Research Article Open Access
In Vitro Test and Molecular Docking of Alkaloid Compound in Marine Sponge Cinachyrella anomala against T47D Cell Cycle
Awik Puji Dyah Nurhayati1*, Rarastoeti Pratiwi2, Subagus Wahyuono3, Istriyati2, Hari Purnomo4 and Syamsudin Abdillah5
1Biology Department, Mathematic and Natural Science Faculty, Sepuluh November Institute of Technology Surabaya, Indonesia
2Biology Faculty, GadjahMada University, Yogyakarta, Indonesia
3Department of Biological Pharmacy, Faculty of Pharmacy, Gadjah Mada University, Yogyakarta, Indonesia
4Department of Chemistry Pharmacy, Faculty of Pharmacy, Gadjah Mada University, Yogyakarta, Indonesia
5Department of Pharmacology, Faculty of Pharmacy, Pancasila University, South Jakarta, Indonesia
- *Corresponding Author:
- Awik Puji Dyah Nurhayati
Biology Department, Mathematic and Natural Science Faculty
Sepuluh November Institute of Technology Surabaya, Indonesia
Tel: +1-031-596-3857 (extn. 123)
Fax: +1-031-596-3857
E-mail: awiknurhayati@gmail.com
Received date February 26, 2015; Accepted date March 28, 2015; Published date March 30, 2015
Citation: Nurhayati APD, Pratiwi R, Wahyuono S, Istriyati, Purnomo H, et al. (2015) In Vitro Test and Molecular Docking of Alkaloid Compound in Marine Sponge Cinachyrella anomala against T47D Cell Cycle. J Marine Sci Res Dev 5:158. doi:10.4172/2155-9910.1000158
Copyright: © 2015 Nurhayati APD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Marine Science: Research & Development
Abstract
The compound 1,4,9-triazatricyclo[7,3,1,0]trideca-3,5(13),10-trien-8-ol (SA2014) was isolated from the marine sponge Cinachyrella anomala. In vitro assay for SA2014 compound was found to be able to induce cell-cycle arrest at the sub-G1 and G2/M phases of T47D cancerous cell. A combined dosage between of SA2014 compound and of doxorubicin was able to induce cell-cycle arrest at sub-G1 and G2/M phases. Molecular docking approach showed that SA2014 compound inhibited cdk2 enzyme. The strength of interaction between SA2014 and cdk2 (docking score = -65,43) was more stable than the interaction between doxorubicin and cdk2 (-36,59).
Keywords
C. anomala; SA2014; cdk2 inhibitor; T47D cell
Introduction
Sponge is a member of Metazoa, which has been successfully through millions of years of evolution. This is evident from the fact that it is widely distributed all over the world, both in the salt water and the fresh water [1]. Sponges that live around the coral reefs are able to produce high-level toxic secondary metabolite as the consequence of extreme water pressure, competition, self-defense mechanism against nudibranch, gastropods, carnivorous fishes [2]. They are also found to have pharmacological potentials [3].
Around 20,000 types of active compounds are produced by sponges. They have wide-ranging chemical class diversity, including sterol, terpenoid, isoprenoid, nonisoprenoid, quinone, brominates, nitrogen heterocyclic, and heterocyclic nitrogen sulfur [4], amino acids, porphyrin and peroxide [5]. There are varied groups of functional compounds from the sponge (OH, OCH3, OAc, OSO3, Na+) [6]. Every shift in the functional groups can potentially shift polarity of each component in a dramatic way. An oncological study on marine sponges found that they could interact with essential components in the cell cycle, enzymes, and other targets [7]. Research conducted by Holland et al showed steroid compounds derived from Cinachyrella sp for anticancer activity through inhibition of aromatase for specific targets. In this study the cytotoxic activity against T47D cells of alkaloids Cinachyrella sp. The first natural 6-hydroximino-4-en-3- one steroids were isolated from Cinachyrella spp and are examples of molecules that can be deployed against a specific type of cancer. They displayed high affinity to aromatase, which is the rate-limiting enzyme that catalyzes the conversion of androgens to estrogens [8].
The development of modern medicine is oriented for specific targets. They are mostly macromolecules, particularly proteins directly involved in the biological activities. Molecular docking is a technique, which provides high accuracy for drug interaction with specific receptors. This way, the technique can be used to predict activities of a molecule (compounds) [9]. The problem discussed in this research is how 1,4,9-triazatricyclo[7,3,1,0]trideca-3,5(13),10-trien-8-ol compound in the marine sponge C. anomala interacts with specific receptors participate in the cell cycle.
Materials and Methods
Sponge C. anomala was taken from the intertidal area of Kukup Beach, Kemadang Village, Tanjungsari Sub-District, Gunung Kidul Regency, DIY using direct collection technique [6]. The sponge samples were put into plastic bags and stored in an Icebox under a temperature of 5°C until the extraction time. Isolation and identification of alkaloid compound of marine sponge C anomala was conducted by Nurhayati [10]. The alkaloid compound from C. anomala is cinachyramine derivative, using molecular formula C10H13N3O 1,4,9-triazatricyclo[7,3,1,0]trideca-3,5(13),10-trien-8-ol [10].
Analysis of cell cycle using flow cytometry method
Cell cycle analysis was conducted using flow cytometry method [11], by distributing 106 cell/well, which is distributed into a 6-well plate. T47D cell was treated using 4,9-triazatricyclo[7,3,1,0]trideca- 3,5(13),10-trien-8-ol and incubated for 24 hours. The cells were harvested using trypsin-EDTA, then centrifuged (at 2000 rpm, 30 seconds at 4°C). The cell suspension was homogenated and transferred to flowcyto-tube. Flow Cytometry data were analyzed using Modfit LT 3,0 program to find out the cell distribution in each phase of G1, S and G2/M.
Molecular docking of 1,4,9-triazatricyclo[7,3,1,0]trideca- 3,5(13),10-trien-8-ol compound with cdk2 inhibitor receptor
Molecular docking was conducted using the software PLANTS (Protein-Ligand ANT-System). The compound 1,4,9-triazatricyclo[7,3,1,0]trideca-3,5(13),10-trien-8-ol was given SA2014 code. Ligand native of the receptor was NS9 (2s,3s)-3-{[7- (benzylamino)-3-(1-methylethyl))(pyrazolo[1,5-a]pyrimidin-5-yl] amino}butane-1,2,4-triol) – an inhibitor of cdk2. Cdk2 inhibitor is a checkpoint component of cell cycle at G1 phase [12]. The comparative compound was Doxorubicin, with code receptor 3NS9.PDB in the Protein Data Bank (PDB). Protein and reference ligand (ref ligand) were prepared using YASARA it is computer programe, while ligand preparation used marvinSketch.
Results and Discussion
The 1,4,9-triazatricyclo[7,3,1,0]trideca-3,5(13),10-trien-8-ol test against T47D cell cycle
Flow Cytometry analysis for T47D cell showed that 1,4,9-triazatricyclo[7,3,1,0]trideca-3,5(13),10-trien-8-ol compound could arrest cell cycle in various phases. At a dosage of 31,25 μg/mL, the compound could induce cell cycle arrest in sub-G1 5,87% and G2/M 50,50% phases. A combined dosage between 12,25 μg/mL of SA2014 compound and 5 μg/mL of doxorubicin could induce cell cycle arrest in sub-G1 5,75 and G2/M 36,89% phases (Table 1). Doxorubicin is a potent anticancer agent for the treatment of breast cancer.
| No | Treatment | Sub-G1(%) | G0/G1 (%) | S (%) | G2/M (%) |
|---|---|---|---|---|---|
| 1 | T47D cell as control | 3,04 | 43,83 | 25,60 | 30,83 |
| 2 | 12,25 μg/mL of SA2014 | 3,99 | 47,51 | 23,20 | 29,44 |
| 3 | 31,25 μg/mL of SA2014 | 5,87 | 30,53 | 18,90 | 50,50 |
| 4 | 5 μg/mL of doxorubicin | 5,35 | 44,07 | 25 | 30,87 |
| 5 | 12,25 μg/ml of doxorubicin | 6,60 | 42,71 | 26,30 | 30,80 |
| 6 | Combination of 12,25 μg/mL of SA2014 and 5 μg/mL of doxorubicin | 5,75 | 43,14 | 19,90 | 36,89 |
Table 1: 1,4,9-triazatricyclo[7,3,1,0]trideca-3,5(13),10-trien-8-ol(SA2014) test against T47D cell cycle.
Interaction of 1,4,9-triazatricyclo[7,3,1,0]trideca-3,5(13),10- trien-8-ol compound in marine sponge C. anomala and cdk2 enzyme
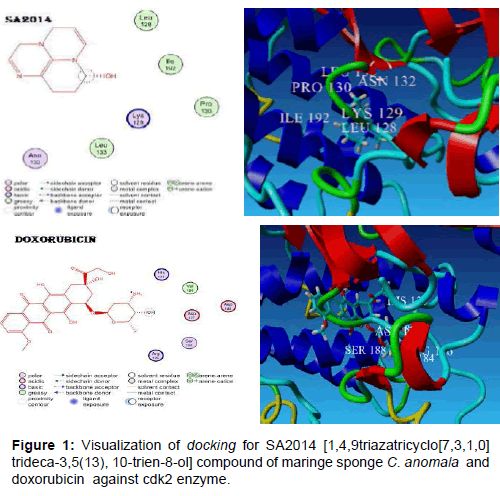
The results of in silico molecular docking test for the interaction between 1,4,9-triazatricyclo[7,3,1,0]trideca-3,5(13),10-trien-8-ol compound of marine sponge C. anomala and cdk2 protein showed a more stable docking score, compared to that of doxorubicin (Table 2 and Figure 1).
| Compounds | Docking score against cdk2 |
|---|---|
| Ligand Native (NS9) | -91.31 |
| doxorubicin | -36.59 |
| SA2014 | -65.43 |
Note: NS9=compound of cdk2 inhibitor, SA2014=structure of,4,9- triazatricyclo[7,3,1,0]trideca-3,5(13),10-trien-8-ol compound of maringe sponge C. anomala
Table 2: Docking score of 1,4,9-triazatricyclo[7,3,1,0]trideca-3,5(13),10-trien-8-ol compound of marine sponge C. anomala against Cell Devision Protein kinase2 (cdk2).
Molecular docking approach showed that 1,4,9-triazatricyclo[7,3,1,0]trideca-3,5(13),10-trien-8-ol compound of maringe sponge C. anomala (SA2014) against cdk2 enzyme showed that SA2014 was related to amino acids of leucine128, lysine129, Prolin130, asparagin132, leucin133, and Isoleucin192, while doxorubicin was related to histidin125, arginin126, asparagin127, valine184, asparagin185, and serin188. In terms of molecular size, 1,4,9-triazatricyclo[7,3,1,0]trideca-3,5(13),10-trien-8-ol compound is smaller than doxorubicin, and its binding to cdk2 enzyme is more stable than doxorubicin. Stability of compound binding with negative docking score showed that its binding to ligand is more stable [13]. In silico assay of molecular docking supported the result of in vivo assay that 1,4,9-triazatricyclo[7,3,1,0]trideca-3,5(13),10- trien-8-ol compound of marine sponge C. anomala could induce cell cycle arrest in G0/G1 phase of T47D cell.
Inhibition of 1,4,9-triazatricyclo[7,3,1,0]trideca-3,5(13),10-trien- 8-ol compound in terms of proliferation and T47D cell cycle in sub-G1 and G2/M phases was assumed to play a role as an inhibitor of cdk2. Cdk2 inhibitor could lead to phosphorylation of Thr-14 and Tyr-15 and degenerate cyclin/cdk2 and cyclin A/Cdk2 complexes, thus interfering with the progress of G1 to S [14].
Cells that are interrupted during the first to the mid-G1 phase would be postponed in the checkpoint G1. Checkpoint G1 depended upon the increasing expression and activation of gene p53 [15]. Gene p53 plays an important role in maintaining genome stability, since p53 serves as a conductor [16]. Gene p53 promoted expression of downstream effector genes, such as p21, gadd45, mdm2 and Bcl-2 associated X protein (Bax) [17] to arrest the cell cycle and improve DNA or apoptosis [18]. The response was due to the fact that effector gene has a certain place in the regulator to recognize P53. After induction, P53 would activate transcription of a number of genes, such as P21 [19]. Gene p21 is related to the inhibitor cyclin A/Cdk2 activity [20]. Over-expression of P21 led to cell arrest at G1 phase [21].
Conclusion
Molecular docking of 1,4,9-triazatricyclo[7,3,1,0]trideca- 3,5(13),10-trien-8-ol compound of maringe sponge C. anomala (SA2014) is smaller than doxorubicin, and its binding to cdk2 enzyme is more stable than doxorubicin.
Acknowledgment
Financial support from the Sepuluh November Institute of Technology Surabaya, Indonesia and Directory of Higher Education.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Utit MJ, Turon X, Becerro MA, GaleroJ (1996)Feedeing deterrence in sponge. the role of toxicity, fisical defenses, energetic contents on life-history stage. Journal ofExperimentMarine Biology and Ecology205:187-204.
- Perdicaris S, Vlachogianni T, ValavanidisA (2013)Bioactive natural substances from marine sponges: new developments and prospects for future pharmaceuticals. Natural Products & Chemistry Research1:114.
- Faulkner DJ (2001) Marine natural Product.Nat Prod Rep18: 1- 49.
- Bhakuni D,Rawat DS (2005) Bioactive marine natural products. Springer, New York.
- Tilvi S, Rodrigues C, Naik CG, Parameswaran PS, Wahidullah S (2004)new bromotyrosine alkaloids from the marine sponge psammaplysillapurpurea. Tetrahedron60: 10207-10215.
- Wright AE (1998) Isolation of Marine Natural Product. Human Press.
- Tohme R, Darwiche N, Gali-MuhtasibH (2011)Review. A Journey Under the Sea: The Quest for Marine Anti-Cancer Alkaloids. Molecules16: 9665-9696.
- Holland HL, Kumaresan S, Tan L, Njar VCO (1992)Synthesis of 6-hydroximino-3-oxo steroids, A new class of aromatase inhibitor. J Chem Soc Perkin Trans1:585-587
- Purnomo H, (2011) Kimia komputasi:molecular docking plants, penambatanmolekul plants [protein-ligand-ant-system]. PenerbitPustakaPelajar.Yogyakarta.
- Dulic V, Stein G, Far DF, Reed SI (1998) Nuclear accumulation of p21 cip at the onset of mitosis a role at the G2/Mphase transition. Mol CellBiol18: 546-557.
- Nurhayati APD, Pratiwi S, Wahyuono S, IstriyatiAbdillah S (2014)isolation and identification of alkaloid compound of marine sponge Cinachyrella sp. (Family Tetillidae). Journal of Advanced Botany and Zoology 2: 1-4.
- Vermes J, Haanen C, Reutelingsperger (2000) Flow cytometry of apoptotic cell death. Journal of Immunological Methods243: 167-190
- Morgan DO (1995) Principles of CDK regulation. Nature374:131-134.
- Kasta MB, Onyekwere O, Sidransky D (1991) Participation of p53 protein in the cellular response to DNA damage. Cancer Res51: 6304-6311.
- Nylander K, Dabelsteen E, Hal PA (2000)The p53 Molecule and its prognosis role in squamous cell carcinomas of the head and neck. Journal of Oral. Pathology & Medicine29: 413-425.
- Miyashita T, Reed JC (1995) tumor suppressor p53 is a direct transcriptional activator of the human bax gen. Cell80: 293-299.
- Malanga M, Pleschke J (1998) Poly (ADP-ribose) binds to specific domains of p53 and alters its DNA binding function. Journal ofBioogical Chemistry273: 11839-11843.
- Zhao H, Antinore S, Lung MJ, Fan F, Blanck P (2000)The central region of GADD45 is required for its interaction with p21/WAF. Experimental Cell Research258: 92-100.
- Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ (1993) The p21 Cdk-interacting protein Cip 1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell75:805-816.
- Rudoltz MS, Gary K, Kenneth R,Ruth JM, Gillies M (1996)Molecular biology of the cell cycle: potential for therapeutic applications in radiation oncology. Seminar in Radiation Oncology6: 284-294.
- Rodriguez J, Nunez L, Peixinho S, Jimenez C (1997)Isolation and synthesis of the first natural 6-hydroximino4-en-3-one-steroids from the spongesCinachyrella spp. Tetrahedron Lett 38: 1833-1836
Relevant Topics
- Algal Blooms
- Blue Carbon Sequestration
- Brackish Water
- Catfish
- Coral Bleaching
- Coral Reefs
- Deep Sea Fish
- Deep Sea Mining
- Ichthyoplankton
- Mangrove Ecosystem
- Marine Engineering
- Marine Fisheries
- Marine Mammal Research
- Marine Microbiome Analysis
- Marine Pollution
- Marine Reptiles
- Marine Science
- Ocean Currents
- Photoendosymbiosis
- Reef Biology
- Sea Food
- Sea Grass
- Sea Transportation
- Seaweed
Recommended Journals
Article Tools
Article Usage
- Total views: 15178
- [From(publication date):
August-2015 - Apr 01, 2025] - Breakdown by view type
- HTML page views : 10529
- PDF downloads : 4649