In vivo Anti-diabetic and Lipid Lowering Activity and In vitro Antimicrobial, Thrombolytic and Cytotoxic Activity of Different Fraction of Methanolic Extract of Solanum melongena
Received: 15-May-2018 / Accepted Date: 31-May-2018 / Published Date: 07-Jun-2018 DOI: 10.4172/2167-065X.1000184
Abstract
Background: In this study was tried to evaluate the in vitro antimicrobial, thrombolytic with cytotoxic activity and in vivo anti-diabetic and lipid lowering activity of different fraction of methanolic extract of Solanum melongena which is widely used in the folkloric treatment in Bangladesh. The aim of this study was to find out the fundamental mechanisms of the plant extracts which might be helpful in the overall folkloric treatments in Bangladesh. Results: In anti-diabetic and lipid lowering assay, plant extract reduce glucose level with Urea, protein and total cholesterol level goes to reduce with prolonged treatment by using plant extract. In thrombolytic assay, positive control streptokinase showed 94.21% lysis of clot and negative control distilled water showed 1.53% of clot. Chloroform soluble fraction (CSF) exhibited highest thrombolytic activity 11.13%. Antimicrobial screening at 400 µg/dise revealed antimicrobial activity in opposition to the tested microorganisms and the inhibition zone range was 8 to 13 mm. In brine shrimp lethality, LC50 µg/ml and LC90 µg/ml were obtained for different extracts after 18, 20 and 22 hours gradually. Such as after 18 hours LC50 µg/ml and LC90 µg/ml for distilled crude extract are 2.055 and 5.083, 3.112 and 6.225 for distilled n-hexane extract, 2.421 and 5.369 for distilled ccl3 extract and 4.00 and 6.948 for distilled Ethyl acetate gradually. Conclusion: Our results yield that crude methanolic extract has better effects than the other in all trials. By reviewing this study, it is very clear that the methanolic extract of Solanum melongena has shown pharmacological effect with its traditional use.
Keywords: Anti-diabetic; Lipid –lowering; Antimicrobial; Thrombolytic; Citotoxic; Solanum melongena
Introduction
Nature is a complete store-house of remedies [1]. So, we have got different types of drugs from nature in the form of herbs, plants and algae which help to cure the incurable diseases [2]. Herbal remedies have good effects and minimal side effects with relatively low cost in clinical experience. So, interest is growing on Herbal drugs. The biological active compounds of this drug may be unknown, but these are prescribed widely [3].
Epilepsy is a chronic neurological disorder and about 52 million people may affect by Epilepsy in the world [4]. Synthetic drugs are used for epilepsy and inflammation. For this reason, side effects are increasing gradually. At present time we need to keep our concentration on the scientific exploration of herbal drugs having fewer side effects [5].
The researchers are seeking medicine extract from plants sources and trying to use in Ayurveda, Allopathy, in traditional medicine and Homeopathy. Synthetic drugs have side effects and high costly. So medicinal plants are used in the traditional and modern system. Due to the lack of modern medicine and hospital the people of rural area are going to depend on traditional medicine for curing their ailment. Now a day about 70% people are using traditional folk medicine [6]. In our country approximately 80% of the population is living in the village with natural resources [7]. Medicinal plants contain various complex chemical substances of different composition [8]. Medicinal plants provide the raw materials which are helpful for internal pharmaceuticals [9]. During this time the phytochemicals research is very helpful to find out new anti-infective agents from higher plants considering physiological action [10]. We should know about the chemical constituent of plant because knowledge about these may help us to discover the actual valuable remedies [11]. Photochemical and polyphenols (phenolic acid, Hydrolysable tannins and Flavonoids) show antioxidant properties, anti-carcinogenic and anti-mutagenic effects [12]. The bioactive chemical constituents into plants like alkaloids, tannin, flavonoids, phenolic composition etc., are responsible for physiological and biochemical actions in the human body [13,14]. Natural products from plants are also show antitumor and antioxidant activity [15].
Solanacae family plants are very important for treatment of primary health care. Many workers in the world have studied about the phytochemical constituents of different plant species in the time being and among them certain authors have tried to report about phytochemical studies of Solanacae family plants [16-18]. The rest workers have also tried to work on phytochemical constituents by collecting different medicinal plants for example [19-28]. The development of antibiotic resistance gradually increasing by pathogenic microorganisms with synthetic drugs failure and side effects. So, recently 80% of population is going to use medicinal plants for their potential antimicrobial activity [29]. There are thousands of species which have antimicrobial activity of plants [30,31]. All plant parts such as (root, stem, flower, fruit etc.) are used as drug extract. So huge study is needed for identifying medicinal properties and should use some medicinal plants against pathogenic microorganisms [32,33]. At past for curing specific diseases different parts of medicinal plants were used [34]. Solanum species belongs to Solanacae family are so effective plants against pathogenic microorganisms. Antibacterial activity of this family was studied [35-37].
Hyperglycemia is an important factor for progressing of the complications and developing of diabetes mellitus. Diabetes mellitus is treated by insulin and oral administration of hypoglycemic drugs like sulfonylureas and Biguanides. Long-time affecting of the kidney, retina and nervous system is also responsible for diabetes mellitus [38-40]. In the world more than 1250 plant species are used in diabetes phytotherapy which contain anti-hyperglycemic activity [41]. Solanum melongena is a medicinal and food plants. As food plant it is hugely used in our country from previous time. Different studies have found that it has anti-hyperglycemic activity with other physiological actions [42].
Solanum melongena plays an important role in the treatment of typical human diseases from the ancient period of time. Solanum melongena (Eggplant) which is commonly known as melongene. In Southeast Asia, South Africa and South Asia, it is called brinjal [43-46]. This plant is also called eggplant because the fruits of the plant are looked like small white eggs (Eu-Sol: Eggplant history). American, Australian English and sometimes Canadian English it is known as “eggplant” or in British English and Canadian English as “aubergine” cause it bears a same name fruit which has a long history of using as a vegetable through cooking [47]. At present, this plant has an important role in the research sector. Different types of studies have presented different types of physiological actions of Solanum melongena such as, Analgesic activity [48], Antipyretic activity [49], Antioxidant Activity [50,51], Anti-inflammatory Activity [52], Anti-asthmatic Activity [53], Action on Anaphylactic Reactions [54], Hypolipidemic Action [55,56], Spasmogenic Activity [57], Action on the Eye [58], Antiplatelet and Calcium Channel Blocking Activities [59], CNS Depressant Activity [60] and Hypotensive Action [61].
Antifungal property [62] of this plant studied over and there is also found some other properties like Flavonoids isolated from Solanum melongena (brinjal) showed potent antioxidant activity [51], In vitro and In vivo. Anticancer Activity of the Fruit Peels of Solanum melongena L. against Hepatocellular Carcinoma [63], Cardio protective properties of raw and cooked eggplants and analgesic effect [64]. It was the first time we had tried to study together about in vivo anti-diabetic and lipid lowering activity and in vitro antimicrobial, thrombolytic and cytotoxic activity of different fractions of methanolic extract of Solanum melongena. Solanum melongena was selected because it is available in Bangladesh and used in rural areas for different medicinal treatments.
Methods
Plant material collection and identification
This vegetable plant was collected from Sherpur, Bangladesh. Then an expert botanist of Bangladesh National Herbarium (DACB), Mirpur-1, Dhak1216, Bangladesh was identified and authenticated the leaves of Solanum melongena. The identification No. of this plant was 41102. For the future reference a voucher specimen was submitted at the herbarium.
Chemicals
The chemicals used in this study were purchased from Merck (Darmstadt, Germany) and Sigma Chemical Co. (St. Louis, MO, USA). The chemicals which used were analytical grade.
Extract preparation: The collected plant parts (leafs) were segregated from plants. They were kept in open air for dry for two weeks under shade. A suitable grinder was used for grinding the dried plant’s parts into a coarse powder. An airtight container was used for storing the powder and kept in a cool, dark and dry place for analysis. About 750 gm. of powder material of leaf was taken in a clean glass container and soaked in 3.5 Litre of Methanol. After keeping the content into the container, it was sealed and stored for a period of 14 days with occasional shaking and stirring. The whole mixture was taken in coarse filtration by a piece of clean, white, cotton material. Then it was filtrate through Whatman filter paper. The filtrate (methanol extract) was evaporated in a water bath and under ceiling fan until dried. It changed into greenish black color. The greenish black color extract was entitled as crude extract of methanol.
Test animals
For the screening of in vivo anti-diabetic and lipid lowering activity young swiss -albino rats (aged 25–30 days), average weight 25-30 g was used. The Animal Resources Branch of ICDDR, B (International Centre for Diarrheal Disease and Research, Bangladesh) was the source of collecting and kept in suitable condition for 10 days for adaptation. Rodent food and water ad libitum formulated by ICDDR, B were used for their feeding. Throughout the experiments, we took care of all animals during our experiment according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals”, 8th edition, prepared by the National Academy of Sciences and published by the National Institute of Health (US).
Test Organism
Artemia salina Leach (brine shrimp). The egg of the shrimp was collected from Katabon University Market.
Thrombolytic assay: With minor modifications of Prasad et al. [65] clot lysis activity in vitro of the leaves of Solanum melongena was carried out. In vitro the thrombolytic activity of the Solanum melongena (eggplant) was conducted by using plant extract and distills water. In a test tube 5 mg of extract was suspended in 10 ml of distilled water and shaked it well. Whole blood (4 ml) was drawn from healthy human volunteer without a history of oral contraceptive or anticoagulant therapy, smoking and then transferred in three different pre-weighed sterilized micro centrifuge tubes. Wait for 5 to 10 min for clot formation in room temperature and after decantation the liquid was removed. Then weight of each test tube and Measure the weight of clot (clot weight = total weight of clot with tube – only weight of tube). To each micro centrifuge tube containing pre-weighed clot, 1 ml of aqueous extract of plant (Solanum melongena) was added separately. 1 ml distilled water is added to clot of tube no. 2, another is for negative control (which is Blank). All the test tube then incubated at 37°C for 90 minutes and observed for clot lysis. After incubation the tubes were kept at lying position for 6 minutes on a tray. At first serum was removed and then liquid was also removed from the tube surface by using the rod of cotton. Each tube was weighed again. Weight of clot lysis was found and calculated by the following way,
Weight of clot lysis = Initial clot weight-resting clot weight. At the final condition weight was measured very minutely, because result may vary for careless weight. So, the balance must be check before weighing. At last, percentage of clot lysis was calculated by the following equation.
% of clot lysis = ((Initial clot weight-Resting clot weight)/ Initial clot weight) × 100
Brine Shrimp Lethality assay: The study was performed according to the Brine shrimp lethality bioassay method Meyer et al. [66] by using Methanol extract of Solanum melongena and Artemia salina Leach (brine shrimp). Stock Solution and Simulated Sea Water were Prepared. After 22 hours hatching the mature nauolii (larve) were taken for bioassay Seven (07) clean test tubes were taken where six (06) of test tube contain different samples concentration respectively 1000 μg/μl, 500 μg/μl, 250 μg/μl, 125 μg/μl, 62.5 μg/μl, 31.25 μg/μl. by adding ½(half) portion of stocksolution, 100 μl DMSO solution and 2.5 ml of sea water and one (1) for negative control test. Finally 10 living shrimps were collected by the help of Pasteur pipette and were kept to each of the test tubes. For n-hexane, CCl3 and ethyl acetate solution prepared by the following above this procedure. After 18 hrs, 20 hrs and 22 hrs the percentage of lethality of brine shrimp nauplii was calculated at each concentration for each sample.
Anti-diabetic and lipid lowering activity: Rats were divided into seven groups containing 5 rats per group. Group I and II received normal diet but Group I served as normal control where Group II consists of alloxan induced rats and serving as diabetic control. Group III consists of alloxan-induced rats by receiving Glibenclamide (synthetic antidiabetic drug) which given 0.5 mg/kg body weight once a day orally for 15 days [67]. While, group IV, V, VI and group VII consist of alloxan induced rats and received concentrated Solanum melongena leaves at a dose of 3 ml/kg body weight) and given once a day orally for 15 days each, respectively. 10 mg/ml solution of Alloxan monohydrate was prepared in citrate buffer (0.1 M pH 4.5). Then the solution was kept in ice and administered into the rats within 5 min. Dose was 50 mg/kg body weight intraperitonally. Glycosuria and hyperglycemia were taken for the experiment from the rats with moderate diabetes after 48 h of alloxan administration.
Antibacterial assay: The disc diffusion method was used for antimicrobial assay [68]. By using standard disc diffusion method, the collected fractions from the plant like ethyl acetate, chloroform and n-hexane were tested along with the methanol extracts. We were collected 16 microorganisms from University of Dhaka, Bangladesh. Blank sterile filter paper disc and Standard Kanamycin (30 μg/disc) were used which were represented as positive and negative controls respectively. To prepare fresh cultures Nutrient agar medium (DIFCO) was used. These fresh cultures were used for testing the sensitivity of the organisms. In the agar plates zones were marked. The marked zones were used for placing the standard antibiotic discs, the sample discs and the control discs gently. At 37ºC for 24 hours the discs on the plate aerobically were then incubated.
Statistical analysis
One-way ANOVA with Dunnett’s post Hoc test for this experiment was carried out with SPSS 16.0 for Windows* software and the results obtained were compared with the control group. P values <0.001 were considered to be statistically significant.
Results and Discussion
Anti-diabetic and lipid lowering activity
The hot water extracts of Solanum melongena produced significant changes in the alloxan induced diabetic rats (Table 1). This plant reduced glucose level considerably in comparison to treatment of the diabetic rats and the results were comparable with that of Glibenclamide (10 mg/kg). The prolonged treatment also reduced urea, protein and total cholesterol (Tables 1 and 2) level in comparison to diabetic rats.
| Group | Treatment | Glucose (mg/dl) | Cholesterol (mg/dl) |
|---|---|---|---|
| Group-1 | Control | 117.2 ± 1.2 | 122.7 ± 1.3 |
| Group-II | Diabetic control | 245.3 ± 1.4 | 236.1 ± 1.3 |
| Group-III | Diabetic+Glibenclamide | 116.1 ± 1.5* | 121.05 ± 1.7 |
| Group-IV | Diabetic + MF | 185.22 ± 1.0** | 125.78 ± 11.0 |
| Group-V | Non-diabetic + MF | 114.2 ± 1.3 | 116.8 ± 1.5 |
| Group-VI | Diabetic +HSF | 189.18 ± 0.4** | 127.1 ± 1.2 |
| Group-VII | Non-diabetic +HSF | 114.7 ± 1.0 | 118.2 ± 1.2 |
| Group-VIII | Diabetic +CSF | 188.5 ± 0.7 | 129.7 ± 1.7 |
| Group-IX | Non-diabetic +CSF | 117.7 ± 1.0 | 119.3 ± 1.4 |
| Group-X | Diabetic +EASF | 184.16 ± 0.4 | 124.1 ± 1.4 |
| Group-XI | Non-diabetic +EASF | 113.7 ± 1.0 | 117.2 ± 1.3 |
| Group-XII | Diabetic +WSF | 180.12 ± 0.4 | 121.1 ± 1.0 |
MF= Methanolic fraction, HSF= n-hexane soluble fraction, EASF= Ethyl acetate soluble fraction and CSF= chloroform soluble fraction WSF= water soluble fraction of the Solanum melongena
Table 1: Glucose and cholesterol content of serum in control and experimental rats.
| Group-1 | Control | 49.1 ± 0.35 | 25.2 ± 1.5 | 28.3 ± 1.6 | 9.9 ± 0.34 |
| Group-II | Diabetic control | 63.5 ± 1.1 | 28.2 ± 1.4 | 28.9 ± 1.5 | 8.3 ± 0.25 |
| Group-III | Diabetic+Glibenclamide | 55.5 ± 1.7 | 33.7 ± 1.3 | 37.3 ± 1.5 | 5.7 ± 0.7 |
| Group-IV | Diabetic + MF | 55.1 ± 1.2 | 55.1 ± 1.2 | 34.1 ± 1.4 | 9.4 ± 0.33 |
| Group-V | Non-diabetic + MF | 46.1 ± 1.2 | 23.5 ± 1.2 | 25.5 ± 1.3 | 9.7 ± 1.2 |
| Group-VI | Diabetic +HSF | 53.7 ± 1.4 | 30.2 ± 2.9 | 33.2 ± 1.5 | 9.1 ± 0.31 |
| Group-VII | Non-diabetic +HSF | 48.5 ± 1.2 | 27.2 ± 1.2 | 29.1 ± 1.7 | 9.4 ± 1.2 |
| Group-VIII | Diabetic +CSF | 55.5 ± 1.2 | 34.2 ± 1.7 | 36.2 ± 1.7 | 8.1 ± 0.29 |
| Group-IX | Non-diabetic +CSF | 45.3 ± 1.3 | 25.1 ± 1.1 | 27.1 ± 1.5 | 7.2 ± 1.3 |
| Group-X | Diabetic +EASF | 50.7 ± 1.5 | 31.2 ± 1.5 | 33.1 ± 1.5 | 8.5 ± 0.25 |
| Group-XI | Non-diabetic +EASF | 42.1 ± 1.1 | 23.1 ± 1.2 | 25.1 ± 1.3 | 8.2 ± 1.7 |
| Group-XII | Diabetic +WSF | 48.5 ± 1.2 | 29.4 ± 1.7 | 31.2 ± 1.3 | 8.1 ± 0.21 |
| Group-XIII | Non-diabetic +WSF | 40.3 ± 1.5 | 21.1 ± 1.5 | 23.1 ± 1.6 | 7.9 ± 1.5 |
MF= Methanolic fraction, HSF= n-hexane soluble fraction, EASF= Ethyl acetate soluble fraction and CSF= chloroform soluble fraction WSF= water soluble fraction of the Solanum melongena
Table 2: Concentration of urea, total protein, SGOT and SGPT in serum of control and experimental rats.
In alloxan induced diabetic rats the blood glucose data obtained clearly indicate significant antihyper-glycemic effect. That means, the fraction of Solanum melongena may potentiate pancreatic secretion or may reuptake glucose. Hyper-cholesterolemia, hyper-triglyceridemia and hyper-uricemia have been reported to occur in alloxan induced diabetic rats [69]. Increase in glycogen in liver may occurred due to increase or decrease of glycogenolysis. In total protein increase (Table 2) depend on amino acids levels circulating changes, muscle output of amino acid concentrations and hepatic amino acids uptake [70]. Serum glutamic pyruvic transaminase (SGPT) and serum glutamic oxaloacetic transaminase (SGOT) were found to be increased in alloxan-induced diabetic rats. The serum level of urea and liver enzymes came to normal upon treatment with Solanum melongena. The result is shown that trans-aminase activity is increased in the serum of a diabetic rat which is active in absence of insulin due to the availability of amino acids in the blood [71]. While on the other hand, we also found that the total cholesterol level as increased in diabetic rats was also significantly reduced upon treatment with the fraction of Solanum melongena. Depressed activities of lipogenic and cholesterogenic enzymes are responsible for these [72,73].
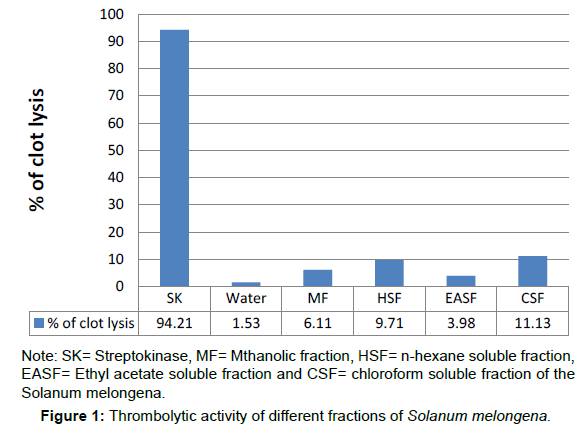
Thrombolytic activity
As a part of discovery of cardio-protective drugs from natural sources the extractives of Solanum melongena were assessed for thrombolytic activity and the results are presented in (Table 3 and Figure 1). 100 μl SK was added, a positive control (30,000 I.U.), to the clots and incubated for 90 minutes at 37°C, showed 94.21% lysis of clot. Distilled water which exhibited negligible lysis of clot (1.53%) and was treated as negative control. In this study; the chloroform soluble fraction (CSF) exhibited highest thrombolytic activity (11.13%).
| Sample | Thrombolytic Activity (% of lysis) |
|---|---|
| SK | 94.21% |
| Water | 1.53% |
| MF | 6.11% |
| HSF | 9.71% |
| EASF | 3.98% |
| CSF | 11.13% |
Table 3: Thrombolytic activity of different fractions of Solanum melongena.
Antimicrobial activity
The crude extract of Solanum melongena was subjected to antimicrobial screening at 400 μg/disc revealed antimicrobial activity in opposition to the tested microorganisms and the zone of inhibition ranging from 8 to 13 mm was measured (Table 4).
| Microorganisms | HSF | EASF | CSF | MF | Kanamycin |
|---|---|---|---|---|---|
| Gram positive bacteria | |||||
| Bacillus cereus | 9 | 7 | - | 8 | 43 |
| Bacillus megaterium | 9 | 8 | 7 | 7 | 43 |
| Bacillus subtilis | 9 | 7 | 12 | 9 | 43 |
| Staphylococcus aureus | 9 | 8 | 7 | 8 | 42 |
| Sarcinalutea | 8 | 7 | - | 8 | 42 |
| Gram negative bacteria | |||||
| Escherichia coli | 9 | 7 | - | 8 | 43 |
| Pseudomonas aeruginosa | 8 | 9 | 7 | 8 | 43 |
| Salmonella paratyphi | 9 | 7 | 12 | 8 | 42 |
| Salmonella typhi | 8 | 8 | - | 9 | 42 |
| Shigellaboydii | 9 | 7 | 7 | 8 | 43 |
| Shigellady senteriae | 8 | 8 | 13 | 7 | 43 |
| Vibrio mimicus | 9 | 7 | - | 9 | 42 |
| Vibrio parahemolyticus | 8 | 7 | - | 8 | 42 |
| Fungi | |||||
| Candida albicans | 9 | 8 | 7 | 7 | 42 |
| Aspergillusniger | - | 7 | 13 | 7 | 42 |
| Sacharomyces cerevacae | 9 | 7 | 5 | 8 | 42 |
Table 4: Antimicrobial activity of Solanum melongena.
Brine shrimp lethality bioassay
In the bioassay, lethality was indicated by methanol extracts by showing the biological activity of the compound which present in the extract. Test samples showed different mortality rate at different concentrations. The LC50 and LC90 value for the extract was obtained from the (Tables 5-7).
| Conc. of Extract µg/ml |
No of Brine shrimp inserted | No of Alive Brine Shrimp | No of death Brine shrimp | % Mortality | LogConc. µg/ml |
LC50 µg/ml |
LC90 µg/ml |
|---|---|---|---|---|---|---|---|
| Dried crude extracts | |||||||
| 0(blank) | 10 | 9 | 1 | 10 | ∞ | ||
| 1000 | 10 | 0 | 10 | 100 | 3 | ||
| 500 | 10 | 0 | 10 | 100 | 2.69 | ||
| 250 | 10 | 1 | 9 | 90 | 2.39 | 2.112 | 5.225 |
| 125 | 10 | 2 | 8 | 80 | 2.09 | ||
| 62.5 | 10 | 2 | 8 | 80 | 1.79 | ||
| 31.25 | 10 | 4 | 6 | 60 | 1.49 | ||
| Distilled n-hexane extracts | |||||||
| 0(blank) | 10 | 9 | 1 | 10 | ∞ | ||
| 1000 | 10 | 0 | 10 | 100 | 3 | ||
| 500 | 10 | 1 | 9 | 90 | 2.69 | ||
| 250 | 10 | 3 | 7 | 70 | 2.39 | 3.728 | 6.274 |
| 125 | 10 | 5 | 5 | 50 | 2.09 | ||
| 62.5 | 10 | 6 | 4 | 40 | 2.79 | ||
| 31.25 | 10 | 8 | 2 | 20 | 1.49 | ||
| Distilled CCl3 extracts | |||||||
| 0(blank) | 10 | 9 | 1 | 10 | ∞ | ||
| 1000 | 10 | 5 | 5 | 50 | 3 | ||
| 500 | 10 | 6 | 4 | 40 | 2.69 | ||
| 250 | 10 | 7 | 3 | 30 | 2.39 | 7.530 | 14.118 |
| 125 | 10 | 7 | 3 | 30 | 2.09 | ||
| 62.5 | 10 | 8 | 2 | 20 | 2.79 | ||
| 31.25 | 10 | 8 | 2 | 20 | 1.49 | ||
| Distilled Ethyl acetate | |||||||
| 0(blank) | 10 | 9 | 1 | 10 | ∞ | ||
| 1000 | 10 | 0 | 10 | 100 | 3 | ||
| 500 | 10 | 4 | 6 | 60 | 2.69 | 4.002 | 7.115 |
| 250 | 10 | 4 | 6 | 60 | 2.39 | ||
| 125 | 10 | 5 | 5 | 50 | 2.09 | ||
| 62.5 | 10 | 5 | 5 | 50 | 2.79 | ||
| 31.25 | 10 | 8 | 2 | 20 | 1.49 | ||
Table 5: After 18 hour later result of Brine shrimp lethality bioassay of distilled crude extracts, distilled n-hexane extracts, distilled CCl3 extracts and distilled Ethyl acetate of the leaves of Solanum melongena.
| Conc. of Extract µg/ml |
No of Brine shrimp inserted | No of Alive Brine Shrimp | No of death Brine shrimp | % Mortality | Log Conc. µg/ml |
LC50 µg/ml |
LC90 µg/ml |
|---|---|---|---|---|---|---|---|
| Distilled crude extracts | |||||||
| 0(blank) | 10 | 9 | 1 | 10 | ∞ | ||
| 1000 | 10 | 0 | 10 | 100 | 3 | ||
| 500 | 10 | 0 | 10 | 100 | 2.69 | ||
| 250 | 10 | 0 | 10 | 100 | 2.39 | 2.055 | 5.083 |
| 125 | 10 | 2 | 8 | 80 | 2.09 | ||
| 62.5 | 10 | 2 | 8 | 80 | 1.79 | ||
| 31.25 | 10 | 4 | 6 | 60 | 1.49 | ||
| Distilled n-hexane extracts | |||||||
| 0(blank) | 10 | 9 | 1 | 10 | ∞ | ||
| 1000 | 10 | 0 | 10 | 100 | 3 | ||
| 500 | 10 | 1 | 8 | 80 | 2.69 | ||
| 250 | 10 | 3 | 7 | 70 | 2.39 | 3.112 | 6.225 |
| 125 | 10 | 5 | 7 | 70 | 2.09 | ||
| 62.5 | 10 | 6 | 6 | 60 | 2.79 | ||
| 31.25 | 10 | 8 | 4 | 40 | 1.49 | ||
| Distilled CCl3 extracts | |||||||
| 0(blank) | 10 | 9 | 1 | 10 | ∞ | ||
| 1000 | 10 | 0 | 10 | 100 | 3 | ||
| 500 | 10 | 0 | 10 | 100 | 2.69 | ||
| 250 | 10 | 1 | 9 | 90 | 2.39 | 2.421 | 5.369 |
| 125 | 10 | 2 | 8 | 80 | 2.09 | ||
| 62.5 | 10 | 4 | 6 | 60 | 2.79 | ||
| 31.25 | 10 | 4 | 6 | 60 | 1.49 | ||
| Distilled Ethyl acetate | |||||||
| 0(blank) | 10 | 9 | 1 | 10 | ∞ | ||
| 1000 | 10 | 0 | 10 | 100 | 3 | ||
| 500 | 10 | 3 | 7 | 70 | 2.69 | 4.000 | 6.948 |
| 250 | 10 | 4 | 6 | 60 | 2.39 | ||
| 125 | 10 | 5 | 5 | 50 | 2.09 | ||
| 62.5 | 10 | 7 | 3 | 30 | 2.79 | ||
| 31.25 | 10 | 7 | 3 | 30 | 1.49 | ||
Table 6: After 20 hour later result of Brine shrimp lethality bioassay of distilled crude extracts, distilled n-hexane extracts, distilled CCl3 extracts and distilled Ethyl acetate of the leaves of Solanum melongena.
| Conc. of Extract µg/ml |
No of Brine shrimp inserted | No of Alive Brine Shrimp | No of death Brine shrimp | % Mortality | Log Conc. µg/ml |
LC50 µg/ml |
LC90 µg/ml |
|---|---|---|---|---|---|---|---|
| Distilled crude extracts | |||||||
| 0(blank) | 10 | 9 | 1 | 10 | ∞ | ||
| 1000 | 10 | 0 | 10 | 100 | 3 | ||
| 500 | 10 | 0 | 10 | 100 | 2.69 | ||
| 250 | 10 | 0 | 10 | 100 | 2.39 | 1.251 | 4.753 |
| 125 | 10 | 1 | 9 | 90 | 2.09 | ||
| 62.5 | 10 | 1 | 9 | 90 | 1.79 | ||
| 31.25 | 10 | 2 | 8 | 80 | 1.49 | ||
| Distilled n-hexane extracts | |||||||
| 0(blank) | 10 | 9 | 1 | 10 | ∞ | ||
| 1000 | 10 | 0 | 10 | 100 | 3 | ||
| 500 | 10 | 1 | 9 | 90 | 2.69 | ||
| 250 | 10 | 1 | 9 | 90 | 2.39 | 2.488 | 5.515 |
| 125 | 10 | 2 | 8 | 80 | 2.09 | ||
| 62.5 | 10 | 3 | 7 | 70 | 2.79 | ||
| 31.25 | 10 | 5 | 5 | 50 | 1.49 | ||
| Distilled CCl3 extracts | |||||||
| 0(blank) | 10 | 9 | 1 | 10 | ∞ | ||
| 1000 | 10 | 0 | 10 | 100 | 3 | ||
| 500 | 10 | 0 | 10 | 100 | 2.69 | ||
| 250 | 10 | 2 | 8 | 80 | 2.39 | 2.771 | 5.645 |
| 125 | 10 | 3 | 7 | 70 | 2.09 | ||
| 62.5 | 10 | 4 | 6 | 60 | 2.79 | ||
| 31.25 | 10 | 5 | 5 | 50 | 1.49 | ||
| Distilled Ethyl acetate | |||||||
| 0(blank) | 10 | 9 | 1 | 10 | ∞ | ||
| 1000 | 10 | 0 | 10 | 100 | 3 | ||
| 500 | 10 | 3 | 7 | 70 | 2.69 | 3.901 | 6.702 |
| 250 | 10 | 3 | 7 | 70 | 2.39 | ||
| 125 | 10 | 5 | 5 | 50 | 2.09 | ||
| 62.5 | 10 | 6 | 4 | 40 | 2.79 | ||
| 31.25 | 10 | 8 | 2 | 20 | 1.49 | ||
Table 7: After 22 hour later result of Brine shrimp lethality bioassay of distilled crude extracts, distilled n-hexane extracts, distilled CCl3 extracts and distilled Ethyl acetate of the leaves of Solanum melongena.
Conclusion
According to the result, it can be expressed that the methanolic extracts of Solanum melongena have tremendous anti-diabetic, lipid-lowering, anti-microbial, thrombolytic and cytotoxic activities. Therefore, further study may be helpful for better understanding the mechanism of such action scientifically.
Competing Interests
The authors declare that they have no competing interests.
Acknowledgments
The authors are grateful to DACB for identifying the plant and thankful to Department of Pharmacy, Manarat International University and Southeast University for providing the laboratory facilities.
Authors’ Contributions
KJ and MA carried out the collection of plants extraction process and conducting the experiments. KJ and MA have equal contribution of this study. MFRM and MSI* carried out conception and design of the study, analysis and interpretation of data. All authors read and approved the final manuscript.
References
- Trease GE, Evans MC (1983) Text book of Pharmacognosy12th edition. Balliere, Tindall London 343-383.
- Gupta RK, Kesari AN, Murthy PS, Chandra R, Tandon V, et al. (2005) Hypoglycemic and antidiabetic effect of ethanolic extract of leaves of Annonasquamosa L in experimental animals. J Ethnopharmacol  99: 75-81.
- Fisher R, Van Emde Boas W, Blume W, Elger C, Genton P, et al. (2005) Epileptic seizures and epilepsy: Definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 46: 470-472.
- Nadkarni KM (1976) Indian Materia Medica. 3rd edition. Bombay, Popular Prakasan 1: 1153-1154.
- Farnsworth NR, Aereele O, Bingel AS (1985) Medicinal Plants in therapy. Bull World Health Organ 63: 965-981.
- Anonymous (1991) Census of India, Primary Census Abstract, Government of India.
- Karthikeyan A, Shanthi V, Nagasathaya A (2009) Preliminary phytochemical and antibacterial screening of crude extract of the leaf of Adhatoda vasica. L Int J Green Pharm 3: 78-80.
- Augusti KT (1996) Therapeutic values of onion and garlic. Indian J Exp Biol 34: 634-640.
- Duraipandiyan V, Ayyanar M, Ignacimuthu S (2006) Antimicrobial Activity of Some Ethnomedical Plants used by Paliyar Tribe from Tamil Nadu, India. BMC Complement Altern Med 6: 35.
- Mojab F, Kamalinijad M, Ghaderi N, Vahidipour H (2003) Phytochemical Screening of Some Iranian Plants. Iranian J Pharma Res 2: 77-82.
- Urquiaga I, Leighton F (2000) Plant poly phenol antioxidant and oxidative stress. Biol Res 33: 159-165.
- Hill AF (1952) Economic Botany: A textbook of useful plants and plant products 2.
- Edeoga HO, Okwu DE, Mbaebie BO (2005) Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotechnol 4: 685-688.
- Karthikumar S, Vigneswari K, Jegatheesan K (2007) Screening of antibacterial and antioxidant activities of leaves Eclipta prostrate (L). Sci Res Essay 2: 101-104.
- Mohi-Ud-Din, Ayesha, Zaheer-Ud DK, Mushtag A, Muhmmad AK (2010) Chemotaxonomic value of Alkaloids in Solanum nigrum complex. Pak J Bot 42: 653-660.
- Ravi VT, Saleem SM, Maiti PP, Gauthaman K, Kamamurthy J (2009) Phytochemical and Pharmacologcal evaluation of Solanum nigrum L. Afr J Pharm Pharmacol 3: 454-457.
- Santhi K, Nadanakunjidam S (2011) Pharmacognostical Studies on Solanum nigrum L. Ad Plant Sci 24: 339-341.
- Amir M, Kumar S (2004) Possible Industrial application of genus Solanum in twenty-first Century-A review.J Sci Ind Res 63: 166-124
- Dash M, Patra JK, Panda PP (2008) Phytochemical and antimicrobial screening of extracts of Aquilaria agallocha Roxb. Afr J Biotechnol 7: 3531-3534.
- Koche D, Shirsat R, Imran S, Bhadange DG (2010) Phytochemical Screening of Eight Traditionally Used Ethnomedicinal plants from Akola District (MS) India. Int J Pharm Bio Sci1: 253-256.
- Harisaranraj R, Suresh K, Saravanbabu S (2009) Evaluation of the Chemical Composition Rauwalfia serpentine and Ephedra vulgaris. Advan Biolog Res 3:174-178.
- Mallikharjuna PB, Rajanna LN, Seetharam YN, Sharanabasappa GK (2007) Phytochemical Studies of Strychnos potatorum L, f-A Medicinal Plant. E J Chem 4: 510-518.
- Parekh J, Chanda S (2007) Antibacterial and phytochemical studies on twelve species of Indian medicinal plants. Afri J Biomed Res 10: 175-181.
- Prasad RN, Viswanathan S, Devi JR, Nayak V, Swetha VC, et al. (2008) Preliminary Phytochemical sreeining and antimicrobial activity of Samanea saman. J Medic Plan Res 2: 268-270.
- Periyasamy A, Mahalingam K (2010) Phytochemical screening and antimicrobial activity from five Indian medicinal plants against human pathogens. Middle East J Scienti Res 5: 477-482.
- Okwu DE, Orji BO (2007) Phytochemical composition and Nutritional Quality of Glycine max and Uignaun guiculata (L): Walp. Ameri J Food Technol 2: 512-520.
- Latha PS, Kanabiran K (2006) Antimicrobial and Phytochemicals of Solanum trilobatum L. Afric J Biotechnol 5: 2402-2404.
- Iwu MW, Duncan AR, Okunji CO (1991) New antimicrobials of plant origin. Int Janick J(Ed) ASHS 5: 457-462.
- Fabry W, Okemo PO, Ansorg R (1999) Antibacterial activity of East African Medicinal plants. J Ethnopharmacol 60: 79-84.
- Samy RP, Ignacimuthu S (2000) Antibacterial activity of some folklore medicinal plants used by tribals in Western Ghats of India. J Ethnopharmacol 69: 63-71.
- Haraguchi H, Kataoka S, Okamoto S, Hanafi M, Shibata K (1999) Antimicrobial triterpenes from Ilex integra and the mechanism of antifungal action. Phytother Res 13: 151-156.
- Sashikumar JM, RemyaM, Janardhanan K (2003) Antimicrobial activity of ethno medicinal plants of Nilgiri biosphere reserve and Western Ghats. Asian J Microbiol Biotechnol Environ Sci 5: 183-185.
- Hashim H, Kamali E L, Mohammed Y (2010) Antibacterial activity and phytochemical screening of ethanolic extracts obtained from selected Sudanese medicinal plants. Curr Res J Bio Sci 2: 143-146.
- John Britto S (2011) Comparative antibacterial activity study of Solanum incanum L. J Swamy Botany Cl 18: 81-82.
- Britto JS, Senthilkumar S (2001) Antibacterial activity of Solanum incanum L leaf extracts. Asian J Microbiol Biotechnol Environ Sci 3: 65-66.
- Pavitra PS, Janani VS, Charumathik H, Indumathy R, Sirisha Potala, et al. (2012) Antibacterial activity of plants used in indian herbal medicine. Int J green Phar 4: 23-28.
- Bhagwat P (2006) Diabetes mellitus causes and treatment (Ayurvedic perspective). Antiseptic 103: 221-222.
- Tiwari AK (2005) Wisdom of ayurveda in perceiving diabetes enigma of therapeutic recognition. Curr Sci 88: 1043-1051.
- Tiwari AK, Rao JM (2002) Diabetes mellitus and multiple therapeutic approaches of phytochemicals, present status and future prospects. Curr Sci 83: 30-38.
- Â Marlos RJ, Farnsworth NR (1995) Antidiabetic plants and their active constituents. Phytomedicine 2: 137-1289.
- Ong HC (2003) Sayuran Khasiat Makanandan Ubatan, Utusan Publication, Distributors sdn Bhd Fakulti Sains University Malaya 92-93.
- Singapore Government Retrieved (2014)Â Brinjal (Solanummelongena), is an easily cultivated plant belonging to the family Solanaceae its fruit is high in nutrition and commonly consumed as a vegetable the fruit and other parts of the plant are used in traditional medicine.
- Chandran, Sheela (2014) The Star Online, Star Publications (Malaysia) Berhad Retrieved  Dr Hashim devotes a large portion of his time tending to his vegetable plot where spinach, lady’s finger, sweet potato, brinjal, sweet corn and long beans grow.
- The Star, Independent Newspapers, South Africa (2011) Retrieved Plant this month beetroot, broccoli, carrots, celery, brinjal (frost-free areas), lettuce (choose heat tolerant varieties), peppers (frost-free areas), spinach, Swiss chard, a first sowing of peas, and in cold gardens a final sowing of beans.
- Vohora SB, Kumar I, Khan MS (1984) Effect of alkaloids of Solanum melongena on the central nervous system. JÂ Ethnopharmacol 11: 331-336.
- Mutalik S, Sulochana B, Chetana M, Udupa N, Uma Devi P (2003) Preliminary studies on acute and subacute toxicity of an antidiabetic herbal preparation Dianex. Indian J Exp Biol 41: 316-320.
- Daglia M, Papetti A, Dacarro C, Gazzani G (1998) Isolation of an antibacterial component from roasted coffee. J Pharm Biomed Anal 18: 219-225.
- Sudheesh S, Sandhya C, Sarah Koshy A, Vijaya lakshmi NR (1999) Antioxidant activity of flavonoids from Solanum melongena. Phytother Res 13: 393-396
- Han S, Humphreys GW (2003) Relationship between uniform connectedness and proximity in perceptual grouping. Sci China C Life Sci 46: 113-26
- Bello D, Kipper S, Valderrábano M, Shivkumar K (2004) Catheter ablation of ventricular tachycardia guided by contrast-enhanced cardiac computed tomography. Heart Rhythm 1: 490-492
- Lee MS, Lee GM (2001) Effect of hypoosmotic pressure on cell growth and antibody Production in recombinant Chinese hamster ovary cell culture. Cytotechnol 36: 61-69
- Sudheesh S, Presannakumar G, Vijayakumar S, Vijayalakshmi NR (1997) Hypolipidemic effect of flavonoids from Solanum melongena. Plant Foods Hum Nutr 51: 321-330
- Guimarães AR, Modesto A, Vieira AR (2000) Formation of alkali-soluble fluoride on the surface of human dental enamel after treatment with fluoridated gels, influence of the pH variation and of the treatment time. J Clin Pediatr Dent 24: 303-307
- Mans BJ, Gothe R, Neitz AW (2004) Biochemical perspectives on paralysis and other forms of toxicoses caused by ticks. Parasitology 129: 95-111
- Igwe SA, Akunyili DN, Ogbogu C (2003) Effects of Solanum melongena (garden egg) on some visual functions of visually active Igbos of Nigeria. J Ethnopharmacol 86: 135-138
- Gönül M, Gül U, Kaya I, Koçak O, Cakmak SK (2011) Smoking, alcohol consumption and denture use in patients with oral mucosal lesions. J Dermatol Case Rep 12: 64-68
- Vohora SB, Kumar I, Khan MS (1984) Effect of alkaloids of Solanum melongena on the central nervous system. J Ethnopharmacol 11: 331-336
- Chiu HF, Shum PS, Lam CW (1991) Psychotropic drug prescribing to chronic schizophrenics in a Hong Kong hospital. Int J Soc Psychiatry 37: 187-191
- Das J, Lahan JP, Srivastava RB (2010) Solanum melongena A potential source of antifungal agent. Indian J Microbiol 50: 62-69
- Shabana A, El-Menyar A, Asim M, Al-Azzeh H, Al Thani H (2013) Cardiovascular benefits of black cumin (Nigella sativa). Cardiovasc Toxicol 13: 9-21
- Braga PC, Ricci D (2011) Thymol-induced alterations in Candida albicans imaged by atomic force microscopy Methods. Mol Biol 736: 401-10
- Prasad S, Kashyap RS, Deopujari JY, Purohit HJ, Taori GM (2007) Effect of Fagoniaarabica (Dhamasa) on in vitro thrombolysis. BMC Complement Altern Med 7: 36
- Meyer BH, Müller FO, Gray IP, Grigoleit HG (1982) Furosemide chronopharmacology. S Afr Med J 62: 975-978
- Das S, Bhattacharya S, Prasanna A, Suresh KRB, Pramanik G (2011) Preclinical evaluation of antihyperglycemic activity of Clerodendron in fortunatum leaf against streptozotocin-induced diabetic rats. Diabetes Ther 2: 92-100
- Bauer AW, Kirby E, Sherris EM, Turk M (1966) Antibiotic by standardized single disc method. Am J Clin Path 45: 493-496.
- Resmi CR, Aneez F, Sinilal B, Latha MS (2001) Antidiabetic effect of a herbal drug in alloxan-diabetic rats. Indian Drugs 38: 319-322
- Felig P, Wahren J, Sherwin R, Palaiologos G (1977) Amino acid and protein metabolism in diabetes mellitus. Arch Intern Med 137: 507-513
- Ghosh R, Sharatohandra KH, Rita S, Thokchom IS (2004) Hypoglycaemic activity of Ficus Hispida (bark) in normal and diabetic albino rats. Indian J Pharmacol 36: 222-225
- Chi MS (1982) Effects of garlic products on lipid metabolism in cholesterol fed rats. Proc Soc Exp Biol Med 171: 174-178
- Yeh YY, Liu L (2001) Cholesterol-lowering effect of garlicextracts and organosulfur compounds: human and animal studies. J Nutr 131: 989-993
Citation: Jannat K, Ashrafudoulla MD, Mizan MDFR, Islam MDS (2018) In vivo Anti-diabetic and Lipid Lowering Activity and In vitro Antimicrobial, Thrombolytic and Cytotoxic Activity of Different Fraction of Methanolic Extract of Solanum melongena. Clin Pharmacol Biopharm 7: 184. DOI: 10.4172/2167-065X.1000184
Copyright: © 2018 Jannat K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6608
- [From(publication date): 0-2018 - Nov 27, 2025]
- Breakdown by view type
- HTML page views: 5579
- PDF downloads: 1029