | 11.86 ± 4.091 | 10.4 ± 0.650 |
Table 4: Effect of ULP of serum biochemical parameter on male wistar rats.
Conclusion
In these acute and sub-acute toxicity studies, similar changes in body weight gain, feed consumption, clinical pathology evaluation, and organ weight were observed. The LD50 did not produce any significant treatment-related changes in clinical observations, and hence haematological, histo-pathological, and serum biochemical parameters were within the normal limits. The toxicity study did not show any toxicological mortality, and no behavioural changes were observed in rats treated with acute and sub-acute studies. Moreover, no irregularity was found in the organs at autopsy. In the present study, the ULP, at all doses tested, produced no changes in the treated animals’ vigorous and reproductive organs in the qualitative analysis. Finally, it is concluded that toxicity of protein extracted from Ulva lactuca L. seaweed does not affect the metabolism and behavior and therefore it can be used for any purpose.
Conflict of Interest
The Author declared no conflict of interest.
Acknowledgement
Funding from University research fellowship, Bharathidasan University, Tiruchirappalli, Tamilnadu, India..
References
- Beaulieu L, Sirois M, Tamigneaux É (2016) Evaluation of the in vitro biological activity of protein hydrolysates of the edible red alga, Palmaria palmata (dulse) harvested from the Gaspe coast and cultivated in tanks. J Appl Phycol 28: 3101-3115.
- Harnedy PA, FitzGerald RJ (2013) In vitro assessment of the cardioprotective, anti-diabetic and antioxidant potential of Palmaria palmata protein hydrolysates. J Appl Phycol 25: 1793-1803.
- Jao CL, Hung CC, Tung YS, Lin PY, Chen MC, et al. (2015) The development of bioactive peptides from dietary proteins as a dipeptidyl peptidase IV inhibitor for the management of type 2 diabetes. Biomedicine 5.
- Aneiros A, Garateix A (2004) Bioactive peptides from marine sources: Pharmacological properties and isolation procedures. J Chromatogr B 803: 41-53.
- Ngo DH, Vo TS, Ngo DN, Wijesekara I, Kim SK (2012) Biological activities and potential health benefits of bioactive peptides derived from marine organisms. Int J Biol Macromol 51: 378-383.
- Daud NA, Ghassem M, Fern SS, Babji AS (2016) Functional bioactive compounds from freshwater fish, edible birdnest, marine seaweed and phytochemical. Am J Food Nutr 6: 33-38.
- Bleakley S, Hayes M (2017) Algal proteins: Extraction, application, and challenges concerning production. Foods 6: 33.
- Fleurence J (1999) Seaweed proteins: Biochemical, nutritional aspects and potential uses. Trends Food Sci Technol 10: 25-28.
- Sousa I, Gouveia L, Batista AP, Raymundo A, Bandarra NM (2008) Microalgae in novel food products. Food Chemistry Research Developments.
- Goldberg M, Gomez-Orellana I (2003) Challenges for the oral delivery of macromolecules. Nature reviews Drug discovery 2: 289.
- Hamman JH, Enslin GM, Kotzé AF (2005) Oral delivery of peptide drugs. BioDrugs 19: 165-177.
- O'Hagan DT, Palin KJ, Davis SS (1988) Intestinal absorption of proteins and macromolecules and the immunological response. Crit Rev Ther Drug Carrier Syst 4: 197-220.
- Shah RB, Ahsan F, Khan MA (2002) Oral delivery of proteins: progress and prognostication. Crit Rev Ther Drug Carrier Syst 19.
- Delaney B, Astwood JD, Cunny H, Conn RE, Herouet-Guicheney C, et al. (2008) Evaluation of protein safety in the context of agricultural biotechnology. Food Chem Toxicol 46: S71-S97.
- Sjoblad RD, McClintock JT, Engler R (1992) Toxicological considerations for protein components of biological pesticide products. Regul Toxicol Pharmacol 15: 3-9.
- Liener IE (1994) Implications of antinutritional components in soybean foods. Crit Rev Food Sci Nutr 34: 31-67.
- Bardocz S, Pusztai A (1995) Lectins: Biomedical Perspectives. Taylor & Francis.
- Delaney B, Zhang J, Carlson G, Schmidt J, Stagg B, et al. (2007) A gene-shuffled glyphosate acetyltransferase protein from Bacillus licheniformis (GAT4601) shows no evidence of allergenicity or toxicity. Toxicol Sci 102: 425-432.
- Mathesius CA, Barnett Jr JF, Cressman RF, Ding J, Carpenter C, et al. (2009) Safety assessment of a modified acetolactate synthase protein (GM-HRA) used as a selectable marker in genetically modified soybeans. Regul Toxicol Pharmacol 55: 309-320.
- Juberg DR, Herman RA, Thomas J, Brooks KJ, Delaney B (2009) Acute and repeated dose (28 day) mouse oral toxicology studies with Cry34Ab1 and Cry35Ab1 Bt proteins used in coleopteran resistant DAS-59122-7 corn. Regul Toxicol Pharmacol 54: 154-163.
- Stagg NJ, Ghantous HN, Ladics GS, House RV, Gendel SM, et al. (2013) Workshop proceedings: Challenges and opportunities in evaluating protein allergenicity across biotechnology industries. Int J Toxicol 32: 4-10.
- Hammond B, Kough J, Herouet-Guicheney C, Jez JM, ILSI International Food Biotechnology Committee Task Force on the Use of Mammalian Toxicology Studies in the Safety Assessment of GM Foods. (2013) Toxicological evaluation of proteins introduced into food crops. Crit Rev Toxicol 43: 25-42.
- Khann P, Nag TN, Chandrajain S, Mohan S, inventors (1976) Process for isolation of insulin from plant source. United States patent US 3,945,988.
- Krishnamoorthi R, Sivakumar SR (2019) Antifungal Activity of seaweed Ulva Lactuca L. Extracted crude protein against pathogenic fungi. Asian J Pharm Clin Res 12: 393-396.
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680.
- Anwer R, Khursheed S, Fatma T (2012) Detection of immunoactive insulin in Spirulina. J Appl Phycol 24: 583-591.
- Patel SB, Rao NJ, Hingorani LL (2016) Safety assessment of Withania somnifera extract standardized for Withaferin A: Acute and sub-acute toxicity study. J Ayurveda Integr Med.; 7: 30-37.
- Collier E, Watkinson A, Cleland CF, Roth J (1987) Partial purification and characterization of an insulin-like material from spinach and Lemna gibba G3. Int J Biol Chem 262: 6238-6247.
- Oliveira AE, Machado OL, Gomes VM, Neto JX, Pereira AC, et al. (1999) Jack bean seed coat contains a protein with complete sequence homology to bovine insulin. Protein Pept Lett 6: 15-22.
- Silva LB, Santos SS, Azevedo CR, Cruz MA, Venâncio TM, et al. (2002) The leaves of green plants as well as a cyanobacterium, a red alga, and fungi contain insulin-like antigens. Braz J Med Biol Res 35: 297-303.
- Duve H, Thorpe A (1979) Immunofluorescent localization of insulin-like material in the median neurosecretory cells of the blowfly, Calliphora vomitoria (Diptera). Cell Tissue Res 200: 187-191.
- LeRoith D, Shiloach J, Roth J, Lesniak MA (1981) Insulin or a closely related molecule is native to Escherichia coli. Int J Biol Chem 256: 6533-6536.
- Robitzki A, Schröder HC, Ugarkovic D, Pfeifer K, Uhlenbruck G, et al. (1989) Demonstration of an endocrine signaling circuit for insulin in the sponge Geodia cydonium. EMBO J 8: 2905-2909.
- Rivas CA, Castillo AA, MartÃnez HS, Zapata EP, Hernández JB, et al. (2013) Acute oral toxicity of Azadirachta indica (Neem Tree). Revista Cubana de Plantas Medicinales 18: 502-507.
- Costa LG, Richter RJ, Li WF, Cole T, Guizzetti M, et al. (2003) Paraoxonase (PON 1) as a biomarker of susceptibility for organophosphate toxicity. Biomarkers 8: 1-2.
- OECD, OECD Environment Data, Compendium (2008) Environmental performance and information division OECD, Environment directorate working group on environmental information and outlooks, Organisation for economic co-operation and development (OECD), Paris, France.
- Brandt AP, Oliveira LF, Fernandes FB, Alba J (2009) Evaluation of prospective hypocholesterolemic effect and preliminary toxicology of crude extract and decoction from Vitex megapotamica (Spreng) Moldenke (V. montevidensis Cham.) in vivo. Revista Brasileira de Farmacognosia 19: 388-93.
- Giknis ML, Clifford CB (2006) Clinical laboratory parameters for Crl: CD (SD) rats. Charles River Laboratories.
Citation: Krishnamoorthi R, Sivakumar SR, Vigneswaren M, Rathinam A, Siva G (2021) In vivo Toxicological Evolution of Protein Extract from Ulva lactuca L. in Marine Seaweed using Male Wistar Rats. Diagnos Pathol Open S7: 027.
Copyright: © 2021 Krishnamoorthi R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 1334
- [From(publication date): 0-2021 - Dec 03, 2024]
- Breakdown by view type
- HTML page views: 993
- PDF downloads: 341