In vitro Plant Regeneration System for Two Middle East Cultivars of Chickpea (Cicer arietinum L.)
Received: 13-Feb-2014 / Accepted Date: 22-Mar-2014 / Published Date: 24-Mar-2014 DOI: 10.4172/2329-8863.1000125
Abstract
This study surveyed a rapid, efficient and reproducible protocol for in vitro shoot regeneration and rooting of two Middle East chickpea cultivars (Balila and Wady). Organogenic ability of various explants (decapitated embryo axes, fragments of embryo axes, fragments of embryo axes with basal part of cotyledons, entire and half of cotyledons) was tested on MS media containing different concentrations and nature of cytokinins (BAP and zeatin) or compound with cytokinin-like activity (TDZ). The highest level of shoot regeneration was achieved in Balila genotype using embryo axes fragments including the basal part of cotyledon on medium with 0.25 mg/L BAP. Higher BAP concentrations than 0.25 mg/L for Balila and 0.5 mg/L for Wady caused a decrease of shoot regeneration. On MS media supplemented with zeatin (1, 3 and 5 mg/L) and thidiazuron (1, 3 and 5 mg/L), the highest level of shoot differentiation was obtained from calli derived from fragments of embryo axes. Organogenic ability of calli from Wady genotype was enhanced by increasing zeatin and TDZ concentrations, up to 5 mg/L. Conversely, a progressive decrease of shoot frequency for Balila and root development for both genotypes, were observed with increasing concentrations of BAP, zeatin and TDZ.
Keywords: Chickpea, Middle East cultivars, Organogenic ability, Cytokinins, Explants types
402793Abbreviations
BAP: 6-Benzylaminopurine; MS: Murashige and Skoog; TDZ: Thidiazuron
Introduction
Chickpea (Cicer arietinum L.), the third most important pulse crop in the world, is valued for its nutritive seeds that provide important source of protein for human consumption and animal feed. Chickpea is a food legume that forms an important constituent of the human diet in Afghanistan, Bangladesh, India, Pakistan, Turkey, Middle-East, and North Africa. It is also a desirable crop rotation that can improve soil fertility by fixing atmospheric nitrogen.
Despite its significant nutritional value, chickpea production has remained static and its yield tends to be low and unstable [1]. This prevailing situation is mainly due to the adverse effects of a number of biotic and abiotic stresses. Among biotic stresses Ascochyta blight (Ascochyta rabiei) and Fusarium wilt (Fusarium oxysporum) are the most destructive and widespread fungal diseases. Some pests such as pod borer insects Helicoverpa armigera and Heliothis virescens cause severe damages [2]. As for the abiotic factors, drought stands to be the most important problem in major chickpea growing regions. Other major abiotic stresses are cold, heat, and salinity [1,3]. Enhanced resistant cultivars are needed in order to increase chickpea productivity. Attempts to improve this crop through conventional plant breeding techniques have been found to have limitations due mostly to the narrow genetic base and variability, cross incompatibility, and unavailability of resistant genotypes against biotic and abiotic factors [4-6].
Genetic transformation technique holds promise in overcoming traditional breeding constraints. It allows the introduction of specific foreign genes conferring desired traits into a crop in order to improve its quality and productivity yields. The most important pre-requisite for successful gene transfer to plants is the compatibility of the transformation protocol with the in vitro plant regeneration systems of the target plant species [7]. Since Legumineous species in general and chickpea in particular are recalcitrant to in vitro plant regeneration, the success of regeneration and transformation procedures is limited compared to other crops [8,9].
In vitro plant regeneration of chickpea has been reported through organogenesis from shoot meristems [10-14], immature cotyledons [15-17] and through embryogenesis from immature cotyledons [18] and leaflet callus [19,20]. In vitro regeneration for chickpea tends to be inefficient and unsatisfactory. Therefore, further investigations to optimize shoot regeneration are required [9]. In vitro regeneration protocols for chickpea have been described in various reports but no report tends to compare systematically the response of various types of explants to different growth regulator concentrations.
The aim of the present research is to establish an efficient and reproducible in vitro regeneration system that could be applied successfully to genetic transformation of chickpea. This report presents successful regeneration through organogenesis from embryo axes of two chickpea cultivars released by ICARDA (International Centre for Agricultural Research in the Dry Areas). These genotypes are widely cultivated in the Middle East. They are chosen for their desirable characters such as good taste and resistance to drought and to fungal disease especially to Ascochyta blight.
Materials and Methods
Chickpea (Cicer arietinum L.) seeds of two Kabuli genotypes Balila and Wady were kindly provided by ICARDA (International Centre for Agricultural Research in the Dry Areas).
Dried mature seeds were treated with 0.1% Tween 20 for 20 min followed by vigorous washing under running tap water. The seeds were surface sterilized by immersion in 0.1 % HgCl2 for 15 min with simultaneous gentle shaking. They were washed thoroughly five times with sterile distilled water and left submerged in water to be soaked overnight under sterile conditions.
On the next day, the seed coats were removed and the following explants were tested in these experimentations:
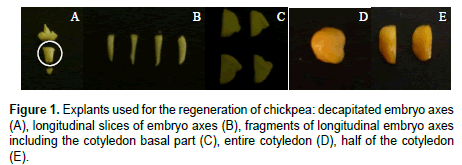
i) decapitated embryo, i.e. embryo axes with removed root apex (Figure 1A).
ii) thin longitudinal slices (3 to 5 slices) from the embryo axes with removed root apex [21] (Figure 1B).
iii) fragment including embryo axis, cotyledonary node and the basal part of a cotyledon, adjacent to meristematic region. For this purpose, embryo root, apical bud, and distal parts of cotyledons were cut out from the seed. The remaining part was cut longitudinally along the axis into four halves (Figure 1C).
iv) entire cotyledon with removed axes cultured with the abaxial surface in contact with the media (Figure 1D).
v) half of the cotyledon with removed axis (Figure 1E).
In order to study shoot regeneration, explants were cultured on Murashige and Skoog medium including macro and micronutrients [22], B5 vitamins [23], and 3 % sucrose. They were supplemented with various concentrations of 6-benzylaminopurine BAP (0.25, 0.5, 1, 2.5 and 5 mg/L), zeatin (1, 3 and 5 mg/L) and thidiazuron TDZ (1, 3 and 5 mg/L). The media were solidified with 0.7 % agar and adjusted to pH 5.8 before autoclaving.
Ten explants per Petri dishes (90 × 15 mm) containing the culture media were incubated under controlled conditions in a 16 h photoperiod (irradiance of 60 μmol.m-2.s-1 light intensity provided by fluorescent tubes), at 25 ± 1°C day and 18 ± 1°C night. The shoots produced (1-1.5 cm in length) were excised and cultured for elongation on B5 medium [23] devoid of growth regulator for 1-3 weeks. The developed shoots were transferred to rooting medium of B5 containing 0.5 mg/L IAA. All other cultural conditions remained the same as described above for shoot culture.
For each treatment, fifty replicated explants were cultured and evaluated. The experiment was repeated twice. Observations were recorded after 4 weeks of culture. Data were subjected to the analysis of variance and the means were evaluated at the P=0.05 level of significance and compared also by using Duncan’s multiple range test.
Results and Discussion
The effect of explants type, nature and concentrations of cytokinins and thidiazuron, and their interactions with the two genotypes were significant (p=0.05) for callus and shoot initiation.
Effect of explants type and different concentration of BAP on shoot regeneration
Entire and half of cotyledons tested over all media for the two genotypes did not produce any shoots. Cotyledons explants showed no reactivity with a progressive necrosis in all BAP concentrations. On medium containing zeatin and TDZ, cotyledons produced big amount of green calli, but these calli were unable to produce shoots or any organogenesis structure. In chickpea, Barna and Wakhlu [19] did not observe any shoot regeneration using cotyledons as explants [19]. While Huda et al. achieved regeneration of multiple shoots via callus induction and organogenesis from cotyledon explants [24].
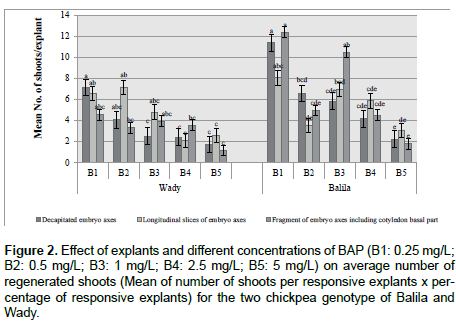
Among all explants and hormonal combinations tested, the best regeneration condition was observed with Balila genotype. When using the embryo axes fragments with basal part of a cotyledon on 0.25 mg/L BAP medium, 75% of explants was responsive and produced 16 to 17 shoots per explant (Figures 2-4A). For Wady genotype, the highest percentage of regeneration was 66% with 10.8 shoots obtained from the decapitated embryo on the medium with 0.25 mg/L BAP. Analysis of variance showed that the percentage of regeneration explants and shoots number per responding explants were significantly affected by explant genotype and BAP concentrations. Explants of Balila gave better results than those of Wady. Higher concentration of BAP than 0.25 mg/L for Balila and 0.5 mg/L for Wady did not improve explants regeneration and decreased the percentage of regenerated explants. Increase of BAP concentration caused also decreasing in the number of shoots of lentils [25]. These results confirm variation in regeneration potential of chickpea explants reported among genotypes and concentrations of BAP [26-28].
Figure 2. Effect of explants and different concentrations of BAP (B1: 0.25 mg/L; B2: 0.5 mg/L; B3: 1 mg/L; B4: 2.5 mg/L; B5: 5 mg/L) on average number of regenerated shoots (Mean of number of shoots per responsive explants x percentage of responsive explants) for the two chickpea genotype of Balila and Wady.
Effect of different concentrations of zeatin and TDZ on explants regeneration
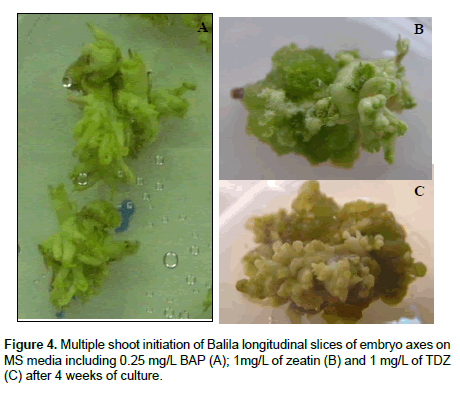
TDZ was explored for its capacity as an effective and more powerful substitute for cytokinin. On the media supplemented with different zeatin and TDZ concentrations, all explants produced big amount of green friable calli. After three weeks, shoot buds were differentiated on the surface of these calli (Figure 4B and 4C). This calli’s capacity to form shoots was different between genotypes, types of explants, concentrations of zeatin or TDZ tested. Variation in shoot forming ability of calli derived from different explant sources, media and genotypes has been noticed in chickpea by Surya-Parkash et al. and Barna and Wakhlu [19,28].
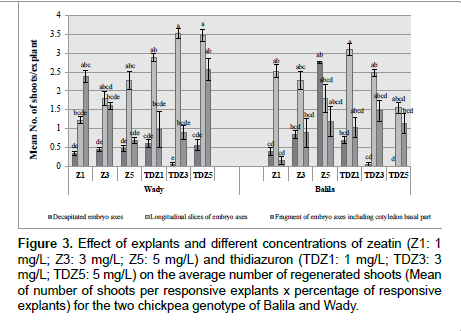
Calli derived from embryo axes fragments presented significantly more shoots per explant than the other two types of explants for Balila and Wady on media containing zeatin and TDZ. They presented also different reactivity between the two genotypes among concentrations of zeatin and TDZ. For Balila, numbers of shoots per explant of embryos fragments decreased with the increase of zeatin and TDZ concentrations. At the opposite, for Wady, their shoots number increased with higher concentration of zeatin and presented better regeneration frequency on 3 and 5 mg/L of TDZ. These explants calli type produced higher number of shoots using TDZ than zeatin. The two other explants types showed weak shoot regeneration response with less than 4 shoots, especially decapitated embryo axes explants which presented less than 2 shoots for Wady and 3 for Balila. Only fragment of axes with cotyledons explants produced higher number of shoots on the medium with 1 mg/L zeatin and 5 mg/L TDZ respectively 5 and 6 shoots. Decapitated axes did not produce any shoots on 5 mg/L TDZ containing medium for Balila genotype, and a very weak shoot production (0.5 shoots) was observed on the medium with 3 mg/L TDZ for Wady and Balila (Figure 3).
Figure 3. Effect of explants and different concentrations of zeatin (Z1: 1 mg/L; Z3: 3 mg/L; Z5: 5 mg/L) and thidiazuron (TDZ1: 1 mg/L; TDZ3: 3 mg/L; TDZ5: 5 mg/L) on the average number of regenerated shoots (Mean of number of shoots per responsive explants x percentage of responsive explants) for the two chickpea genotype of Balila and Wady.
Some morphogenic structures were produced in addition to shoot formation on the calli derived from the three types of explants cultured on 1 and 3 mg/L TDZ medium for the two genotypes. These structures had no regeneration potential. Previous reports observed that chickpea cotyledons explants gave rise to white cotyledon structure (CLS) as a response to cytokinin zeatin [15] and TDZ [17]. Shoot initiation occurred near the base of these CLS. In this study, the histogenic origin of these structures cannot be determined. Rather they resembled to ‘abnormal embryoids’ as observed in soybean [30] or deformed shoots with stunted growth [31].
Effect of shoot induction media on the elongation of regenerated shoots
Elongation of shoots transferred to B5 medium was better when they were cultured on different concentrations for BAP than for zeatin or TDZ, for which 35% of shoots could not initiate elongation and died after three weeks. Increasing of TDZ concentration has been reported to induce stunted growth of shoot buds [13,31]. Sarker et al. observed on MS medium supplemented with 0.7 mg/L TDZ that regenerated shoots did not develop any elongated shoots [9]. It was reported that the development and elongation of shoots on a medium without phytohormone prior to transferring them to a rooting medium lessened rooting problems encountered on chickpea shoots [13].
Effect of shoot induction media on rooting
Well growing shoots can produce roots on B5 medium [23] supplemented with 0.5 mg/L IAA. The highest percentage of rooting, 72%, was obtained from the shoots produced on 0.25 mg/L and 0.5 mg/L BAP. There was no significant effect on rooting percentage between the two genotypes. Previously regenerated shoots on the media supplemented with BAP (up then 0.5 mg/L) and TDZ rooted with respectively 64% and 60%. While shoots initiated on the medium supplemented with zeatin were more efficient in promoting rhizogenesis with 70% of rooting. Shoots of chickpea showed low rooting response when regenerated on the medium containing BAP [14,32]. TDZ and BAP were found to inhibit root development more than zeatin on lentils. No significant differences in rhizogenesis were observed between the two genotypes. A progressive decrease in root development was observed with the increase of BAP, TDZ and zeatin concentrations on shoots initiation media. Increasing phytohormone concentration of BAP and TDZ has been reported to alter root regeneration in chickpea [13,31]. Many reports indicate that rooting of chickpea is extremely difficult and rooting failed shoots were subjected to micrografting on rootstocks of in vitro germinated seedlings.
Conclusion
The procedure reported here for the regeneration of the recalcitrant crop chickpea constitutes an efficient and reproducible tissue culture protocol as a prerequisite for efficient application of genetic strategies. The best condition was obtained with fragments of embryo axes including part of the cotyledon of Balila genotype on 0.25 mg/l BAP level. Among all the combinations tested, fragments of embryo axes including part of the cotyledon constituted a regenerative type of explants. Medium with BAP exceeding 1 mg/L did not improve the regeneration response for the two chickpea genotypes. TDZ and zeatin induced calli formation in all explants. To overcome problems related to TDZ as stunted and deformed shoots and a decrease in regeneration, explants should be induced with the lowest but effective TDZ concentration (1 mg/L for Balila and 3 mg/L for Wady) and kept on TDZ containing medium for the least necessary duration (3 weeks).
References
- Millan T, Clarke HJ, Siddique KHM, Buhariwalla HK, Gaur PM et al. (2006) Chickpea molecular breeding: New tools and concepts. Euphytica 147: 81-103.
- Cherry AJ, Rabindra RJ, Parnell MA, Geetha N, Kennedy JS, et al. (2000) Field evaluation of Helicoverpaarmigeranucleopolyhedrovirus formulations for control of the chickpea pod-borer, Harmigera (Hubn.), on chickpea (Cicerarietinum var. Shoba) in southern India. Crop Protection 19: 51-60.
- Singh KB (1994) Current status and future strategy in breeding chickpea for resistance to biotic and abiotic stresses. Euphytica 73: 137-149.
- Ahmad F, Slinkard AE, Scoles GJ (1988) Investigations into barrier(s) to interspecific hybridization between cicerarietinum L. and eight other annual species. Plant Breeding 100: 193-198.
- Kumar VD, Davey MR (1991) Genetic improvement of legumes using somatic cell and molecular techniques. Euphytica 55: 157-169.
- Van Rheenen HA, Pundir RPS, Miranda JH (1993) How to accelerate the genetic improvement of a recalcitrant crop species such as chickpea. CurrSci 654: 414-417.
- Kar S, Johnson TM, Nayak P, Sen SK (1996) Efficient transgenic plant regeneration throughAgrobacterium-mediated transformation of Chickpea (Cicerarietinum L.). Plant Cell Rep 16: 32-37.
- Somers DA, Samac DA, Olhoft PM (2003) Recent advances in legume transformation. Plant Physiol 131: 892-899.
- Sarker RH, Ferdous T, Hoque MI (2005) In vitro direct regeneration of three indigenous chickpea (Cicerarietuinum L.) varieties of Bangladesh. PTC&B 15: 135-144.
- Bajaj YPS, Dhanju MS (1979) Regeneration of plant from apical meristem tips of some legumes. CurrSci 48: 906-907.
- Sharma DR, Kumari R, Chowdhury JB (1979) Plant regeneration in Cicer species through tissue culture. Indian J ExpBiol 17: 607-609.
- Kartha KK, Pahl K, Leung NL, Mroginski LA (1981) Plant regeneration from meristems of grain legumes: soybean, cowpea, peanut, chickpea, and bean. Can J Bot 59: 1671-1679.
- Malik KA, Saxena PK (1992) Thidiazuron induces high-frequency shoot regeneration in intact seedlings of pea (Pisumsativum), chickpea (Cicerarietinum) and Lentil (Lens culinaris). Planta 186: 384-388.
- Polisetty R, Paul V, Deveshwar JJ, Khetarpal S, Suresh K, Chandra R (1997) Multiple shoot induction by benzyladenine and complete plant regeneration from seed explants of chickpea (Cicerarietinum L.). Plant Cell Rep 16: 565-571.
- Shri PV, Davis TM (1992) Zeatin induced shoot regeneration from immature chickpea (Cicerarietinum L.) cotyledons. Plant Cell Tissue Organ Cult 28: 45-51.
- Islam R, Riazuddin S (1993) Shoot organogenesis in chickpea (Cicerarietinum L.) from callus culture of hypocotyl explants. J. Bio. Sci. 1: 1-5.
- Srivastava K, Tiwari KN, Singh BD, Jaiswal HK (2001) Shoot regeneration from immature cotyledons of Cicerarietinum. Biol Plant 44: 333-337.
- Sagare AP, Suhasini K, Krishnamurthy KV (1993) Plant regeneration via somatic embryogenesis in chick pea (Cicerarietinum L.). Plant Cell Rep 12: 652-655.
- Barna KS, Wakhlu AK (1994) Whole plant regeneration of Cicerarietinum from callus cultures via organogenesis. Plant Cell Rep 13: 510-513.
- Schroeder HE, Schotz AH, Wardley-Richardson T, Spencer D, Higgins T (1993) Transformation and Regeneration of Two Cultivars of Pea (Pisumsativum L.). Plant Physiol 101: 751-757.
- Murashige T, Skoog FA (1962) Revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473-479.
- Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50: 151-158.
- Huda S, Siddique NA, Khatun N, Rahman MH, Morshed M (2003) Regeneration of shoot from cotyledon derived callus of chickpea (Cicerarietinum L.). Pak J BiolSci 6: 1310-1313.
- Omran VG, Bagheri A, Moshtaghi N (2008) Direct in vitro regeneration of lentil (Lens culinarisMedik.). Pak J BiolSci 11: 2237-2242.
- Rubluo A, Kartha KK, Mroginski LA, Dyck J (1984) Plant Regeneration from Pea Leaflets Cultured in vitro and Genetic Stability of Regenerants. J Plant Physiol 117: 119-130.
- Gulati A, Jaiwal PK (1994) Plant regeneration from cotyledonary node explants of mungbean (Vignaradiata (L.) Wilczek). Plant Cell Rep 13: 523-527.
- Chakraborti D, Sarkar A, Das S (2006) Efficient and rapid in vitro plant regeneration system for Indian cultivars of chickpea (Cicerarietinum L.). Plant Cell Tissue Organ Cult 8: 117-123.
- Surya-Parkash, Chowdhury JB, Jain RK (1992) Factors affecting plant regeneration in chickpea, Cicerarietinum L. Indian J ExpBiol 30: 1149-1153.
- Lazzeri PA, Hildebrand DF, Collins GB (1985) A procedure for plant regeneration from immature cotyledon tissue of soybean. Plant MolBiol 3: 160-167.
- Jayanand B, Sudarsanam G, Sharma KK (2003) An efficient protocol for the regeneration of whole plants of chickpea (Cicerarietinum L.) by using axillary meristem explants derived from in vitro-germinated seedlings. In Vitro Cell Biol Plant 39: 171-179.
- Polowick PL, Baliski DS, Mahon JD (2004) Agrobacterium tumefaciens-mediated transformation of chickpea (Cicerarietinum L.): gene integration, expression and inheritance. Plant Cell Rep 23: 485-491.
- Polanco MC, Ruiz ML (2001) Factors that affect plant regeneration from in vitro culture of immature seeds in four Lense cultivars. Plant Cell Tissue Organ Cult 66: 133-139.
Citation: Kadri A, Chalak L, El Bitar A, Nicolas N, Mroué S, Grenier De March G (2014) In vitro Plant Regeneration System for Two Middle East Cultivars of Chickpea (Cicer arietinum L.). Adv Crop Sci Tech 2: 125. DOI: 10.4172/2329-8863.1000125
Copyright: © 2014 Kadri A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 17676
- [From(publication date): 4-2014 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 12449
- PDF downloads: 5227