Research Article Open Access
In Vitro Degradation Behavior of Ternary Mg-Zn-Se and Mg-Zn-Cu Alloys as Biomaterials
Dharam Persaud-Sharma1* and Noah Budiansky2
1Department of Biomedical Engineering, Florida International University, Miami, FL, USA 33199
2Exponent Failure Analysis Associates, Natick, MA, USA 01760 Noah Budiansky2
- Corresponding Author:
- Dharam Persaud-Sharma
Department of Biomedical Engineering
Florida International University, Miami, FL, USA 33199
E-mail: Dpers001@fiu.edu
Received date June 02, 2013; Accepted date July 05, 2013; Published date July 15, 2013
Citation: Persaud-Sharma D, Budiansky N (2013) In Vitro Degradation Behavior of Ternary Mg-Zn-Se and Mg-Zn-Cu Alloys as Biomaterials. J Biomim Biomater Tissue Eng 18:101. doi: 10.4172/1662-100X.1000101
Copyright: © 2013 Persaud-Sharma D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biomimetics Biomaterials and Tissue Engineering
Abstract
In this study, the corrosion behavior of Mg-Zn-Se and Mg-Zn-Cu alloys was investigated to evaluate their corrosion behavior related to use as implantable biomaterials. The corrosion behavior of these alloys and a commercially available Mg-Zn alloy were examined using static solution electrochemical testing, dynamic solution gravimetric testing, ion leaching testing, and microscopic evaluation. Fluctuations in the pH of the Dulbecco’s Modified Eagles Medium (DMEM) used for the gravimetric and ion leaching immersion testing were also recorded over the 30-day duration to assess whether the media conditions induced by the alloy degradation would permit for cellular survival. Weight loss experimentation and electrochemical tests revealed the Mg-Zn-Cu alloy to have the greatest corrosion rate.
Keywords
Magnesium alloys; ICPMS; Corrosion; Tafel extrapolation; pH
Introduction
This study explores the corrosion behavior of novel as-cast Mg- Zn-Se and Mg-Zn-Cu alloys first presented by Persaud-Sharma et al. [1]. Magnesium alloys have been widely explored as suitable materials for biomedical implantation due to their ability to degrade in situ, demonstrate a non-toxic behavior with living tissues, and possess the physical and mechanical properties that are a required for a multitude of applications [2-6]. However, one of the main limitations of using magnesium alloys in many applications is its unfavorable degradation rate with physical and mechanical properties that change during degradation resulting in unsuitable qualities needed for specific biomedical applications [6]. The high susceptibility to corrosion can be mitigated using techniques such as surface coatings, anodizing and alloying techniques have been explored in an effort to increase the applicability of magnesium and its alloys [7]. In attempts to address the problem of corrosion and unsuitable mechanical properties of magnesium alloys that have been previously explored such as the ascast AZ31 and AZ91D alloys, novel as- cast magnesium alloys Mg-Zn- Se and Mg-Zn-Cu alloys were developed and have shown to possess enhanced mechanical properties when compared with other as-cast magnesium alloys [1].
In order to determine the corrosion rate of the magnesium alloys, it is best practice to evaluate materials using both gravimetric weight loss and electrochemistry techniques. The advantages and limitations for gravimetric and electrochemical methods complement each other, thus permitting for a more complete understanding of the corrosion behavior of a material. One of the main purposes of in-vitro testing is to simulate in situ conditions. However, conventional corrosion testing fails to simulate such realistic environments, as is the case for materials tested for endovascular medical devices. In this case, corrosion testing procedures for materials used for endovascular applications are usually performed under biological conditions of 37°C, and under static conditions, which is not representative of the dynamic fluid conditions seen in biological subjects. This fails to account for the influence of fluid kinetic contributions to the corrosion rate of the material which has been shown to alter the corrosion mechanism and corrosion rate of the material as high six times of that determined under static conditions [8]. This shortfall may grossly underestimate the corrosion performance of the biomaterial once implanted within a patient.
Additionally, testing to simulate vascular conditions fail to use an aqueous media which is truly representative of human blood and/or plasma. Through widely reported studies, it is known that the inclusion of proteins within the testing solution greatly influences the corrosion rate regardless of the electrolytic medium utilized [9-12]. However, the inorganic components included in such media do not accurately represent those found in human plasma. One such media that accurately mirrors the ionic concentrations and pH of human plasma is Kokubo’s solution developed by Kokubo et al. [13]. A comparison of chemical formulation of Kokubo’s solution, Dulbecco’s solution, and Human Plasma is shown in Table 1. Performing corrosion tests using Kokubo’s solution will provide an accurate simulation of the corrosion rate that could be expected once implanted in vivo, as it considers the influence of the various organic and inorganic ions on the corrosion rate of a metal.
| Ion | Concentration (mmol/dm3) | ||
|---|---|---|---|
| Kokubo’s Simulated Body Fluid | Human Blood Plasma | Dulbecco's Modified Eagle's Medium | |
| Na+ | 142.0 | 142.0 | 55.28 |
| K+ | 5.0 | 5.0 | 2.81 |
| Mg+2 | 1.5 | 1.5 | 0.16 |
| Ca+2 | 2.5 | 2.5 | 0.65 |
| Cl- | 147.8 | 103.0 | 68.19 |
| HCO-3 | 4.2 | 27.0 | 31.99 |
| HPO4-2 | 1.0 | 1.0 | 0.63 |
| SO4-2 | 0.5 | 0.5 | 0.65 |
See reference [13,14]
Table 1: Comparison of ion concentration for Kokubo’s solution and human blood plasma.
The experimental study evaluates the corrosion behavior of as-cast magnesium alloys Mg-Zn-Se and Mg-Zn-Cu, characterized using both gravimetric weight loss methods over a 30-day period under dynamic conditions and electrochemical potentiodynamic tests using Kokubo’s solution as an in vivo simulation solution. These results were compared with a binary Mg-Zn alloy used as a control composition. The aim was to characterize the corrosion behavior of the experimental Mg-Zn-Se and Mg-Zn-Cu alloys.
Materials and Methods
Alloy manufacturing and sample preparation
The alloy compositions were synthesized using an Arc-melting method in inert argon gas at ACI alloys, Inc. (CA, USA). Both experimental alloy compositions were composed of wt.% ratios (98/1/1) Mg-Zn-Se/Cu.
Materials were cast in a 1 in. × 1 in. × 6 in. mold. Test specimens were cut into testing dimensions of approximately 10 × 10 × 0.95 mm3. Samples were mechanically dry ground to a #1200 grit finish using silicon carbide abrasive paper. After mechanical polishing, test specimens were cleaned in acetone and rinsed with deionized water and blown dry with nitrogen gas. Test specimens were stored in a plastic bag until further testing.
Electrochemical testing procedures
To assess the corrosion properties and the susceptibility of the magnesium alloys to localized corrosion, electrochemical testing procedures were conducted using ASTM standard F2129-08 as a guide. Samples were prepared by spot welding a nickel tab to the magnesium alloy samples and soldering an electrical wire to the nickel tab to allow electrical control of the samples. The samples were mounted in metallographic epoxy with the large cross sectional area exposed to the solution. The samples were dry polished to #1200 grit finish. The electrical conductivity between the sample and the electrical wire was checked using a handheld multi- meter. The interface between the magnesium alloy and the epoxy mount were masked off to prevent liquid ingress into the interface between the epoxy and the sample. The approximate exposed surface was 5 mm × 5 mm for a total of approximately 25 mm2. The exact surface area was determined by measuring the surface area from stereo-optical microscope images and image analysis software Image J.
A Gamry potentiostat and Gamry DC 105 software was used for cyclic potentiodynamic corrosion testing. A Gamry style electrochemical cell with a saturated calomel reference electrode (SCE) and graphite counter electrode were used for testing. Kokubo solution was used for electrochemical testing with ion concentrations shown in Table 1. 1 liter of fresh test solution was used for each electrochemical test. The test cell was heated to 37°C in a heated water bath. Deaeration was performed using ultra high purity nitrogen at a flow rate of 150 cc/min. The system was deaerated for at least 30 minutes prior to testing. The open circuit potential or rest potential was monitored for approximately 15 minutes prior to cyclic polarization testing. Cyclic polarization testing was measured from -0.250 VSCE to +1VSCE with a scan rate of 1 mV/sec. The potential scan was reversed upon reaching the vertex potential (+1VSCE) or a preset current density (100 mA). Six tests were conducted for each alloy.
Electrochemical testing
The corrosion rate was calculated from cyclic potentiodynamic curves using Tafel extrapolation techniques in accordance with ASTM G-102. The Tafel regions of the curves were analyzed with tangential lines determined to start at ± 50 mV from the open circuit potential to determine the Ecorr and Icorr values. The corrosion rate (C.R.) in mm/ year, for each alloy was determined in accordance with Faraday’s law as shown in equation 1. Accordingly, K1 = 3.27 × 10-3 mm· g/μ A, icorr in μ A/cm2, ρ is the density of the alloy and is measured in g/cm3, and the equivalent weight (EW). The equivalent weight for each alloy was calculated according to equation 2. Accordingly, ‘ni’ is the valence state of the alloying element, ‘fi’ is the mass fraction of the alloying elements, and ‘Ai’ is the atomic weight of the alloying element.
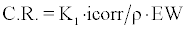 (1)
(1) 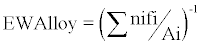 (2)
(2)
Gravimetric weight loss tests
Gravimetric weight loss testing was conducted in Dulbecco’s modified eagles medium (SH30081.01) as a simulated body fluid (SBF) under aerated conditions. Ionic concentrations of the Dulbecco SBF are shown in Table 1. 300 mL of Dulbecco’s modified eagles medium was placed in a container and heated and maintained at 37°C for 30 days. The test containers were continuously agitated at 300 rpm for 30 days using a MaxQ 4000 Barnstead labline orbital shaker to expose the materials to a shear forces normally found in physiologic conditions. Normal physiologic levels of shear stress within arteries range from 10 to 70 dynes/cm2 in vivo, whereas shear stress in veins is lower and ranges between 1 to 6 dynes/cm2 [15-17]. To achieve a nominal value within the shear stress range found in an artery that could be simulated using available lab equipment, a shear force of (τmax) of 14.5 dynes/ cm2 was exerted on the testing specimens as determined by equation 3. Accordingly, ‘a’ is the orbital radius of the shaker, ‘’ is the viscosity of the culture medium (0.0075 poise), ‘ρ’ is the density of the culture medium (1.09 g/mL), and ‘f’ is the frequency of rotation.
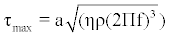 (3)
(3)
The weight of the sample was measured before and after the 30 day exposure in the SBF solution. Samples were dried with dry nitrogen gas prior to final weight loss measurements. During the duration of the 30 day SBF exposure, an aliquot of SBF test solution was collected every 24-hour for ICP-MS ion leaching analysis. The pH of the aliquot was measured immediately after collection using an Accumet Research AR50 pH meter.
Inductively coupled plasma-mass spectrometry
ICP-MS analysis was performed on the collected aliquots of Dulbecco’s solution. Prior to ICP-MS measurements, the SBF samples were acid digested using nitric acid and hydrogen peroxide at 90°C to remove all organic components from the SBF that cannot be quantified or tolerated by mass spectrometry equipment and to solubilize all the metallic ions. 1000 μL of the aliquot for each period of analysis was diluted with 9975 μL of nitric acid (0.8M Optima Grade), vortexed and sonicated for 15 minutes. Rhodium was used as the internal standard with concentrations of 50 parts per billion (ppb). The aliquot samples were measured for the detection of trace elements commonly found within magnesium alloys such as Be (4 ppm), Ni (20-50 ppm), and Fe (35-50 ppm) [18,19]. Subsequently, a further dilution was then applied to 100 μL of the previously diluted volumetric samples to attain a final dilution factor of 1:1000 to detect elements at higher concentrations such as Mg, Zn, Cu, and Se. Yttrium was added as the internal standard. Each sample was analyzed in triplicate. Deionized water (18 mohms) and optima grade nitric acid was used to prepare the calibration curve with 0, 10, 25, 50, 60, 80, and 100 ppb of the standards. Calibration was ensured by obtaining good linearity for calibration elements and isotopes (r2=0.99).
Results
Table 3 shows the corrosion rates as determined by gravimetric and electrochemical methods for the experimental magnesium alloys Mg- Zn-Cu, Mg-Zn-Se, and the binary Mg-Zn alloy used as a comparative control.
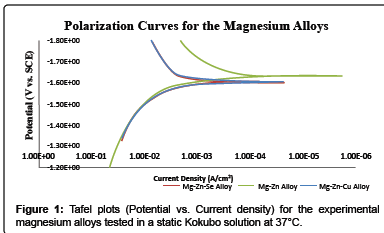
Such results shows the Mg-Zn alloy to possess the lowest corrosion rate as determined by Tafel extrapolation methods 19.5 ± 5.26 mm · yr-1. Both Mg-Zn-Se and Mg-Zn-Cu possessed very similar electrochemical corrosion rates with 44.8 ± 8.87 mm · yr-1 and 44.4 ± 14.9 mm · yr-1, respectively. Exemplary polarization curves for each alloy tested in static Kokubo’s solution at 37°C can be seen in Figure 1.
Gravimetric tests, revealed the Mg-Zn alloy to possess a corrosion rate of 2.16 mm · yr-1, while the Mg-Zn-Se alloy showed to have the lowest corrosion rate of 0.428 ± 0.19 mm · yr-1. The gravimetric weight loss values for the Mg-Zn-Cu alloy revealed to possess the highest corrosion rate with a value with a calculated gravimetric degradation rate of 46.2 mm · yr-1 which is calculated based upon complete degradation within a period of 96 hours and an average mass of 214 mg.
Optical images of the magnesium alloys before and after immersion in the Dulbecco’s SBF solution for 30 days are shown in Figure 2. Figures 2 A, C, and E show Mg-Zn, Mg-Zn-Se, and Mg-Zn- Cu before immersion, respectively. It can be seen in these figures that the surfaces are consistent with a #1200 grit surface finish. Surface defects are can be seen on the surface of all alloys, although Mg-Zn- Cu shows more pronounced pit defects (Figure 2E). After the 30 day period of immersion, both the Mg-Zn and the Mg-Zn-Se alloys show a corroded surface. Both the Mg-Zn alloy and the Mg-Zn-Se alloys show corroded surfaces with deep circular pits. However, such pits are more pronounced on the surface of the Mg-Zn alloy.
| Alloy Composition | Mg | Zn | Cu | Se |
|---|---|---|---|---|
| Mg-Zn (98/01) | 99 | 1 | - | - |
| Mg-Zn-Se (98/01/01) | 98 | 1 | - | 1 |
| Mg-Zn-Cu (98/01/01) | 98 | 1 | 1 | - |
Table 2: Weight percentage of alloy compositions (wt. %).
| Alloy Composition | Corrosion Rate Determined by Electrochemical Tests | Corrosion Rate Determined by Gravimetric Tests |
|---|---|---|
| mmyr-1 | mmyr-1 | |
| Mg-Zn | 19.5 ± 5.26* | 2.16 ± 0.855* |
| Mg-Zn-Se | 44.8 ± 8.87* | 0.428 ± 0.19* |
| Mg-Zn-Cu | 44.4 ± 14.9* | 46.2 ± 19.1* |
* Data represented as mean ± standard deviation
Table 3: Corrosion rate for magnesium alloys determined by electrochemical and gravimetric tests.
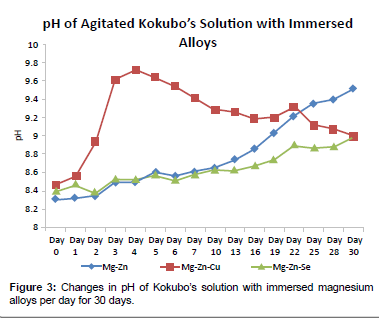
The pH fluctuations of the SBF containing the magnesium alloys are shown in Figure 3. Both the Mg-Zn-Se and the Mg-Zn alloy follow an increase in pH toward more basic values over the 30 day exposure period, although Mg-Zn solution showed to be 10x greater or 1 pH unit greater than the Mg-Zn-Se alloy after 30 days (Figure 3). The pH of the SBF containing the Mg-Zn-Cu alloy showed a rapid increase in alkalinity immediately after immersion until day 4. Afterwards, the pH increased in acidity towards a more neutral pH of 7.5 for the remaining 30 day duration. The differences in pH values of the leachate collected from the 30-day period of alloy immersion, reached statistical difference with a p-value of 0.0001, when evaluated with an alpha value of 0.05 using a one-way ANOVA test.
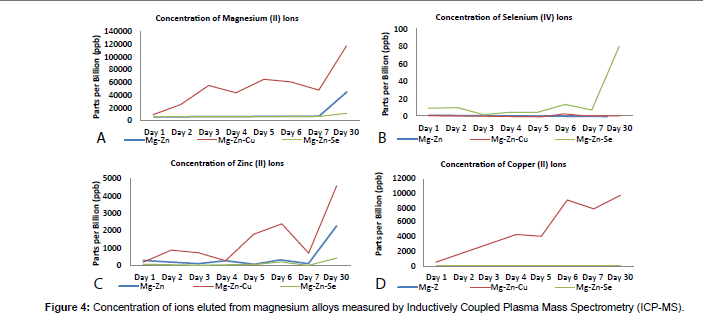
ICP-MS analysis was performed on aliquot samples collected from SBF solutions with the immersed alloys for every 24 hour period for the first 7 days of the 30 day experimental duration, and every 72 hours thereafter. This was performed to analyze the immediate elemental ion release from the alloys following immersion in a SBF. Results from these analyses can be seen in Figure 4.
The concentration of Mg+2 ions released from the magnesium alloys during the 30 day experimental period is shown in Figure 4A. Analysis of the gravimetric data for the Mg-Zn-Cu alloy released the highest concentration of the Mg+2 ions as compared to the Mg-Zn and the Mg-Zn-Se alloys with a final Mg+2 concentration reaching 100 ppm. This continual increase in Mg+2 ion concentrations occurred throughout the 30-day period of analysis despite the Mg-Zn-Cu alloy degrading within 4 days. The Mg-Zn and Mg-Zn-Se alloys showed lower concentrations of Mg+2 ions 45 ppm and 9 ppm, respectively. Figures 4B shows that the Mg-Zn-Se alloy released the greatest concentration of the Se+4 ions with slight increases in concentrations per day over the 30 day period. Figure 4C shows the release of Zn+2 ions from all magnesium alloys. It can be seen that the Mg-Zn-Cu alloy showed the highest release of the Zn+2 ions, which is expected as this alloy completed corroded during the first X days of immersion. Similarly to the release of Mg+2 ions from the alloys (Figure 4A), the Mg-Zn-Cu alloy showed to release a higher concentration of Zn+2 ions as compared to the Mg-Zn-Se and Mg-Zn alloy as seen in Figure 4C. The amount of Cu+2 ions released was nearly undetectable for the Mg-Zn and the Mg-Zn-Se alloy. The Mg- Zn- Cu alloy showed to have substantially greater releases of Cu+2 ions over the 30 day period. However, it was not expected that the copper concentration would continue to increase after the Mg-Zn-Cu alloy completely degraded after 4 days (Figure 4D). Of the common trace elements which are commonly detectable in magnesium alloys and are considered impurities (Be, Ni, Fe), only Fe+2 ions were detectable by mass spectrometry methods. Interestingly, the Mg-Zn-Cu alloy showed to release the greatest concentration of Fe+2 ions over the 30 day period with concentration reaching 1 ppm (1000 ppb), while the Mg-Zn-Se alloy reached Fe+2 concentrations of 0.4 ppm (400 ppb), while the Mg- Zn alloy reached concentrations of 0.2 ppm (200 ppb) at 30 days.
Discussion
There exists a significant disparity in the corrosion rate of the alloys between the gravimetric and electrochemical methods. The main contributing factors of differences between the corrosion rates measured likely originate from the difference stirring rate (300 RPM orbital versus no stirring). In addition, the electrolyte and aeration conditions also contribute to the corrosion rate. According to Zhang, two other contributing factors are 1) time effects (30 day exposure versus approximately 1 hour of exposure) and 2) regions of analysis selected on the Tafel plots made during the determination of Icor values [20]. Stern and Weisert, have shown that without precise knowledge of the Tafel slopes, corrosion rates can be estimated within a 50% error [21,22]. Thus, such an phenomenon cannot explain the percent differences of 170% and 196% for the Mg-Zn and Mg-Zn-Se alloys, respectively. A more likely explanation for the large differences between the gravimetric corrosion rates and the electrochemical corrosion rates for the magnesium alloys is due to the differences in the experimental parameters used. Primarily, gravimetric testing used Dulbecco’s solution as a SBF, whereas electrochemical testing used Kokubo’s solution as a SBF. Comparatively, the ion concentrations between the elements of the Kokubo’s solution and the Dulbecco’s solution are very different. The higher ionic salt concentrations found in the Kokubo’s solution could attribute to the more aggressive corrosion rates seen in the electrochemical testing results, because the electrolyte media provides for higher rates of oxidation of the testing specimen. Additionally, the effects of the vitamins and organic compounds found within Dulbecco’s media on metal are not well understood.
Another parameter that was not adequately controlled was the effects of stirring in the gravimetric tests and the static media conditions used in the electrochemical testing. Recognizing that conventional corrosion tests are not a true representation of in situ condition, gravimetric tests within this study were conducted under fluid conditions with a simulated wall shear stress of 14.5 dynes/cm2. Li et al. [8] conclude that magnesium alloys under stirring conditions exhibited the lowest corrosion rate under, while the flowing conditions promote local corrosion with rates three to six times faster [8]. However, the two sets of data cannot truly be compared as the gravimetric tests were performed under dynamic conditions, whereas the electrochemical tests were performed under static conditions. This phenomenon would partially explain the lower corrosion rates seen for the Mg-Zn and Mg- Zn-Se alloys when determined by gravimetric methods, as compared to electrochemically determined corrosion rates (Table 3). More so, the electrochemical corrosion rates determined for Mg-Zn (19.5 mm · yr-1), Mg-Zn-Se (44.8 mm · yr-1), and Mg-Zn-Cu (44.4 mm · yr-1) are higher than pure Mg (0.09652 mm · yr-1), AZ31 (0.05842 mm · yr-1), and AZ91D (0.11938 mm · yr-1) alloys reported by Xue et al. [2]. However, these results fail in comparison because both testing conditions were different between the two experiments as Xue et al., used a Phosphate buffer solution, and an exposed sample area of 20 mm2; whereas, the study presented herein, used a Kokubo’s solution with a greater salt concentration and a exposed surface area of 25 mm2.
Another important parameter that was not accounted for during experimentation was the mimicry of the partial pressure of oxygen (pO2) and partial pressure of carbon dioxide (pCO2) concentrations in-vitro during the gravimetric tests to those that are normally found within the human body. Such parameters can greatly influence the corrosion rate of a metal. Bundy, highlights that sometimes an implant surface can be in contact with areas of different pO2, which would create the possibility for different aeration cells to develop [9]. This increased amount of oxygen species would lead to the formation of an oxide layer on the surface of the alloy, thus reducing the effects of corrosion. Whereas, a deaerated solution as was used for electrochemical testing, would inhibit an oxide layer from developing, leading to a more aggressively observed corrosion behavior. Likewise, pCO2 also influences the rate of corrosion because of its effects on the pH of the solution [9].
ICP-MS analysis on the samples revealed unexpected results for the solutions containing the Mg-Zn-Cu alloy. It’s observed in such solutions, that the concentrations of Cu, Mg, and Zn, continue to increase for the 30 day immersion period in SBF beyond the point of complete degradation for the alloy. The Mg-Zn-Cu alloy was seen to degrade completely around day 4 of the period, which is consistent with the pH trend which increases to day 4 and then migrates back to neutrality for the remaining experimental period. The ICP-MS results that show the Mg, Zn, and Cu concentrations increasing beyond day 4 can be explained by a transitional state of the alloy degrading into a precipitate or residue in the SBF that continues to exchange ions within solution with other elemental species to continuously release ions. The increased ion concentration is what is detected by the ICP-MS analysis as the 30 day period progresses.
Additionally, ICP-MS detected Fe+2 ions in solutions obtained from all the magnesium alloys. This shows that Fe+2 is a common impurity that is difficult to be removed during the alloy forming process. The ionic concentration of the Fe+2 ions seen in the magnesium alloys (Mg- Zn- Cu > Mg-Zn-Se > Mg-Zn), correlate to their electrochemically determined corrosion rate. Thus, it is possible that even in minute quantities, Fe+2 does have an influential effect on the corrosion rate of magnesium alloys.
Future experimentation would improve upon the data obtained from experimentation presented in this manuscript mainly through the use of more representative bio-corrosion studies of the human body for biomaterial testing. Such improvements would include performing both electrochemical and gravimetric techniques using aerated SBF solutions, as well as varying the types of electrolyte solutions used for corrosion to include water, Kokubo’s solution, and a conventionally used SBF. More so, a parallel set of experiments should be conducted which explores the same parameters listed above, under varying degrees of media conditions such as stirring, static and flow conditions.
Conclusions
In this work, the corrosion behavior of Mg-Zn-Se and Mg-Zn-Cu alloys was investigated to assess the potential of these alloys to serve as implantable biomaterials. Designing experimental magnesium alloys to include 1 wt.% of Cu and Se, allow for the gross effects of the materials to be determined through biocompatibility testing and corrosion analysis. In this paper, the corrosion behavior of these experimental alloys was shown to be more aggressively corroding than pure Mg, and other as-cast AZ31 and AZ91D alloys when determined by electrochemical testing. However, a comparison between such results is not accurate, because of varying testing parameters for the experiments that need to be standardized to emulate bio- corrosion environments (aeration vs. deaeration, SBF compositions, stirring vs. static). Independent electrochemical results show that the Mg-Zn-Se and Mg-Zn-Cu have an aggressive initial corrosion rate. While, gravimetric tests show the Mg-Zn-Se alloy to be corrosion resistant. In both tests, Mg-Zn-Cu showed to possess the highest corrosion rate.
Acknowledgement
This project was developed by Dharam Persaud (PI), who is also a recipient of a grant from the National Brain Aneurysm Foundation which financially supports this project. Dharam Persaud is also supported by NIH Fellowship number NIH/ NIGMS R25 GM061347. D.P. would like to extend his gratitude to Noah Budiansky for his collaboration in obtaining results for this study.
References
- Persaud-Sharma D, Budiansky N, McGoron AJ (2013) Mechanical Properties and Tensile Failure Analysis of Novel Bio-absorbable Mg-Zn-Cu and Mg-Zn-Se Alloys for Endovascular Applications. Metals (Basel) 3: 23-40.
- Xue D, Yun Y, Tan Z, Dong Z, Schultz M (2012) In Vivo and In Vitro degradation behavior of magnesium alloys as biomaterials. J Mater Sci Tech 3: 251-267.
- Song G, Atrens A (2003) Understanding magnesium corrosion-a framework for improved alloy performance. Advanced Engineering Materials 5: 837- 858.
- Zeng RC, Dietzel W, Witte F, Hort N, Blawert C (2008) Progress and challenges of magnesium alloys as biomaterials. Advanced Engineering Materials 10: B3-B14.
- Staiger MP, Pietak AM, Huadmai J, Dias G (2006) Magnesium and its alloys as orthopedic biomaterials: a review. Biomaterials 27: 1728-1734.
- Persaud-Sharma D, McGoron A (2012) Biodegradable Magnesium Alloys: A Review of Material Development and Applications. J Biomim Biomater Tissue Eng 12: 25-39.
- Poinern GE, Brundavanam S, Fawcett D (2012) Biomedical magnesium alloys: A review of material properties, surface modifications, and potential as a biodegradable orthopedic implant. American Journal of Biomedical Engineering 2: 218-240.
- Li N, Guo C, Wu YH, Zheng YF, Ruan LQ (2012) Comparative study on corrosion behavior of pure Mg and WE43 alloy in static, stirring and flowing Hank’s solution. Corrosion Engineering Science and Technology 47: 346-351.
- Bundy KJ (2005) Corrosion testing in invivo environments. In: Baboian R (Ed.), Corrosion tests and standards: application and interpretation (pp. 500-508). USA: ASTM international.
- Williams RL, Brown SA, Merritt K (1988) Electrochemical studies on the influence of proteins on the corrosion of implant alloys. Biomaterials 9: 181-186
- Gu XN, Zheng YF, Chen LJ (2009) Influence of artificial biological fluid composition on the biocorrosion of potential orthopedic Mg-Ca, AZ31, AZ91 alloys. Biomed Mater 4: 065011.
- Sun D, Wharton JA, Wood RK (2008) Effects of proteins and pH on tribocorrosion performance of cast CoCrMo- a combined electrochemical and tribological study. Tribology 2: 150-160.
- Kokubo T, Kushitani H, Sakka S, Kitsugi T, Yamamuro T (1990) Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W. J Biomed Mater Res 24: 721-734.
- Dulbecco’s modified eagle’s medium liquid formulation: Hyclone Thermo Scientific (SH30081).
- Paszkowiak JJ, Dardik A (2003) Arterial wall shear stress: observations from the bench to the bedside. Vasc Endovascular Surg 37: 47-57.
- Lipowsky HH (1995) Shear stress in the circulation. American Physiological Society. In: Bevan JA (Ed.), Flow Dependent Regulation of Vascular Function, Oxford: University Press 28-45.
- Malek AM, Alper SL, Izumo S (1999) Hemodynamic shear stress and its role in atherosclerosis. JAMA 282: 2035-2042.
- Persaud-Sharma D, McGoron A (2012) Biodegradable Magnesium Alloys: A Review of Material Development and Applications. J Biomim Biomater Tissue Eng 12: 25-39.
- Witte F, Hort N, Vogt C, Cohen S, Kainer K, et al. (2008) Degradable Biomaterials Based on Magnesium. Curr Opin Solid State Mater Sci 12: 63-72.
- Zhang XG (1996) Corrosion and electrochemistry of Zinc, Plenum Press NY: USA.
- Stern M, Weisert ED (1959) Experimental observations on the relation between polarization resistance and corrosion rate. Proceedings of the American Society for Testing and Materials 59: 1280-1291.
- Pardo A, Feliu S, Merino MC, Arrabal R, Matykina E (2010) Electrochemical estimation of the corrosion rate of Magnesium/Aluminum alloys. International Journal of Corrosion 1-8.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 20422
- [From(publication date):
July-2013 - Apr 19, 2025] - Breakdown by view type
- HTML page views : 15705
- PDF downloads : 4717