In vitro Evaluation of the Interaction between Amoxicillin and Hydro Ethanolic Extracts of Annickia chlorantha
Received: 18-Jul-2017 / Accepted Date: 05-Sep-2017 / Published Date: 08-Sep-2017 DOI: 10.4172/2476-2067.1000133
Abstract
The discovery of antibiotics has contributed significantly to improving the life expectancy of the global population. Nowadays, the emergence of multi resistant strains of bacteria worldwide is a major concern as it dramatically reduces the choice of effective antibiotics for prevention and treatment of a very common infection in both hospitals and our local communities. The aim of this study was to evaluate the in vitro interaction of amoxicillin and Annickia chlorantha against some Multi resistant bacteria strains. The interaction between Amoxicillin and Annickia chlorantha was studied using the Checkerboard technique. Fractional Inhibitory Concentration (FIC) indices showed that Amoxicillin/Annickia chlorantha combination was synergistic against the clinical stock of Salmonella typhi at concentrations of 5: 15 and 25 mg/mL. The extracts of the stem barks of Annickia chlorantha decreased the minimum inhibitory concentration (MIC) of amoxicillin against Salmonella typhi by 2: 4 and 66 folds respectively. At the same concentrations the combination showed no difference against the reference stocks of Staphylococcus aureus and a dose dependent antagonism against clinical stock of Escherichia coli. The results of this study revealed that the concomitant use of Annickia chlorantha and amoxicillin may potentiate and restore the antibacterial effect of amoxicillin against resistant strains of Salmonella typhi. This implies that the intake of Annickia chlorantha in traditional medicines may affect the effectiveness of a co-administered amoxicillin, which makes this plant a good, environmentally friendly and promising candidate for the development of an improved traditional medicine.
Keywords: Amoxicillin; Multi-resistant bacteria strains; Annickia chlorantha; Synergy
23645Introduction
The discovery of antibiotics has contributed significantly to increase the life expectancy of the population. Nowadays, the emergence of multi-resistant strains of bacteria worldwide is a major concern as it dramatically reduces the choice of effective antibiotics for prevention and treatment of a very common infection in both hospitals and our local communities [1]. This situation leads to a significant increase in the costs of health care due to increase morbidity and mortality linked to infectious diseases in Africa [2]. With the prevailing circumstances of multi-resistance the need for new antimicrobial becomes greater than ever but the cost of pharmaceutical research and development and a number of factors make antimicrobial agents less economically attractive targets for development than other drug classes [3]. The discovery of new conventional antibiotics have always been systematically followed by bacterial resistance giving us the obligation to investigate an alternative solution.
Natural products from plants could be an interesting alternative [4] notably in developing nations where the use of phytomedicines as the main source of treatment of various diseases is estimated by the world health organization (WHO) to be greater than 80 % of the population [5]. There has been in the last years great scientific interest in chemical and pharmacological investigations of the biological properties of medicinal plants [6,7] that have proven the efficacy of many plants used in traditional medicine. Furthermore, the traditional use of fractions of plant extracts as antimicrobial agents show a low risk of increase resistance to their action because they are complex mixtures making microbial adaptability very difficult [8]. With most the natural products, the MIC value described is over 1 mg/mL which from a clinical perspective has little relevance [9].
Concurrent uses of pharmaceuticals with herbal remedies in selfmedications are reported [10] and interactions are inevitable. Looking for the nature of these interactions hoping that allopathic medicines can solve problems of antimicrobial therapy either by restoring the effectiveness of existing antibiotics on resistant strains or by diminishing some adverse effects, many studies have been conducted to assess not only for their direct antimicrobial activity of plant extract but also its activity as resistance – modifying agents [11].
Several chemical compounds derived from plants such as Glycyrrhizic acid isolated from Glycyrrhiza glabra are useful for the enhancement of antibiotic activity or the reversal of antibiotic enhancers and bio-availability facilitators for a variety of molecules including anti- infective and anti- cancer agents [12].
Annickia chlorantha Oliver (Annonaceae), also known as the African yellow wood, found in the dense forest widely distributed in Cameroon, Nigeria and Gabon, is commonly used in folk medicine for the traditional treatment of stomach problems, jaundice, urinary tract infections, malaria, tuberculosis and hepatitis [13]. The stem bark of this plant features amongst the common plants sold in local markets in Cameroon [14] phytochemical studies of the stem bark of Annickia chlorantha have resulted in the isolation of berberine and protoberberine alkaloids possessing antimalarial [15] antibacterial trypanosomicidal, anti HIV and antihepatotoxic properties. Studies have demontrated the cytoprotective and the healing actions of the stem bark aqueous extract of Enantia chlorantha [16]. Amoxicillin is a wide spectrum antibiotic which belongs to the betalactam group. It is on the list of WHO essential medicines. In this work we tested the Hydro ethanolic extract of A. chlorantha as a resistance modifying agent in amoxicillin resistant strains.
Materials and Methods
Study sites and plant identification
A. chlorantha (Annonaceae) is a tree of about 12-30 m in height. The plant bark was harvested at Kala mountain in the Centre Region of Cameroon and a sample was identified in the National Herbarium under the voucher number HNC 2133. The rest of the study was conducted at the different laboratories in Cameroon, the extraction of the crude product was carried out in the Pharmacognosy and Pharmaceutic Chemistry laboratory of the Faculty of Medicine and Biomedical Sciences Yaoundé–Cameroon, and the antimicrobial activity was tested in the laboratory of microbiology, (Clinique Universitaires des Montagnes), Bangangté-Cameroon.
Preparation of extracts of Annickia chlorantha
The bark of the stems of Annickia chlorantha was cut into pieces, air- dried for ten consecutive days and pulverized. Approximately 1300 g of the powdered bark was extracted by maceration with ethanol 70% for 72 h, the extract was filtered and concentrated to obtain a solid residue using a Heidolph rotary vapor.
Phytochemical screening
The phytochemical tests were performed to detect the presence of saponins, tannins, flavonoids, steroids triterpenes and alkaloids according to the method described by Zintchem et al. [17]. The tests were based on the visual observation of a change in color or formation of precipitate after the addition of specific reagents.
Standardization of test micro organisms
The test organisms used in this investigation were selected on the basis of their clinical significance, these include, S. aureus (ATCC BAA 1026), and clinical stocks of E. coli and S. typhi both resistant to Amoxicillin, amoxicillin-clavulanic acid fluoroquinolon which were obtained from the laboratory of Microbiology, (Clinique Universitaires des Montagnes) (Université des Montagnes, Bangangté-Cameroon).
All strains used in this experiment were cultured on Mueller Hinton agar (Liofilchem) and incubated at 37°C for 18-24 h. From the resulting overnight pure culture, a bacterial suspension equal to 0.5 McFarland (106- 108 cells/mL) was prepared and adjusted to the final density required for susceptibility tests according to the (Comité de l’Antibiogramme de la Société Francaise de Microbiologie, CA-SFM (2014)) [18].
Susceptibility test
Antimicrobial susceptibility test of the isolates was performed by Kirby- Bauer Diffusion technique [11], in which the test isolate was swabbed uniformly onto the surface of the Muller Hinton Agar plates. Antibiotic and plant extract sterile disc were then placed on the plate.Following incubation, a bacterial lawn appeared on the plate with zone of inhibition around the antibiotic discs.
Determination of Minimum and Fractional Inhibitory Concentrations (MIC and FIC)
The Minimum inhibitory concentrations (MIC) of A. chlorantha , Amoxicillin and combination of both were determined by the macrodilution assay as described by Jai et al [8]. The antibiotic concentrations ranged from 0.0475-25 mg/mL for amoxicillin and 0.475-250 mg/mL for plant extract.
The titer plates were inoculated with bacteria having 0.5 Macfarland turbidity and incubated aerobically at 37°C for 24 h. MIC was defined as the lowest concentration at which no growth was observed as described by Spoorthi et al. [19].
For the evaluation of Annickia chlorantha as a modulator of Antibiotic resistance, we determined the MIC of mixture of Amoxicillin and Annickia chlorantha at different sub inhibitory concentrations (5: 15 and 25 mg/mL). Therefore Fractional Inhibitory Concentration (FIC) was used to understand the effect of the combination. This was determined by checkerboard method as described by Esimone et al. [20] and Bhone Myint Kyaw et al. [21]. FIC indices for the all combinations were calculated using the formula below.
FIC index were calculated as follows : FIC index = FIC A + FIC B, where FIC A is the MIC of drug A in the combination/ MIC of drug A alone and FIC B is the MIC of drug B in the combination/ MIC of drug B alone. The combination is considered synergistic when the fractional inhibitory index is ≤0.5 Indifference was indicated by a FIC index >0.5 to ≤4 while antagonism when ΣFIC is >4 [22].
Results and Discussion
Phytochemical composition of hydro ethanolic extracts of A. chlorantha
The phytochemical screening of the bark extract of A chlorantha showed the presence of alkaloids, flavonoids and polyphenols as shown in Table 1, that was aligned with previous studies conducted by Dawudo et al. [23] and Salman et al [24].
| A. chlorantha | ||
|---|---|---|
| Alkaloids | Valse-mayer | ++++ |
| Hager | ++++ | |
| Wagner | ++++ | |
| Steroids | - | |
| Polyphenols | +++ | |
| Flavonoids | ++ | |
| Saponosids | - | |
| Tanins | +++ | |
| Mucilage | - | |
Table 1: Phytochemical composition of hydro ethanolic extracts of A.chlorantha .
The concept of compound that inhibits resistance in a bacterium which may be employed with a conventional antibiotic is illustrated by clavulanic acid a microbial derived inhibitor of beta lactamase which is used in combination with amoxicillin to increase the stability of this antibiotic to its degradation by the beta lactamase enzyme [9]. Piperine, an alkaloid isolated from (Trikatu) glycyrrhizin isolated from roots of Glycyrrhiza glabra are examples of plants derived resistance modifying agents.
Minimum inhibitory concentration evaluation (MIC)
The MIC recorded with amoxicillin against the tested bacteria confirmed the sensitivity profile of the microbes to this molecule Table 2. The MIC obtained with Annickia chlorantha alone showed activity against all the isolates tested. Reference E. coli was the most sensitive 15.62 mg/mL and the clinical stock of E. coli the least sensitive with a recorded MIC of 62.5 mg/ mL. These results confirmed the results of Razaq et al. [25]. The antibacterial activity of the plant extract is probably due to its phytochemical composition as established by Bassole et al., Erasto et al., Doss et al. [26-28].On the other hand these results confirmed the superiority of conventional antibiotics in terms of potency compared to the plant extracts.
| S. aureus reference | E. coli reference | E. coli Clinique | S. typhi Clinique | |
|---|---|---|---|---|
| Amoxicillin | 0.78 | 1.56 | 3.125 | 12.5 |
| A. chlorantha | 31.5 | 15.62 | 62.5 | 31.25 |
Table 2: MIC (mg/mL) of Amoxicillin and plant extracts against the strains.
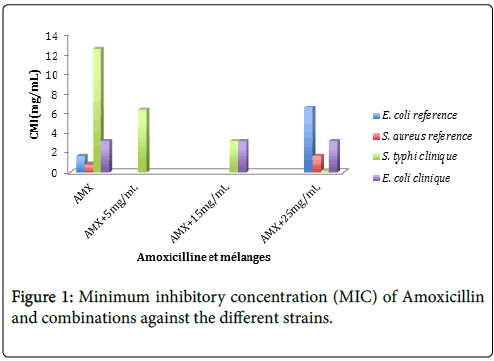
The results of the combined activity of Amoxicillin with Annickia chlorantha extract at concentrations of 5 mg/mL, 15 and 25 mg/mL, showed an important decrease in the MIC of Amoxicillin Figure 1. The plant extract decreased the MIC of Amoxicillin against S. typhi by 2; 4 and 66 times respectively at concentrations of 5, 15 and 25 mg/mL. Based on these results we can extrapolate that in vivo for an adult of 70 kg, 200 g of A. chlorantha have to be absorbed in order to reduce the concentration of amoxicillin required by half, 600 g in order to obtain a reduction by quarter and 1000 g to obtain a reduction by 66 of the amount necessary for Salmonellosis treatment.
From toxicological point of view, with regards to acute and subacute toxicological studies made by Tan et al. [13] which found that the LD50 of A chlorantha was higher than 5000 mg/mL, we can conclude that an amount of 200 g of the plant extract (to obtain a reduction by half of the amount of Amoxicillin required for treatment) can be safe but not very applicable in practice. In regards to the increase rate of reduction of the amount of amoxicillin when we increase the amount of plant extract, we can say that the substance responsible for the potentiation is present in a small amount in the plant, and further studies should be made to isolate it.
MIC of extract and amoxicillin mixture
The mixture of amoxicillin and extract of E. chlorantha was effective on all the bacteria strains tested. The clinical strains of Salmonella were the most sensitive at all level of MIC as shown in Table 3.
| E coli reference | S. aureus reference | S. typhi clinic | E. coli clinic | ||
|---|---|---|---|---|---|
| MIC (mg/mL) | AMX+5 mg/mL Extract. | - | - | 6.25 | - |
| AMX+15 mg/mL Extract. | - | - | 3.125 | 3.125 | |
| AMX+25 mg/mL Extract. | 6.5 | 1.56 | 0.19 | 3.125 | |
| MBC (mg/mL) | AMX+5 mg/mL Extract. | - | - | 25.0 | - |
| AMX+15 mg/mL Extract. | - | - | 12.5 | 12.5 | |
| AMX+25 mg/mL Extract. | 25.0 | 12.5 | 1.56 | 12.5 | |
Table 3: Minimum inhibitory concentration of extract mixture with amoxicillin.
Fractional inhibitory concentration (FIC)
The FIC study showed that a high value of 4.582 for Escherichia coli followed by Staphylococcous aureus as an indication of antagonism. For Salmonella typhi clinical stock the FIC value was 0.021 that showed synergism as opposed to E coli clinical stock (1.05) that was indifferent Table 4.
| Strains | E. coli reference | S. aureus reference | S. typhi clinic | E coli clinic |
|---|---|---|---|---|
| FIC at 25 mg/mL | 4.582 | 2.049 | 0.021 | 1.05 |
| Interpretation | Antagonism | Antagonism | synergy | indifference |
Table 4: Fractional Inhibitory Concentration (FIC).
Conclusion
Our results showed that the concomitant intake of Annickia chlorantha and amoxicillin could lead to the potentiation of the antibacterial effect of amoxicillin on multi resistant strains of Salmonella typhi , no effect on Staphylococcus aureus and virtually antagonism on multi-resistant strains of Escherichia coli . These results may imply using a lower dose of amoxicillin to achieve the same therapeutic effect when given in combination with Annickia chlorantha . This study showed a promising potential traditional use of this plant extract to treat different infectious diseases and also suggests that in combination with amoxicillin could restore the antibacterial activity of amoxicillin with respect to resistant strains. The results obtained indicate that A. chlorantha could serve as a source of plant derived natural products with antibiotic resistance-modifying activity to be used against multi-resistant bacteria. It is therefore a good candidate to reduce the drug dosage towards circumventing the problem of drug resistance and the other side effects possible during anti-infective therapy.
References
- Gould IM (2005) The clinical significance of methicillin resistant Staphylococcus aureus. J Hosp Infect 61: 277-282.
- Bossi P (2004) Epidemiologie des maladies infectieuses conference medicale 4: 555-563.
- Projan SJ (2003) Why is Big Pharma getting out of antibacterial drug discovery? Curr Opin Microbiol 6: 427-430.
- Mbwambo ZH, Moshi MJ, Masimba PJ, Kapingu MC, Nondo RS (2007) Antimicrobial activity and brine schrimp toxicity of extracts of Terminalia brownii roots and stem. BMC Complement Altern Med 7:9.
- Tintino SR, Souza CES, Guedes GMM, Costa JIV, Duarte FM, et al. (2014) Modulatory antimicrobial activity of Piper arboreum extracts. Acta Bot Croat 73: 281-289.
- Raja G, Ivvala AS, Inampudi S, Swaminathan R, Saleem B, et al. (2012) Phytochemical screening and reducing power Assay of Nut extracts of Juglans regia L. Int J Chem Life Sci 1: 1026-1032.
- Kuate TCR, Fotsing KPR, Ngoupayo J, Ndelo J, Kouamouo J, et al. (2013) Phytochemical screening, antibacterial and antifungal activity of crude aqueous and methanol extracts of stem bark of Garcinia brevipedicellata. Int J Pharm Biomed Res 4: 221-226.
- Jai BF, Ngoupayo J, Fotsing KPR, Kuate TT, Kuoamouo J, et al. (2014) Phytochemical screening and antimicrobial activity of extracts from the stem bark of Oldfieldia africana Benth & Hook (Euphorbiaceae): preliminary study. Int J Curr Res Chem Pharma Sci 1: 101-109.
- Gibbons S (2004) Anti-staphylococcal plant natural products. Nat Prod Rep 21: 263-277.
- Kassler WJ, Blanc P, Greenblattt R (1991) The use of medicinal herbs by human deficiency virus infected patients. Arch Int Med 151: 2281-2288.
- Olajuyigbe OO, Afolayan AJ (2012) Synergistic interactions of Methanolic Extract of Acacia mearnsii De Wild. With Antibiotics aginst bacteria of Clinical relevance. Int J Mol Sci 13: 8915-8932.
- Paul V, Boda M, Enow-Orock EG, Etoa FX, Bitolog P (2007) Acute and sub-acute toxicity profile of the aqueous stem bark of Enantia chlorantha Oliver (Annonaceae) in laboratory animals. Pharmacology online 1: 304-313.
- Betti JL (2002) Medicinal plants sold in Yaoundé markets, Cameroon. African Study Monographs 23: 47-64.
- Olanlokun JO, Akomolafe SF (2013) Antioxidant potential of various solvent extracts from stem bark of Enantia chlorantha. J Biomed Sci Eng 6: 877-884.
- Mimosette Mesmine KT, Christophe M, Ernestine N, Paul Vernyuy T (2015) Cytoprotective and antioxydant properties of the stem bark aqueous extract of Enantia chlorantha (Annonaceae) in rats. World J Pharm Pharma Sci 2: 84-92.
- Zintchem R, Njinkio B, Kamgang R, Fokunang C, Tsala DE, et al. ( 2013) Antioxidative properties of Mallotus oppositifolium decoction leaves extracts using in vitro models. Int J Bio Chem Sci 6: 2396-2408.
- Antibiogram Committee of the French Society of Microbiology (2014) CA-SFM.
- Spoorthi NJ, Vishwanatha T, Reena V, Divyashree BC, Aishwarya S, et al. (2011) Antibiotic synergy test: checkerboard method on multidrug resistant Pseudomonas aeruginosa. Int Res J Pharm 2: 196-198.
- Esimone CO, Okoye FBC, Nworu CS, Agubata CO (2008) In vitro interaction between caffeine and some penicillin antibiotics against Staphylococcus aureus. J Pharm Res 2: 969-974.
- Kyaw BM, Arora S, Lim CS (2012) Bactericidal antibiotic- phytochemical combinations against Methicillin resistant Staphylococcus aureus. Braz J Microbiol 23: 938-945.
- Nworu CS, Esimone CO (2006) Evaluation of three in vitro techniques in the interaction of Ampicillin and Ciprofloxacin against Staphylococcus aureus and Escherichia coli. Trop J Pharm Res 5: 605-611.
- Dawodu AO, Moses UD, Apena A, Adetoro A, Dairo JO (2014) The proximate evaluation and phytochemistry of Enantia chlorantha Stem bark in aqueous and ethanolic Extract. Middle–East J Scient Res 21: 2145-2148.
- Salman TM, Adesokan AA (2008) Sperm quality of male rats treated with aqueous extract of Enantia chlorantha stem bark. Afr J Biotech 7: 8.
- Razak FA, Sani A, Ajewole SM (2003) Effect of stem bark extracts of Enantia chlorantha on some clinical isolates. Biochem 15: 84-92.
- Bassole IH, Ouattara AS, Nebie R, Ouattara CA, Kabore ZI, et al. (2003) Chemical composition and antimicrobial activities of the essential oil of Lippia chevelieri and Lippia mutiflora from Burkina faso. Phytochemistry 62: 209-212.
- Erasto P, Bojase-Moleta G, Majinda RR (2004) Antimicrobial and antioxidant flavonoids from the root wood of Bolusanthus speciosus. Phytochemistry 65: 875-880.
- Doss A, Mubarack HM, Dhanabalan R (2009) Antibacterial activity of tannins from the leaves of Solanum tribatum Linn. Ind J Sci Tech 2: 41-43.
Citation: Ntungwen FC, Ronel TA, Estella TF, Nobert N, Rahmane NA, et al. (2017) In vitro Evaluation of the Interaction between Amoxicillin and Hydro Ethanolic Extracts of Annickia chlorantha. Toxicol Open Access 3: 133. DOI: 10.4172/2476-2067.1000133
Copyright: © 2017 Ntungwen FC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 8781
- [From(publication date): 0-2017 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 7913
- PDF downloads: 868