In vivo Studies of Anti-Inflammatory and Anti-Diabetic Activities of the Methanolic Extract of Pilea microphylla on Experimental Mice
Received: 24-Jun-2020 / Accepted Date: 09-Jul-2020 / Published Date: 16-Jul-2020 DOI: 10.4172/2573-4555.1000286
Abstract
Introduction: Ethnopharmacological relevance: Pilea microphylla is an important medicinal plant belonging to family Urticeaceae. This plant is used in traditional medicine to balance insulin levels and cure diabetes. This herb is beneficial in treating disorder related to breathing problem including asthma.
Aim of the study: The aim of the present study was to assess the in vivo anti-inflammatory activity and Antidiabetic activity of methanolic extracts of Pilea microphylla.
Materials and methods: The anti-inflammatory effect of the extracts was evaluated on paw edema in mice induced by carrageenan and the anti-diabetic activity was determined by oral glucose tolerance test (OGTT).
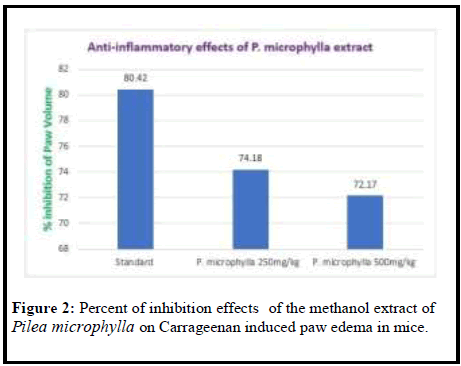
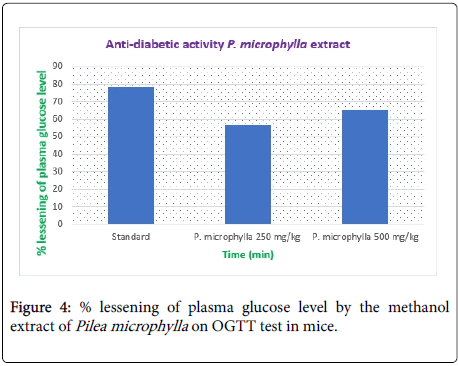
Results: In the present study the anti-inflammatory effect was observed of methanol extract in doses of 250 mg/kg and 500 mg/kg and the results were 74.18% and 72.17% inhibition of paw edema respectively compared to the standard drug Diclofenac-Na 80.42% inhibition of paw edema. The anti-diabetic effect was observed of methanol extract in doses of 250 mg/kg and 500 mg/kg and the results were 56.44% and 62.26% inhibition of paw edema respectively compared to the standard drug Metformin hydrochloride 78.05% inhibition of paw edema.
Conclusion: These findings suggest the use of Pilea microphylla as therapeutic agents to treat diabetes and inflammation as an alternative option of synthetic drugs particularly among poor people of developing countries.
Keywords: Pilea microphylla; Carrageenan anti-inflammatory activity; Anti-diabetic activity; OGTT
Introduction
Since time immemorial, plant-based drugs have been used for various diseases ranging from the common cold to cancer. Traditional herbal medicine is an important component that provides primary health care systems in developing countries [1]. Medicinal plants are playing significant role in the daily lives of many people and are an essential part of the South African cultural heritage [2]. The World Health Organization (WHO) has calculated that 80% of the world’s inhabitants utilize traditional medicine for meeting their primary health care needs and the majority of this therapy requires the use of herbal extracts and their active components. The estimation of 20,000 herbal plants used for medicinal purposes in the world [3]. There are 30,000 species of higher plants in South Africa and about 350 species are traded as medicinal plants [2]. In recent years, there has been an increasing interest in botanical description and biological activities from plants used traditionally to treat many diseases and those used as food [4].
Various medicinal plant bioactive extracts and their isolated active constituents have shown different medicinal pharmacological properties against acute and chronic diseases and disorders [5]. Pain is a direct response to a problematic event in association with inflammation, cancer, tissue damage arising from injury [6].
Inflammation, a confined physical condition, is a complex biological response of vascular tissues to invasion or harmful stimulation by an infectious agent, antigen challenge, chemical, physical, or traumatic impairment [7]. The complex cases and mediators involved in the inflammatory reactions can develop many diseases which include rheumatoid arthritis, chronic asthma, multiple sclerosis, inflammatory bowel disease and psoriasis [8]. Free radicals including reactive oxygen species (ROS) and reactive nitrogen species (RNS) raise a highly reactive state that can damage many biomolecules, leading to several chronic diseases like diabetes mellitus, atherosclerosis, inflammation and cancers [9,10]. Non-steroidal anti-inflammatory drugs (NSAIDs) are often used for treating several inflammatory diseases, but these drugs have side effects that include aspirin-induced asthma and disturbances of the gastrointestinal (GI) tract [11,12].
Diabetes mellitus is the most common of the endocrine disorders which is characterized by chronic hyperglycemia due to relative or absolute lack of insulin secretion or actions [13]. Hyperglycemia resulting from an accumulation of free glucose in the blood that leads to a generation of free radicals due to autoxidation of glucose and glycosylation of proteins [14]. Oxidative stress plays a vital role in the diabetic pathophysiology [13]. Moreover, oxidative stress is thought to be a major contributing factor to the development and progression of diabetic complications, which is also associated with insulin resistance and impaired insulin secretion that results in the development of diabetes mellitus [15].
Currently, there are approximately 347 million people suffering from diabetes, and 90%-95% of all diabetes patients have type 2 diabetes mellitus (T2DM) [16,17]. T2DM results from the ineffective use of insulin, or insulin resistance [17]. Chronic hyperglycemia syndrome, which is a common complication of T2DM, can results from conditions like obesity, kidney failure, nerve damage, fatty liver, hypertension, hyperlipidemia, and cardiovascular diseases [18]. As of 2014, the number of diabetes patients only in China soared to 96 million [16]. Therefore, there is a huge demand for the development of therapeutic agent with minimum side effects for the prevention, control and treatment of diabetes.
The present study investigated the anti-inflammatory and anti-diabetic effects of methanol extract of Pilea microphylla whole plant.
Methods
Drugs and chemicals
The standard drug, Metformin hydrochloride was the generous gift samples from Beximco Pharmaceuticals Limited, Bangladesh. Alloxan monohydrate was purchased from Loba Chemie, India. Carrageenan was purchased from Otto Chemika, India. Blood samples analyzed for blood glucose content by using OK meter Match glucose test meter (Hsinchu, Taiwan). Acetic acid was collected from laboratory of Bangladesh University.The standard drug Diclofenac-Na was purchased from Square Pharmaceuticals Limited, Bangladesh.
Experimental animals
Eight weeks-old Swice albino mice (27 g-30 g) purchased from Jahangirnagar University, Dhaka, Bangladesh and were housed in animals’ cages under standard environmental conditions (22°C-25°C, humidity 60%-70%, 12 h light: 12 h dark cycle). The mice were feed with standard pellet diet taken from, Jahangirnagar University, Dhaka. The animals used in this study were cared in accordance with the guidelines on animal experimentation of our institute.
Plant materials
Pilea microphylla plants were collected from Mohammadpur, Dhaka and identified by experts at Bangladesh National Herbarium, Dhaka, Bangladesh.
Drying and grinding
The collected plants were separated from undesirable materials or plants or plant parts. They were dried in the sun for one week after cutting into small pieces. The plant parts were crushed into coarse powder with the help of a suitable grinder. The powder was stored in an airtight container and kept in a cool, dark and dry place until analysis commenced.
Preparation of plant extract
About 350 g of powdered sample was taken in a clean, flat-bottomed glass container and soaked in 1200 ml of 90% methanol. The container with its contents was sealed and kept for a period of 10 days accompanying occasional shaking and stirring. The whole mixture then underwent a coarse filtration by a piece of clean, white cotton material. Then it was through whatman filter paper. The filtrate was kept in an open space to evaporate the solvent thus crude extract was obtained. Fine powders of the plant of Pilea microphylla are dissolved in 90% methanol and then evaporation the solvent.
Anti-inflammatory activity
Inflammation (paw edema) was induced by injecting 0.1 ml of 1% Carrageenan in physiological saline into the sub plantar tissues of the left hind paw of each mouse [19]. The methanol extract of Pilea microphylla 250 mg/kg and 500 mg/kg were administered orally 30 min prior to Carrageenan administration. The paw edema size was measured at 0, 1, 2, 3 and 4 hours by using dial caliper [20]. The percentage reduction of paw edema in drug treated group was compared with the control group. Diclofenac sodium (10 mg/kg p.o.) was used as reference standard. 0 (zero) hour reading was considered as an initial normal paw size. Data was collected from the paw thickness and percentage reduction of paw edema of the treated animals. Percentage reduction of paw edema was calculated by using the formula.
Anti-inflammatory activity (%)=(1-T/C) × 100
Where T is the change of paw diameter in treated group and C is the change of paw diameter in control group.
Anti-diabetic activity
This is done by oral glucose tolerance test (OGTT) in diabetic mice. After fasting 16 hours, diabetes was induced into mice by in intraperitoneal injection (i. p.) of alloxanmonohydride (90 mg/kg) dissolved in saline. After 72 hours, plasma glucose levels were measured by glucometer using a blood sample from tail-vein of mice. Mice with blood sugar higher than 8.5 mmol/L were considered as diabetic. Agematched healthy mice were used to examine the effects of extract on normal mice [21].
All the mice were divided into 4 groups, each group containing 5 mice. The divided groups are NC (normal control), DC (diabetic control), STD (diabetic mice receiving 100 mg/kg Metformin), ME (diabetic mice receiving 250 mg/kg and 500 mg/kg methanol extract). The mice were fasted over-night and next day blood samples were taken from all groups of animals to estimate fasting blood glucose level (0 min). All mice received 1 gm/kg glucose, after 1 h of feeding of extract and drug and four more blood samples were collected at 30,60,90 and 120 minutes intervals and blood glucose level was estimated in all the experiments by using OK meter Match glucose test meter [22].
Results
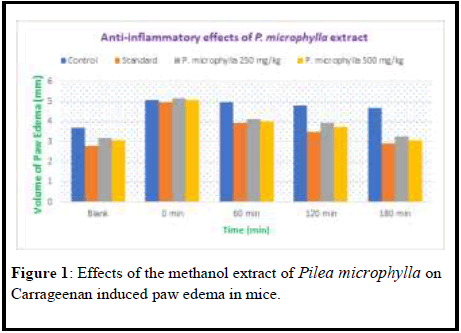
Extract of Pilea microphylla reduced inflammation in mice
Values were expressed in (Mean ± SEM) value. Each group comprised 4 animals. Control Group received normal water and standard group received 10 mg/kg Diclofenac sodium. Extract administered group was treated with 250 mg/kg and 500 mg/kg of the crude extract of Pilea microphylla (Tables 1, 2 and 3, Figures 1 and 2).
| Group | Volume of Paw Edema (mm) | ||||
|---|---|---|---|---|---|
| Blank | 0 min | 60 min | 120 min | 180 min | |
| Control | 3.7 ± 0.10 | 5.1 ± 0.11 | 4.95 ± 0.06 | 4.8 ± 0.07 | 4.7 ± 0.07 |
| Standard | 2.8 ± 0.10 | 4.95 ± 0.02 | 3.93 ± 0.06 | 3.50 ± 0.04 | 2.9 ± 0.05 |
| P. microphylla 250 mg/kg | 3.18 ± 0.08 | 5.17 ± 0.17 | 4.13 ± 0.11 | 3.92 ± 0.13 | 3.25 ± 0.07 |
| P. microphylla 500 mg/kg | 3.08 ± 0.04 | 5.08 ± 0.10 | 4.02 ± 0.08 | 3.72 ± 0.08 | 3.05 ± 0.07 |
Table 1: Anti-inflammatory effects of the methanol extract of Pilea microphylla extract on 1% Carrageenan induced paw edema on mice.
| Group | % inhibition of paw volume |
|---|---|
| Control | 0 |
| Standard | 80.42 |
| P. microphylla 250 mg/kg | 74.18 |
| P. microphylla 500 mg/kg | 72.17 |
Table 2: % inhibition of paw volume.
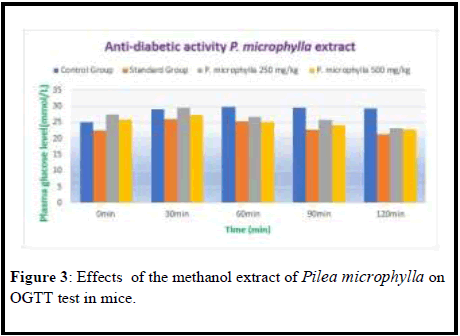
| Animal Group | 0 min | 30 min | 60 min | 90 min | 120 min |
|---|---|---|---|---|---|
| Control Group | 25.22 ± 0.32 | 29.05 ± 0.39 | 29.85 ± 0.35 | 29.55 ± 0.42 | 29.40 ± 0.45 |
| Standard Group | 22.45 ± 0.25 | 26.06 ± 0.22 | 25.43 ± 0.35 | 22.70 ± 0.25 | 21.25 ± 0.18 |
| P. microphylla 250 mg/kg | 27.45 ± 0.25 | 29.62 ± 0.30 | 26.85 ± 0.35 | 25.75 ± 0.29 | 23.20 ± 0.27 |
| P. microphylla 500 mg/kg | 25.92 ± 0.32 | 27.25 ± 0.25 | 25.15 ± 0.30 | 24.15 ± 0.27 | 22.85 ± 0.22 |
Table 3: Plasma glucose level (mmol/L) of Pilea microphylla extract in alloxan-induced diabetic (mM/L) in mice.
Anti-diabetic effec t o f Pile a microphyl l a extr ac t in allox aninduced diabetic mice
Values were expressed in (Mean ± SEM) value. Each group comprised 4 animals. Control Group received normal water and standard group received 100 mg/kg Metformin Hydrochloride. Extract administered group was treated with 250 mg/kg and 500 mg/kg of the crude extract of Pilea microphylla (Table 4, Figures 3 and 4).
| Group | % lessening of plasma glucose level |
|---|---|
| Control | 0 |
| Standard | 78.05 |
| P. microphylla 250 mg/kg | 56.44 |
| P. microphylla 500 mg/kg | 65.26 |
Table 4: % lessening of plasma glucose level.
Discussion
Alternative medicine is getting more popular for the treatment of inflammation [23]. Plant based drugs have become the main focus of current research due to the greatest therapeutic potential without many of the side effects associated with synthetic drugs [7]. A wide variety of mediators (e.g nitric oxide, eicosanoids and pro- inflammatory cytokines) have been associated critically in the development of inflammatory diseases [24]. Most of the phytochemicals that exert anti-inflammatory effects have been studied extensively for their potential to inhibit pro-inflammatory cytokines [25,26]. Methanol extract of Pilea microphylla extract has been shown to possess good anti-inflammatory potential.
T2DM is a common form of diabetes which is caused by insufficient use of insulin that eventually results in hyperglycemia [27]. Hyperglycemia has abnormal impacts on normal physiological functions in the body as well as results in macro and micro-vascular dysfunction in diabetes [28]. At present available anti-diabetic agents include sulphonylureas and the rapidly-acting secretagogues that stimulate insulin secretion, biguanides that is associated to reduce hepatic glucose production, α-glucosidase inhibitors which delay digestion and absorption, and thiazolidinediones which promotes insulin action. Although there are a large number of drugs to treat T2DM, many of these may cause drug tolerance and severe secondary side-effects such as hepatic disease [29,30]. The present investigation, concentrate of Pilea microphylla had set apart in hypoglycemic impacts in diabetic mice (Tables 3 and 4).
Conclusion
These finding suggests that administration of extract of Pilea microphylla is beneficial in reducing blood glucose levels as well as to alleviate inflammation which may arise from different acute and chronic diseases. However, further research is warranted to find out whether it does modulate the insulin secretion or directly affect the blood glucose in terms of metabolism. Status of cytokines and other targets in the pathway of developing inflammation should be studied following administration of Pilea microphylla extract to elucidate the mechanism of its anti-inflammatory effect.
References
- Sowjanya KM, Narendra K, Swathi J, Satya K (2013) Phytochemical extraction andantimicrobial efficiency of crude leaf extract of medicinal plant. Int J Res Pharm BiomedSci 4:465-470
- Van Wyk BE, Oudtshoorn BV, Gericke N (2009) Medicinal plants of South Africa. BrizaPublications, Pretoria.
- Baytop T (1999) Therapy with medicinal plants in Turkey (past and present). Pub Ist Univ 312.
- Erdem F, Doğan G, Kıran Y, Evren H (2017) Morphological, anatomical, palynologicalandkaryological characters of endemic Sideritisvulcanica Hub.-Mor. (Lamiaceae) fromTurkey. Int J Nat LifeSci 1:1-11.
- World Health Organization (2005) National policy on traditional medicine and regulation ofherbal medicines: Report of a WHO Global Survey.
- Rang HP, Dale MM, Ritter JM, Flower RJ, Henderson G (2012) Pharmacology.seventh ed. Elsevier Churchill, Livingstone 200-215.
- Anosike CA, Obidoa O, Ezeanyika LUS (2012) The anti-inflammatory activity of garden egg(Solanumaethiopicum) on egg albumin-induced oedema and granuloma tissue formation in rats. Asian Pac J Trop Med 5:62-66.
- Gautam R, Jachak SM (2009) Recent developments in anti-inflammatory natural products. Med Res Rev 29:767-820.
- Lei Y, Wang K, Deng L, Chen Y, Nice, et al. (2014) Redox regulation of inflammation: Old elements,a new story. Med Res 35:306-340.
- Thanan R, Oikawa S, Hiraku Y, Ohnishi S, Ma N, et al. (2014) Oxidative stress and its significant roles in neurodegenerative diseases and cancer. Int J MolSci 16(1):193-217.
- Carin ED, Priya G (2006) Nonsteroidal anti-inflammatory drugs. Phys Med RehabilClin N Am 17:34-54.
- Wallace JL, Vong L (2008) NSAID-induced gastrointestinal damage and the design of GI-433 sparing NSAIDs. CurrOpinInvestig Drugs 9:1151-1156.
- Ghosh S, Bhattacharyya S, Rashid K, Sil PC (2015)Â Curcumin protects rat liver from streptozotocin-induced diabetic pathophysiology by counteracting reactive oxygen species and inhibiting the activation of p53 and MAPKs mediated stress response pathways. Toxicol Rep 2:365-376.
- Shahidi S, Jabbarpour Z, Saidijam M, Esmaeili R, Komaki A, et al. (2016) The effects of the synthetic antioxidant, tempol, on serum glucose and lipid profile of diabetic and non-diabetic rats. Avicenna J Med Biochem 4:1-7.
- Kayama Y, Raaz U, Jagger A, Adam M, Schellinger IN, et al. (2015)Â Diabetic cardiovascular disease induced by oxidative stress. Int J Mol Sci. 16:25234-25263.
- Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, et al. (2014)Â Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 103:137-149.
- Yang XZ, Yang J, Xu C, Huang M, Zhou Q, et al. (2015) Hypoglycemic Activity and the potential mechanism of the flavonoid rich extract from sophoratonkinensisgagnep in kk-ay mice. Ethnopharmacol 171:161-170.
- Sijian Z, Shihao D, Yun H, Mi H, Ping Z (2017) Anti-diabetic Activity of a Polyphenol-Rich Extract From PhellinusIgniarius in KK-Ay Mice With SpontaneousType 2 Diabetes Mellitus. Food Funct9(1):614-623.
- Winter CA, Risley EA, Nuss GW (1962) Carrageenin-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Proceedings of the Society for experimental Biology and Medicine. 111(3):544-547.
- Al-Haboubi HA, Zeitlin IJ. (1983) Re-appraisal of the role of histamine in carrageenan-induced paw oedema. Eur J Pharmacol88(2-3):169-176.
- Ramdas P, Sangameswaran B, PopatM, Shantaram K (2016) Antidiabetic and antihyperlipidaemic potential of Amaranthus viridis. Asian Pacific Journal of Tropical Disease2:S180-S185.
- Hossain MS, Asadujjaman M, Khan MR, Ahmed M, Islam A (2011) Antidiabetic and glycogenesis effects of different fractions of methanolic extract of Momordicacharantia (Linn.) in alloxan induced diabetic rats. Int J Pharmaceutical Sci Res 10(4):404.
- Babu NP, Pandikumar P, Ignacimuthu S (2009) Anti-inflammatory activity of AlbizialebbeckBenth, an ethnomedicinal plant, in acute and chronic animal models of inflammation. J Ethnopharmacol 125:356-360.
- Lee S, Shin S, Kim H, Han S, Kim K, et al. (2011) Anti-inflammatory function of arctiin by inhibiting COX-2 expression via NF-kB pathways. J Inflamm 8:16.
- Kunnumakkara AB, Anand P, Aggarwal BB (2008) Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett 269:199-225.
- Kundu JK, Surh YJ (2005) Breaking the relay in deregulated cellular signal transduction as arationale for chemoprevention with anti-inflammatory phytochemicals. Mutat Res 591:123-146.
- Clark CM, Anthony LD, Engl N (1995) Duloxetine versus routine care in the long-term management of diabetic peripheral neuropathic pain. J Med 332:1210-1217.
- UK Prospective Diabetes Study Group (1998) Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. Br Med J 317(7160):703-713.
- Browne DL, Avery L, Turner BC, Kerr D, Cavan DA (2000) Evidence-based Management of Diabetes. Diabetic Med 17528-17531.
- Betsy S, Debra R, Betty C, Bonnie S (1999) Patient race and ethnicity in primary care management of child behavior problems: an important nonfinding. Med Care 1169-1173.
Citation: Chowdhury IH, Wahab SMR, Islam O, Shiara M, Faysal F, et al. (2020) In vivo Studies of Anti-Inflammatory and Anti-Diabetic Activities of the Methanolic Extract of Pilea microphylla on Experimental Mice. J Tradit Med Clin Natur 9: 286. DOI: 10.4172/2573-4555.1000286
Copyright: © 2020 Chowdary IH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4189
- [From(publication date): 0-2020 - Apr 16, 2025]
- Breakdown by view type
- HTML page views: 3320
- PDF downloads: 869