Research Article Open Access
In vitro Aseptic Culture Establishment of Sugarcane (Saccharum officinarum L.) Varieties Using Shoot Tip Explants
| Belay Tolera1*, Mulugeta Diro2 and Derbew Belew3 | ||
| 1 Ethiopian Sugar Corporation, Research and Training Division, Variety Development Directorate, Biotechnology Research Team, Wonji Research Center, P.O Box 15, Wonji, Ethiopia | ||
| 2 Capacity Building for Scaling up of Evidence-based Best Practices in Agricultural Production in Ethiopia (CASCAPE) Project, Addis Ababa, Ethiopia | ||
| 3 Jimma University, College of Agriculture and Veterinary Medicine, Jimma, Ethiopia | ||
| Corresponding Author : | Belay Tolera Ethiopian Sugar Corporation Research and Training Division Variety Development Directorate Biotechnology Research Team Wonji Research Center, Wonji, Ethiopia Tel: +251910181644 E-mail: belaytolera@yahoo.com |
|
| Received March 25, 2014; Accepted April 25, 2014; Published April 27, 2014 | ||
| Citation: Belay Tolera, Mulugeta Diro and Derbew Belew (2014) In vitro Aseptic Culture Establishment of Sugarcane (Saccharum officinarum L.) Varieties Using Shoot Tip Explants . Adv Crop Sci Tech 2:128. doi: 10.4172/2329-8863.1000128 | ||
| Copyright: © 2014 Tolera B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Advances in Crop Science and Technology
Abstract
Two sugarcane varieties namely, ‘B41-227’ and ‘N14’ were tested for in vitro culture establishment response
under five levels of BAP (0, 1, 2, 3 and 4 mgL-1) and kinetin (0, 0.5, 1, 1.5 and 2 mgL-1) in a completely randomized design with 2*5*5 factorial treatment combinations. The objective was to determine the optimum concentration and combination of BAP and kinetin for in vitro culture establishment of shoot tip explants. Among the different concentrations and combinations of BAP and kinetin tested, Murashige and Skoog (MS) medium supplemented with 3 mgL-1 BAP without kinetin for B41-227; and 3 mgL-1 BAP along with 1.5 mgL-l kinetin for N14 exhibited a better response than the other concentrations in percent initiated shoot tip explants, percent of dead or not responding cultures, number of shoots per explant and average shoot length of the sugarcane varieties. These media established 93.33% of shoot tip explants with 4.5 ± 0.11 shoots per explant and 3.15 ± 0.28 cm shoot length in B41-227; and initiated 76.67% shoot tip explants with 3.3 ± 00 shoots per explant and 4.58 ± 0.28 cm shoot length in N14 after 30 days of culture.
| Abstract |
| Two sugarcane varieties namely, ‘B41-227’ and ‘N14’ were tested for in vitro culture establishment response under five levels of BAP (0, 1, 2, 3 and 4 mgL-1) and kinetin (0, 0.5, 1, 1.5 and 2 mgL-1) in a completely randomized design with 2*5*5 factorial treatment combinations. The objective was to determine the optimum concentration and combination of BAP and kinetin for in vitro culture establishment of shoot tip explants. Among the different concentrations and combinations of BAP and kinetin tested, Murashige and Skoog (MS) medium supplemented with 3 mgL-1 BAP without kinetin for B41-227; and 3 mgL-1 BAP along with 1.5 mgL-l kinetin for N14 exhibited a better response than the other concentrations in percent initiated shoot tip explants, percent of dead or not responding cultures, number of shoots per explant and average shoot length of the sugarcane varieties. These media established 93.33% of shoot tip explants with 4.5 ± 0.11 shoots per explant and 3.15 ± 0.28 cm shoot length in B41-227; and initiated 76.67% shoot tip explants with 3.3 ± 00 shoots per explant and 4.58 ± 0.28 cm shoot length in N14 after 30 days of culture. |
| Keywords |
| Conventional propagation; In vitro shoot tip explant establishment; Sugarcane; BAP and kinetin |
| Introduction |
| Sugarcane (Saccharum officinarum L.), the major source of sugar and alcohol, is a crop of prime importance owing to its high agro-economic [1] and global importance as a high valued multipurpose agro-industrial cash crop. Thus, much research has been focused on Sugarcane crop improvement through conventional breeding and recently through biotechnological approaches [2-4]. The conventional breeding method takes 10 to 15 years of work to complete a selection cycle [5-7] and once a desired variety is developed or clone is identified, it usually takes six to seven years to produce sufficient quantity quality planting materials via conventional propagation method [8]. As varieties of sugarcane are highly heterogeneous, multiplication for commercial purpose is usually carried out vegetatively by stem cuttings [9]. In the conventional propagation method where nodal sections with two or three nodes or sets are used as planting material have a wide-ranging of limitations. |
| The seed multiplication rate is very slow, usually 1:10 [6,12] which makes the spread of a newly released variety too slow to scale up to commercial level [5-6,10-11] and can result in degeneration before commercialization[12]. Additionally, sugarcane stocks can be infected by various pathogens without exhibiting any symptoms [13] and may result in epidemics. Moreover, the conventional propagation method in sugarcane requires large quantity of seed (1.2-1.5 tonnes/ha) and land demanding (seedcane demands 10% of the next planting plan). Besides the costly transport of the bulky cane cuttings, harbor many pests and diseases with accumulation of disease over vegetative cycles leading to further yield and quality decline over the years [6,12] and hence increase cost of production. Generally, the conventional method of sugarcane planting material propagation is wasteful in terms of time and money. |
| In spite of its various limitations, the conventional propagation method is exclusively used for propagation of sugarcane planting materials in the Ethiopian Sugar Estates since the establishment of the first sugar industry in 1954. Regardless of the huge production potentials and market opportunities, the current sugar production of the sugar industry in Ethiopia covers only 60% of the annual demand for domestic consumption while the remaining 40% is imported from abroad. To reverse the current situation, the Ethiopian Sugar Corporation is undertaking massive development endeavors at nine sugar estates (six new development and 3 expansion projects) located in different regions of the country. However, availability of adequate quantity quality and disease free planting materials of selected sugarcane varieties within a short time is the major limitation to realize the intended plan. On the other hand, the yield of the existing few and old commercial cane varieties is declining alarmingly and some productive varieties were also obsolete due to lack of alternative technologies for disease cleansing and rejuvenation. Moreover, due to shortage of sufficient quantity planting material, commercialization of introduced and adapted improved varieties of sugarcane takes several years using the conventional route of planting material multiplication. |
| Nowadays, unlike the conventional method, micropropagation technique is the only realistic and viable alternative for achieving rapid and large scale production of disease free quality sugarcane planting materials to facilitate the commercialization process [14-16] and improves the quality and productivity of sugarcane [17-23]. Furthermore, in contrary to the four to five shoots per bud in a year in the conventional propagation method [24], micropropagation in sugarcane exhibited a potential production of 76,500 shoot from a single shoot apex in about 5.5 months [25] which is sufficient to plant 5.5 ha. Moreover, a small laboratory area of 20 m2 is sufficient to propagate up to 500,000 plantlets, save space, planting materials, and the planting materials area easy to handle and transport [26] and the rate of propagation is 1:22-25 in 8-10 months [27]. Similarly, micropropagation using shoot tip or apical meristem culture has been widely used to produce virus-free plants [13, 28] with rejuvenation and mass production of true to type and uniform planting materials from old diseased sugarcane plants [20,29]. Moreover, tissue culture raised sugarcane plants were reported to give superior in cane and sugar yield as compared to their donors from conventional seed source under similar climate and management practices [17-23]. To solve the multitude problems of the conventional propagation method facing the sugar industry and utilize the advantage of micropropagation technology, the corporation is establishing its own sugarcane micropropagation facilitates with estimated annual production capacity of 45 million plantlets for which efficient and rapid starter culture initiation protocols is a prerequisite that will help utilize the full capacity of the facilities within the shortest possible time. In line with this, many researchers have developed in vitro propagation protocols for sugarcane. But, every genotype needs specific protocol for rapid and efficient propagation [30-32]. In addition, the universal application of the protocols was limited by various factors like genotype [30-31] and explants type [31,33]. Position of explant on the stock plant, age, endogenous levels of plant growth regulators, time of the year and physical growth factors [10,33] and explant size [34]. One of the major obstacles is genotype to media or plant growth regulators interactions [8]. In addition, there is no report on tissue culture study of sugarcane varieties grown in Ethiopian Sugar estates. Therefore, this study was carried out with the objective of evaluating the response of the two sugarcane varieties (B4-227 and N14) to different levels BAP and kinetin on in vitro aseptic culture establishment using shoot tip explants. |
| Materials and Methods |
| The stem cuttings (setts) of the two sugarcane varieties namely N14 and B41-227, were obtained from Metahara and Wonji sugar estate seedcane nurseries and treated with hot water at 50oC for 2 hours to use as source of explants for the study. The varieties were selected as they are well adapted and have high cane and sugar yield and are among a few very productive ones. The sets of these varieties were planted in the greenhouse of Jimma University College of Agriculture and Veterinary Medicine (JUCAVM) where the study was carried out. Then, actively growing shoot tips with apical meristem were collected from three months old stock plants and used as source of shoot tip explants. Full strength Murashige and Skoog (1962), (MS) medium along with different concentration and combinations of BAP and kinetin was used for initiation or establishment of the shoot tip explants of the sugarcane varieties. The MS medium contained 30 g/l sucrose (table sugar) as a carbon source and pH of the media was adjusted to 5.8 using before gelled with 8 g/l agar and autoclaved at 121oC, 15 psi for 20 minutes while molten medium of 40 ml was dispensed per each glass culture jar having 6 cm diameter and 9 cm height. Explant preparation and surface sterilization method were adopted from [12] and [35] with some modification. Shoot tops were cut from stock plants at the base with some nodes, the leaves were trimmed and taken to the laboratory for surface sterilization and explant preparation. Trimmed shoot tops were washed thoroughly under running tap water, outer leaf sheath removed and cut in to about 10 cm length. Thereafter, the shoot tips were further washed three times each for 15 minutes with tap water containing liquid soap solution (Top) and three drops of Tween-20. This step was done twice. |
| Then, explants taken to laminar airflow chamber, immersed in 0.3% (w/v) Kocide solution for 30 minutes followed by three times washing each for five minutes with sterile distilled water. The shoot tops were again, rinsed in 70% alcohol for one minute and washed with sterile distilled water three times each for five minutes. Finally, the explants were treated with 10% (v/v) sodium hypochlorite solution (4% active chlorine) for 20 minutes. After discarding the sodium hypochlorite solution, the explants were washed with sterile distilled water three times each for five minutes and the surface sterilized explants were excised and sized to 1 cm long and 0.5 cm diameter and cultured on initiation medium. The experiment was carried out at a temperature of 25 ± 2oC under 16-hours light and eight hours dark photoperiod regimes maintained under fluorescent light having 2500 µmol m-2S-1 light intensity with 75-80% relative humidity of the incubation chamber. The experiment was laid out in a completely randomized design (CRD) with a three factor factorial treatment combinations arrangements forming 2*5*5 factorial (2 varieties with five levels of BAP and kinetin each). |
| Each treatment replicated three times with 15 explants per treatment combination. Collection of data on percent initiation or establishment of shoot tip cultures, percent dead or not responding cultures, number of shoots per explant and average shoot length (cm) were collected after 30 days. Then, data were subjected to analysis of variance (ANOVA) using SAS statistical software (version 9.2) and treatments’ means were separated using the procedure of REGWQ (Ryan-Einot-Gabriel-Welsch Multiple Range Test). |
| Results and Discussion |
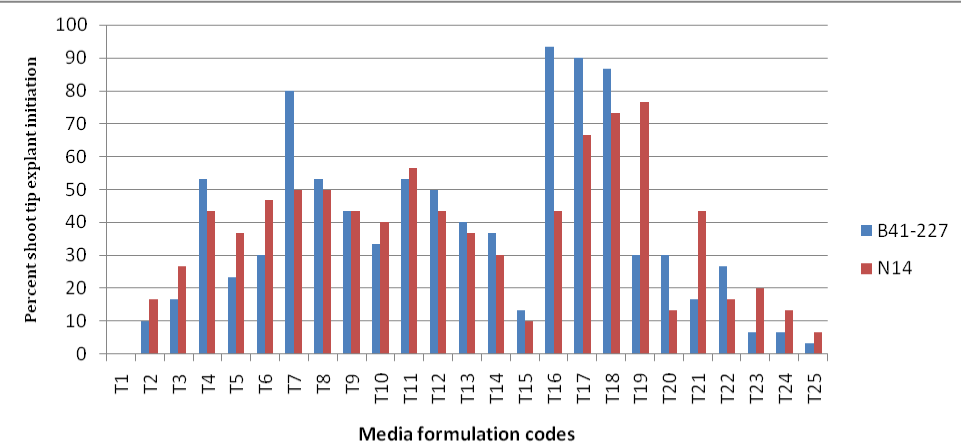
| Analysis of variance (ANOVA) revealed that the effects of sugarcane varieties (genotype), BAP and kinetin was very highly significant (Genotype * BAP * kinetin=P<0.001) on percent shoot tip culture initiation, number of shoots per explant and average shoot length of the sugarcane varieties. The two sugarcane varieties also showed significant variation in all the responses tested: percent shoot initiation, number of shoots per explant and average shoot length. The results confirmed that shoot tip culture initiation or establishment responses in the sugarcane varieties was dependent on the interaction effect of sugarcane varieties (genotype), BAP and kinetin. There were no culture initiation responses within 30 days of culture on initiation medium lacking the plant growth regulators BAP and kinetin in both varieties of sugarcane (Figure 1). Among the different concentrations and combinations of BAP and kinetin tested, sugarcane variety B41-227 gave the highest (93.33%) shoot initiation responses on MS medium containing 3 mgL-1 BAP without kinetin (Figures 1 and 4) while N14 gave only 43.33% on this medium composition (Figure 1). N14 gave the highest (76.67%) shoot initiation on MS medium supplemented with 3 mgL-1 BAP with 1.5 mgL-1 Kinetin (Figures 1 and 4) while B41-227 gave only 30% shoot initiation of shoot tip explants on the same medium composition (Figure 1). |
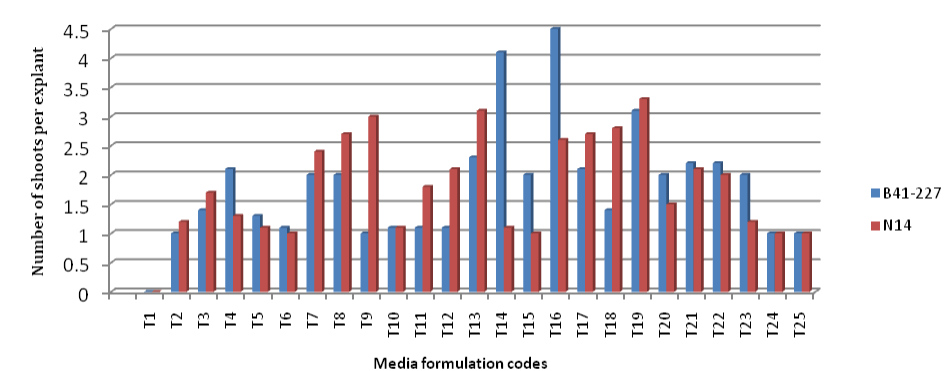
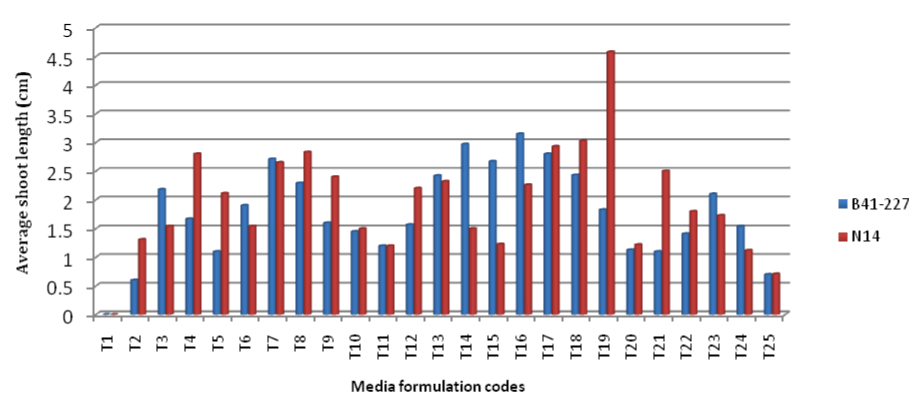
| B41-227 gave the largest shoot multiplication responses (4.5 ± 0.11 shoots per explant) on MS medium supplemented with BAP (3 mgL-1) alone (Figures 2 and 4), which is significantly better than that of N14 (2.6 ± 0.03 shoots per explant). N14 produced relatively larger number of shoots (3.3 ± 0.00 shoots per explant) when MS medium was supplemented with 3 mgL-1 BAP along with 1.5 mgL-1 kinetin (Figures 2 and 4). The two varieties respond differently to same media composition. The difference in the responses of the genotypes might be accounted by the differences in levels of naturally occurring endogenous plant growth regulators and their interaction with exogenously applied plant growth regulators or differences in the metabolic capabilities of the cells [33]. The difference in the concentration of receptor molecules (protein) to plant growth regulators of target tissue of the genotypes or varieties also determines the response potential [35]. It is also reported that the nutritional requirement for each sugarcane genotype is different and exact [37]. In addition, B41-227 has better sett germination and produces larger number of live buds and hence more shoots per stalk than N14 under the conventional propagation and showed the same tendency within in the in vitro condition. Maintaining the concentration of BAP at 0 mgL-1 and increase in the concentration of kinetin from 0 to 0.5 mgL-1 significantly increased the percent initiation of shoot tip cultures from 0 to 10% in B41-227 and from 0 to 16.67% in N14 (Figure 1). There is also a significant increase in the average number of shoots per explant (from 0.00 ± 0.00 to 1.0 ± 0.17); and average shoot length (from 0.00 ± 0.00 to 0.6 ± 0.29) in B41-227 for increased concentration of kinetin from 0 to 0.5 mgL-1 while BAP kept at 0 mgL-1 (Figures 3 and 4). |
| Remark: See Table 1 below for description of media formulation codes (T1-T25). |
| Similarly, increase in kinetin concentration from 0 to 0.5 mgL-1 while withholding BAP at 0 mgL-1, showed a considerable increase in the number of shoots per explant (from 0.00 ± 0.00 to 1.20 ± 0.02) and average shoot length (from 0.00 ± 0.00 to 1.31 ± 0.29) in N14 just because of 0.5 mgL-1 kinetin. Keeping the concentration of BAP at 3 mgL-1 and increase in the concentration of kinetin from 0 to 0.5 mgL-1, significantly decreased the percent initiated shoot tip cultures (from 93.33% to 90%), number of shoots per explant (from 4.5 ± 0.11 to 3.11 ± 0.17) and average shoot length(from 3.15 ± 0.28 to 2.80 ± 0.11 cm) in B41-227. Similarly, maintaining BAP concentration at 3 mgL-1 while increasing the concentration of kinetin from 1.5 to 2 mgL-1 significantly reduced the percent initiated shoot tip cultures (from 76.67 to 13.33%), number of shoots per explants ( from 3.3 ± 0.00 to 1.5 ± 0.00) and average shoot length (from 4.58 ± 0.28 to 1.22 ± 1.15 cm) in N14. This might be related to the fact that higher levels of cytokinin inhibit cell division and hence organogenesis [33]. The current results in B41-227 at 3 mgL-1 BAP without kinetin were in contrast with the findings of [6] who reported the highest (72%) shoot initiation on MS medium containing 2 mgL-1 BAP in sugarcane variety CoC-671 after 28 days. It was reported that 80% shoot initiation with 1.3 shoots per explant and 1.5 cm average shoot length on MS medium supplemented with 2.5 mgL-1 BAP in sugarcane variety CP-77-400 after 12 days [5]. The results obtained in N14 at 3 mgL-1 BAP and 1.5 mgL-1 kinetin were different from the findings of [38] who reported 4.25 to 5.75 shoots per explant within 10 days of culture on MS medium containing BAP and kinetin each at 1.5 mgL-1. |
| Conclusion |
| From these results, it can be concluded that, MS medium containing 3 mgL-1 6 -benzylaminopurine (BAP) alone for B41-227 and 3 mgL-1 6 - benzylaminopurine (BAP) along with 1.5 mgL-l kinetin for N14 could give optimum responses for in vitro aseptic culture establishment or initiation of shoot tip explants of the two sugarcane varieties. |
| Acknowledgements |
| We would like to express our gratefulness to Ethiopian Sugar Corporation for financing the study and Jimma University College of Agriculture and Veterinary Medicine for provision of Tissue Culture Laboratory and facilities. |
| References |
References
|
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
 |
 |
|||
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Agricultural science
- Agronomy
- Climate impact on crops
- Crop Productivity
- Crop Sciences
- Crop Technology
- Field Crops Research
- Hybrid Seed Technology
- Irrigation Technology
- Organic Cover Crops
- Organic Crops
- Pest Management
- Plant Genetics
- Plant Breeding
- Plant Nutrition
- Seed Production
- Seed Science and Technology
- Soil Fertility
- Weed Control
Recommended Journals
Article Tools
Article Usage
- Total views: 16200
- [From(publication date):
August-2014 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 11317
- PDF downloads : 4883
