Research Article Open Access
In Vitro Antibacterial Effects of Crateva adansonii, Vernonia amygdalina and Sesamum radiatum Used for the Treatment of Infectious Diarrhoeas in Benin
Agbankpe AJ1 , Dougnon TV1*, Bankole SH1, Houngbegnon O1 , Dah-Nouvlessounon D2 and Baba-Moussa L2
1Laboratoire de Recherche en Biologie Appliquée (LARBA), Ecole Polytechnique d’Abomey-Calavi (EPAC), Université d’Abomey-Calavi (UAC), 01 BP 2009 Cotonou, Bénin
2Laboratoire de Biologie et de Typage Moléculaire en Microbiologie, Faculté des Sciences et Techniques/Université d’Abomey-Calavi, 05 BP 1604 Cotonou, Bénin
- *Corresponding Author:
- Dougnon T Victorien
Laboratoire de Recherche en Biologie Appliquée (LARBA)
Ecole Polytechnique d’Abomey-Calavi (EPAC)
Université d’Abomey-Calavi (UAC)
01 BP 2009 Cotonou, Bénin
Tel: 00 22997736446
E-mail: victorien88@hotmail.com
Received date: March 20, 2016; Accepted date: May 30, 2016; Published date: June 02, 2016
Citation: Agbankpe A, Dougnon TV, Bankole SH, Houngbegnon O, Dah- Nouvlessounon D, et al. (2016) In Vitro Antibacterial Effects of Crateva adansonii, Vernonia amygdalina and Sesamum radiatum Used for the Treatment of Infectious Diarrhoeas in Benin. J Infect Dis Ther 4:281. doi:10.4172/2332-0877.1000281
Copyright: © 2016 Agbankpe A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Infectious Diseases & Therapy
Abstract
Diarrheal and infectious diseases are the leading causes of morbidity and mortality worldwide. With the emergence of multidrug resistant bacteria, the treatment of these diseases is problematic. This situation stresses the need to search for alternative antibacterial sources notably medicinal plants. The present study aimed to assess the antibacterial activity of three leafy vegetables commonly used to treat diarrheal diseases. Therefore, aqueous and hydro-ethanolic extracts of the leaves of Crateva adansonii, Vernonia amygdalina and Sesamum radiatum were prepared and tested against 12 clinical isolates and 4 reference strains. The antibacterial activities were measured using a microdilution method to determine the Minimal Inhibitory Concentration, Minimal Bactericidal Concentration and the antibiotic power. Susceptibility tests of the extracts were carried out using well diffusion method.
The hydro-ethanolic extracts of the leaves of S. radiatum and C. adansonii and the aqueous extract of S. radiatum had an effective antibacterial effect on the clinical and reference strains isolates. This was supported by Minimal Inhibitory Concentration values ranging between 0.3125 and 5 mg/ml, Minimal Bactericidal Concentration between 0.3125 and 10 mg/ml, a bactericidal power on S. aureus ATCC 25923, Pseudomonas mirabilis A 24974 (reference strains); Staphylococcus aureus, Vibrio cholera and Salmonella Typhi (clinical isolates). For the active extracts, the inhibition zone diameters were significantly different (p<0.05) and greater than 9 mm. Extracts of the leaves of S. radiatum showed the best antibacterial effects on the clinical and reference strains isolates, although reference strains and most of the clinical isolates still more sensitive to antibiotics.
Keywords
Antibacterial activity; Well diffusion; S. radiatum; C. adansonii; V. amygdalina
Introduction
In developing countries, infectious diarrhoeas represent a serious public health challenge because of their frequency and gravity. They are responsible for over 17 million deaths every year across the world with more than half of this burden occurring in Africa [1]. This problem affects all age groups but particularly the most sensitive ones such as infants and the elderly, as well as immunedepressed people. Up to date, vaccinations and antibiotherapy are still the common means used to combat diarrheal infections. These remedies have contributed to the reduction of the impact of these infections especially in developed countries [2]. However, resource limited populations still rely on medicinal plants whenever these infections occur. Moreover, the use of medicinal plants has become the main alternative therapeutic solution against infectious diarrhoeas because of the steadily high cost of antibiotics together with the emergence of multidrug resistant microorganisms and the lack of vaccines for many enteric pathogens [3,4].
Plant resources occupy an important place in the life of rural populations of developing countries [5]. Furthermore, the African continent harbours a wide diversity of medicinal plants [6]. According to the World Health Organization, more than 80% of African populations depend on traditional medicine and pharmacopeia to solve their health problems [7]. Out of the more or less 300000 medicinal plants species identified on the planet, more than 200000 are found in tropical Africa [8,9]. Some of these medicinal plants, mainly the leafy vegetables are used by populations as food and are very beneficial for health maintenance and for the prevention of many diseases [10,11].
An ethnobotanical survey conducted by Agbankpé et al. in southern Benin revealed 27 species of leafy vegetables commonly used in traditional medicine against infectious diarrhoeas [12]. Rural populations and traditional healers basically use them to combat diarrheal bacterial infections and against several other diseases. Some of these vegetables such as Vernonia amygdalina, Crateva adansonii and Sesamum radiatum possess high nutritional values that can help the rural populations to circumvent malnutrition [13]. Apart from the nutritional potentials of these vegetables, it is necessary to evaluate their antibacterial activity in order to validate their utilisation as phyto-medicines in the treatment of diarrheal diseases by rural populations and traditional healers.
The objective of the present study was to assess the antibacterial properties of the aqueous and hydro-ethanolic extracts of these three vegetables on bacteria species that are responsible for diarrheal infections.
Materials and Methods
Materials
Common consumables and materials of Bacteriology laboratory were used during the manipulations. The study also involved powders of the leaves of Crateva adansonii, Vernonia amygdalina and Sesamum radiatum. The used bacteria strains were obtained from the Bacteriology section of the National Laboratory of the Ministry of Health (LNMSP).
They were constituted of 12 clinical strains isolated from diarrheal faeces samples: Escherichia coli, Staphylococcus aureus, Salmonella Typhis, Salmonella choleraesius, Shigella spp, Shigella flexneri, Vibrio cholerae, Citrobacter spp, Proteus mirabilis, Proteus vulgaris, Klebsiella pneumoniae, Klebsiella rhinoscleromatis and 4 reference strains (Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 25923, Pseudomonas aeruginosa ATCC 27853 and Proteus mirabilis A 24974).
Methods
a) Preparation of the extracts: 100 g of the vegetables’ powder was boiled for 30 minutes in 1000 ml of distilled water. The brew was cooled and filtered once using absorbent cotton and once with Whatman N°1 filters paper. The obtained filtrate was then dried at 50°C in an incubator and served as the aqueous extract (AE).
To make the hydro-ethanolic extract (HE), 100 g of powder was agitated for 72 hours in 1000 ml of 70% diluted ethanol. It was then filtered once on absorbent cotton and once on Whatman N°1 filters paper. The hydro-ethanolic phase was lastly dried at 50°C.
The aqueous and hydro-ethanolic extracts of the vegetables were reconstituted in distilled water at a concentration of 20 mg/ml. The prepared solutions were sterilized by filtration using filter-syringes on 0.22 μm Millipore membrane. The sterility of the stock solutions was verified by culturing aliquots of each solution on Mueller Hinton II media and incubated at 37°C for 24 to 48 hours.
b) Preparation of bacterial suspensions: A pure 24 hours colony of each bacteria strain was
emulsified in 5 ml of physiological water and adjusted to McFarland 0.5 standard.
c) Determination of minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC): This was performed using 96 well plates method described by Houngbeme et al. [14]. 100 μl of the stock solution of each extract was added to 100 μl of the different bacterial suspensions in a liquid media containing 100 μl of Mueller-Hinton broth. Positive and negative controls were prepared and respectively made of, 100 μl of MH broth + 100 μl of bacterial suspension and 100 μl of MH broth + 100 μl of the stock solutions of the extracts being tested. The micro-plates were covered with parafilm paper and incubated at 37°C for 24 hours. The MIC was estimated with naked eyes compared to the controls and each well was cultured on MH II agar and incubated at 37°C for 24 hours for the determination of the MBC.
The MBC is the smallest concentration of extract at which no bacteria colony can be observed. The antibiotic power (ap) of each extract was thereafter calculated with the formula CMB/CMI.
d) Susceptibility tests: A swab of each inoculum was cultured onto Mueller-Hinton II agar plates [15]. Using the tip of sterile pastor pipette, wells of 6 mm of diameter were dug in the agar and 50 μl of each extract was transferred to the wells. A well containing sterile distilled water served as negative control. The petri dishes were kept at ambient temperature for 30 min to 1h for a pre-diffusion of the substances before being incubated at 37°C for 24 h [16]. Meanwhile, swabs of each inoculum were cultured onto MH II plates and reference antibiotic disks were used as positive controls. The tests were repeated three times and the antibacterial activity of the extracts was determined by measuring the inhibition zone diameters around each well (Table 1).
e) Statistical analyses: Susceptibility tests were repeated thrice and the results analysed using Stata 11.0 software and presented as Mean ± standard error. An analysis of variance (ANOVA single factor) was used to compare the means of the inhibition zone diameters between the two extracts of the same plant, between the aqueous extracts of the three plants, between the hydro-ethanolic extracts of the three plants and between each reference antibiotic and the plant extracts. The level of significance was defined at 5%.
| Inhibition zone diameter (Δ) | Degree of susceptibility of the germ | Symbol |
|---|---|---|
| Δ <7 mm | Resistant | − |
| 7 mm ≤ Δ <8 mm | Susceptible | + |
| 8 mm ≤ Δ <9 mm | Fairly Susceptible | ++ |
| Δ ≥ 9 mm | Very Susceptible | +++ |
Table 1: Standard values used to interpret the results of the susceptibility tests of the plant extracts. Source: Tsirinirindravo and Andrianarisoa [17], WHO [18].
Results
Yield of extractions and sterility tests
The yields of the aqueous extraction were 12.7%; 10.5% and 12.4% being 12.7 g of Crateva adansonii, 10.5 g of Vernonia amygdalina and 12.4 g of Sesamum radiatum. The yields of the hydro-ethanolic extracts were 14.3%; 10.7% and 13.2% for Crateva adansonii, Vernonia amygdalina and Sesamum radiatum, respectively. The sterility tests revealed that both extracts of C. adansonii, V. amygdalina and S. radiatum do not contain any contaminant.
Minimal Inhibitory Concentration (MIC), Minimal Bactericidal Concentration (MBC) and the antibiotic power (ap)
The results of MIC and MBC are displayed in Tables 2 and 3. All the reference strains were sensitive to the hydro-ethanolic extract of the leaves of S. radiatum with MIC values ranging from 0.3125 to 1.25 mg/ ml. This extract showed a bactericidal effect on S. aureus ATCC 25923 and P. mirabilis A24974 and a bacteriostatic activity on E. coli ATCC 25922 and P. aeruginosa ATCC 27853. S. aureus ATCC 25923 was sensitive to the aqueous extract of S. radiatum and the hydro-ethanolic extract of C. adansonii with the same MIC of 0.625 mg/ml. None of the reference strains was susceptible to the extracts of V. amygdalina. However, a MIC of 2.5 mg/ml was recorded for these extracts on S. aureus ATCC 25923 (Table 2).
| Reference strains | |||||
|---|---|---|---|---|---|
| Extracts | Parameters | E. Coli ATCC 25922 | S.aureus ATCC 25923 | P.mirabilis A 24974 | P.aeruginosa ATCC 27853 |
| Ca AE Ca HE |
MIC | 5 | 1.25 | - | - |
| MBC | >10 | >10 | - | - | |
| a. p. | - | - | - | - | |
| MIC | 5 | 0.625 | 10 | 5 | |
| MBC | >10 | 2.5 | > 10 | >10 | |
| a. p. | - | 4 | - | - | |
| Va AE Va HE |
MIC | - | - | - | - |
| MBC | - | - | - | - | |
| a. p. | - | - | - | - | |
| MIC | - | 2.5 | - | - | |
| MBC | - | >10 | - | - | |
| a. p. | - | - | - | - | |
| Sr AE Sr HE | MCI | 2.5 | 0.625 | 2.5 | 5 |
| MBC | >10 | 2.5 | >10 | >10 | |
| a. p. | - | 4 | - | - | |
| MIC | 0.625 | 0.3125 | 0.625 | 1.25 | |
| MBC | 2.5 | 0.625 | 1.25 | 10 | |
| a. p. | 4 | 2* | 2* | 8 | |
Table 2: MIC (mg/ml), MBC (mg/ml) and a. p. of the aqueous and hydro-ethanolic extracts of the three tested vegetables on reference bacteria strains.
S. aureus, E. coli, V. cholerae, S. Typhi and P. vulgaris were the only clinical isolates susceptible to the hydro-ethanolic extract of S. radiatum with MIC values from 0.3125 to 1.25 mg/ml and a bactericidal effect on S. aureus, V. cholerae and S. Typhi. At the same MIC of 0.625 mg/ ml, S. aureus, V. cholerae, S. Typhi and P. vulgaris were susceptible to the aqueous extract of S. radiatum with a bactericidal power on S. aureus. Similarly, V. cholerae and P. vulgaris were susceptible to the hydroethanolic extract of C. adansonii (MIC = 0.625 mg/ml). No MBC was recorded for the extracts of the leaves of V. amygdalina. Nevertheless, some MIC values were obtained on S. aureus (V. amygdalina AE = 5 mg/ ml; V. amygdalina HE = 1.25 mg/ml) (Table 3).
| Clinical isolates | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Extracts | Parameters | S. aureus | E. coli | V. cholerae | S. Typhi | S. Choleraesius | S. Spp | S. flexneri | C. spp | P. vulgaris | P. mirabilis | K. pneumoniae | K. rhinoscleromatis |
| Ca AE | CMI | 2.5 | - | 2.5 | - | - | 5 | - | - | 1.25 | - | - | - |
| CMB | > 10 | - | >10 | - | - | > 10 | - | - | >10 | - | - | - | |
| a. p. | - | - | - | - | - | - | - | - | - | - | - | - | |
| Ca HE | CMI | 1.25 | 10 | 0.625 | 5 | - | 1.25 | - | - | 0.625 | - | - | - |
| CMB | >10 | >10 | 2.5 | >10 | - | >10 | - | - | 5 | - | - | - | |
| a. p. | - | - | 4 | - | - | - | - | - | 8 | - | - | - | |
| Va AE | CMI | 5 | - | - | - | - | - | - | - | - | - | - | - |
| CMB | >10 | - | - | - | - | - | - | - | - | - | - | - | |
| a. p. | - | - | - | - | - | - | - | - | - | - | - | - | |
| Va HE | CMI | 1.25 | - | - | - | - | - | - | - | - | - | - | - |
| CMB | >10 | - | - | - | - | - | - | - | - | - | - | - | |
| a. p. | - | - | - | - | - | - | - | - | - | - | - | - | |
| Sr AE | CMI | 0.63 | - | 0.625 | 0.625 | - | - | - | - | 0.625 | 2.5 | - | - |
| CMB | 1.25 | - | 2.5 | 5 | - | - | - | - | 2.5 | >10 | - | - | |
| a. p. | 2* | - | 4 | 8 | - | - | - | - | 4 | - | - | - | |
| Sr HE | CMI | 0.31 | 1.25 | 0.625 | 0.625 | - | 2.5 | 2.5 | - | 0.625 | - | 5 | - |
| CMB | 0.31 | 5 | 1.25 | 1.25 | - | >10 | >10 | - | 2.5 | - | >10 | - | |
| a. p. | 1* | 4 | 2* | 2* | - | - | - | - | 4 | - | - | - | |
Table 3: MIC (mg/ml), MBC (mg/ml) and a.p. of the aqueous and hydro-ethanolic extracts of the three tested vegetables on clinical isolates.
Susceptibility tests
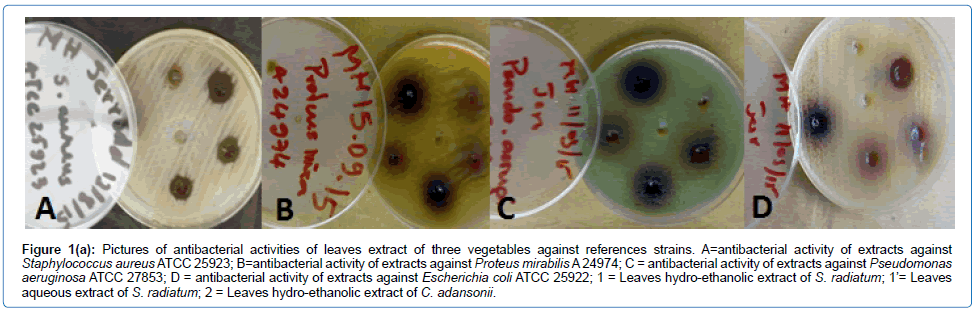
As shown in Table 4 and Figure 1a, S. aureus ATCC 25923 is susceptible to the hydro-ethanolic extract of the leaves of C. adansonii, and S. radiatum and the aqueous extract of S. radiatum.
| Inhibition zone diameters (mm) of the extracts | ||||||
|---|---|---|---|---|---|---|
| Reference strains | Ca AE | Ca HE | Va AE | Va HE | Sr AE | Sr HE |
| S. aureus ATCC 25923 | 0 ± 0a | 10.33 ± 0,57b | 0 ± 0c | 0 ± 0d | 13 ± 1.15e | 18.67 ± 0.57f |
| E. coli ATCC 25922 | 0 ± 0 | 0 ± 0a | 0 ± 0 | 0 ± 0b | 0 ± 0c | 13 ± 1d |
| P. mirabilis A 24974 | 0 ± 0 | 0 ± 0a | 0 ± 0 | 0 ± 0b | 0 ± 0c | 09 ± 1d |
| P.aeruginosa ATCC 27853 | 0 ± 0 | 0 ± 0a | 0 ± 0 | 0 ± 0b | 0 ± 0c | 11 ± 1d |
Means of the inhibition zone diameters followed by the same letters in the same row are not significanty different (p< 0.05); Ca = Crateva adansonii; Va = Vernonia amygdalina; Sr = Sesamum radiatum ; AE= aqueous extract; HE = hydro-ethanolic extract; E. coli = Escherichia coli ; S. aureus = Staphylococcus aureus ; P. mirabilis = Proteus mirabilis ; P. aeruginosa = Pseudomonas aeruginosa
Table 4: Inhibition zone diameters (mm) of the aqueous and hydro-alcoholic extracts of the studied vegetables on reference bacteria strains.
Figure 1a: Pictures of antibacterial activities of leaves extract of three vegetables against references strains. A=antibacterial activity of extracts against Staphylococcus aureus ATCC 25923; B=antibacterial activity of extracts against Proteus mirabilis A 24974; C = antibacterial activity of extracts against Pseudomonas aeruginosa ATCC 27853; D = antibacterial activity of extracts against Escherichia coli ATCC 25922; 1 = Leaves hydro-ethanolic extract of S.radiatum; 1’= Leaves aqueous extract of S. radiatum; 2 = Leaves hydro-ethanolic extract of C.adansonii
The inhibition zone diameters obtained with the hydro-ethanolic extract of the leaves of S. radiatum were significantly higher than (p < 0.05) those recorded using the aqueous extract of the same plant but also higher than those obtained with the hydro-ethanolic extracts of C. adansonii and V. amygdalina. Apart from S. aureus ATCC 25923, the other reference strains were only sensitive to the hydro-ethanolic extract of the leaves of S. radiatum with inhibition zone diameters varying between 9 ± 1 and 13 ± 1 mm.
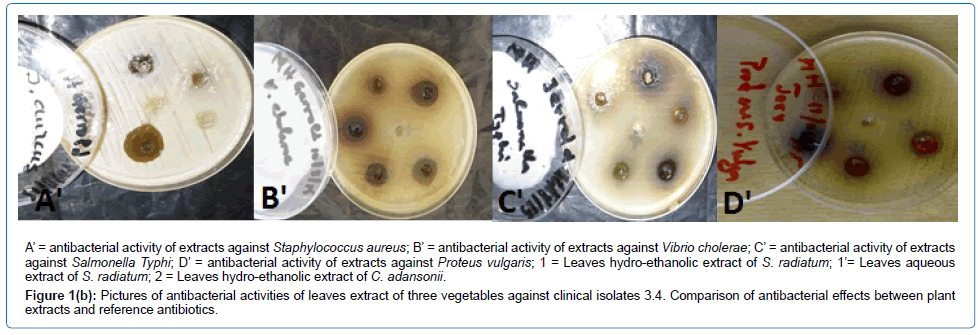
For the clinical isolates, V. cholerae and P. vulgaris were sensitive to the hydro-ethanolic extract of the leaves of C. adansonii (10.67 ± 0.57 mm; 10.33 ± 0.57 mm) and S. radiatum (15.67 ± 0.57 mm; 14 ± 1 mm) and to the aqueous extract of the leaves of S. radiatum (11.67 ± 0.57 mm; 11 ± 1 mm). The inhibition zone diameters of the hydro-ethanolic extract of the leaves of S. radiatum were significantly higher than (p < 0.05) those induced by the same type of extract of the two other vegetables. S. aureus and S. Typhi were susceptible to the aqueous extract (12.67 ± 0.57 mm; 9.67 ± 0.57 mm) and the hydro-ethanolic extract (17 ± 1 mm; 13.33 ± 0.57 mm) of S. radiatum, with inhibition zone diameters of the latter being significantly higher than (p < 0.05) those of the aqueous extract. E. coli was only susceptible to the hydro-ethanolic extract of S. radiatum with a diameter of 9.66 ± 1.15 mm (Table 5 and Figure 1b).
| Inhibitionzone diameters (mm) of the extracts | ||||||
|---|---|---|---|---|---|---|
| Clinicalisolates | CaAE | CaHE | VaAE | VaHE | SrAE | SrHE |
| Staphylococcusaureus | 0 ± 0a | 0 ± 0b | 0 ± 0c | 0 ± 0d | 12.67 ± 0.57e | 17 ± 1f |
| Escherichiacoli | 0 ± 0 | 0 ± 0a | 0 ± 0 | 0 ± 0b | 0 ± 0b | 09.67 ± 0.57c |
| Vibriocholerae | 0 ± 0a | 10.67 ± 0.57b | 0 ± 0c | 0 ± 0d | 11.67 ± 0.57e | 15.67 ± 0.57f |
| SalmonellaTyphi | 0 ± 0a | 0 ± 0b | 0 ± 0c | 0 ± 0d | 9.67 ± 0.57e | 13.33 ± 0.57f |
| Salmonellacholeraesius | 0 ± 0a | 0 ± 0a | 0 ± 0a | 0 ± 0a | 0 ± 0a | 0 ± 0a |
| Shigellaspp | 0 ± 0a | 0 ± 0a | 0 ± 0a | 0 ± 0a | 0 ± 0a | 0 ± 0a |
| Shigellaflexneri | 0 ± 0a | 0 ± 0a | 0 ± 0a | 0 ± 0a | 0 ± 0a | 0 ± 0a |
| Citrobacterspp | 0 ± 0a | 0 ± 0a | 0 ± 0a | 0 ± 0a | 0 ± 0a | 0 ± 0a |
| Proteusmirabilis | 0 ± 0a | 0 ± 0a | 0 ± 0a | 0 ± 0a | 0 ± 0a | 0 ± 0a |
| Proteusvulgaris | 0 ± 0a | 10.33 ± 0.57b | 0 ± 0c | 0 ± 0d | 11 ± 1e | 14 ± 1f |
| Klebsiellarhinoscleromatis | 0 ± 0a | 0 ± 0a | 0 ± 0a | 0 ± 0a | 0 ± 0a | 0 ± 0a |
| Klebsiellapneumoniae | 0 ± 0a | 0 ± 0a | 0 ± 0a | 0 ± 0a | 0 ± 0a | 0 ± 0a |
Table 5: Inhibition zone diameters (mm) of the aqueous and hydro-alcoholic extracts of the studied vegetables on clinical isolates.
Comparison of antibacterial effects between plant extracts and reference antibiotics
A significant statistical difference (p < 0.05) was observed between the antibacterial activity of reference antibiotics and the effective plant extracts (hydro-ethanolic extract of C. adansonii, aqueous and hydroethanolic extracts of S. radiatum). Inhibition zone diameters induced by reference antibiotics on reference bacteria strains were greater than (21 ± 1 to 32.67 ± 0.57 mm) those obtained with plant extracts (0 ± 0 to 18.67 ± 0.57 mm) (Table 6).
| Plant extracts | Reference antibiotic discs | |||||
|---|---|---|---|---|---|---|
| Reference strains | Sr AE (1 mg) | Sr HE (1 mg) | Ca HE (1 mg) | Cotrimoxazole (25 µg) | Chloramphenicol (30 µg) | Ciprofloxacin (30 µg) |
| E. coli ATCC 25922 | 0 ± 0a | 13 ± 1b | 0 ± 0c | 22.67 ± 0.57d | 27.33 ± 0.57e | 32.67 ± 0.57f |
| S. aureus ATCC 25923 | 13 ± 1a | 18.67 ± 0.57b | 10.33 ± 0.57c | 24.33 ± 0.57d | 23.33 ± 0.57e | 30.33 ± 0.57f |
| P. mirabilis A 24974 | 0 ± 0a | 9.67 ± 0.57b | 0 ± 0c | 22 ± 1d | 26.33 ± 0.57e | 30.33 ± 0.57f |
| P. aeruginosa ATCC 27853 | 0 ± 0a | 11.33 ± 0.57b | 0 ± 0c | 21 ± 1d | 25 ± 1e | 28.67 ± 0.57f |
Table 6: Comparison of inhibition zone diameters (mm) of the active plant extracts and those of reference antibiotic discs on reference bacteria strains.
Furthermore, there was a significant difference between the antibacterial activity exercised by plant extracts on clinical isolates as compared to that of reference antibiotics (p < 0.05). The inhibition zone diameters produced by reference antibiotics were higher than (0 ± 0 to 38.33 ± 0.57 mm) those recorded from the active plant extracts (0 ± 0 to 17 ± 1 mm). V. cholerae was not susceptible to Cotrimoxazole but to the three active plant extracts. Likewise, Citrobacter sp were resistant to Cotrimoxazole but sensitive to Chloramphenicol and Ciprofloxacin (Table 7).
| Plant extracts | reference antibiotic discs | |||||
|---|---|---|---|---|---|---|
| Clinical isolates | Ca HE (1 mg) | Sr AE (1 mg) | Sr HE (1 mg) | Cotrimoxazole (25 µg) | Chloramphenicol (30 µg) | Ciprofloxacin (30 µg) |
| Staphylococcus aureus | 0 ± 0a | 12.67 ± 0.57b | 17 ± 1c | 24.67 ± 0.57d | 22.33 ± 0.57e | 30.33 ± 0.57f |
| Escherichia coli | 0 ± 0a | 0 ± 0b | 09.66 ± 0.57c | 23.33 ± 0.57d | 28 ± 1e | 32.33 ± 0.57f |
| Vibrio cholerae | 10.67 ± 0.57a | 11.67 ± 0.57b | 15.67 ± 0.57c | 0 ± 0d | 23 ± 1e | 26.33 ± 0.57f |
| Salmonella Typhi | 0 ± 0a | 09.67 ± 0.57b | 13.33 ± 0.57c | 30 ± 1d | 27 ± 1e | 38.33 ± 0.57f |
| Salmonella choleraesius | 0 ± 0a | 0 ± 0b | 0 ± 0c | 24.33 ± 0.57d | 25.67 ± 0.57e | 28.67 ± 0.57f |
| Shigellaspp | 0 ± 0a | 0 ± 0b | 0 ± 0c | 22 ± 1d | 23.33 ± 0.57e | 26 ± 1f |
| Shigellaflexneri | 0 ± 0a | 0 ± 0b | 0 ± 0c | 23.33 ± 0.57d | 24.33 ± 0.57e | 26.33 ± 0.57f |
| Citrobactersp | 0 ± 0a | 0 ± 0b | 0 ± 0c | 0 ± 0 | 22.67 ± 0.57d | 30 ± 1e |
| Proteus mirabilis | 0 ± 0a | 0 ± 0b | 0 ± 0c | 26 ± 1d | 22 ± 1e | 25 ± 1f |
| Proteus vulgaris | 10.33 ± 0.75a | 11 ± 1b | 14.33 ± 0.57c | 28 ± 1d | 23 ± 1e | 26 ± 1f |
| Klebsiellarhinoscleromatis | 0 ± 0a | 0 ± 0b | 0 ± 0c | 22 ± 1d | 28 ± 1e | 32.67 ± 0.57f |
| Klebsiellapneumoniae | 0 ± 0a | 0 ± 0b | 0 ± 0c | 24 ± 1d | 27.33 ± 0.57e | 30 ± 1f |
Table 7: Comparison of inhibition zone diameters (mm) of the active plant extracts and those of reference antibiotic discs on clinical isolates.
Discussion
The present study aimed to assess the antibacterial activity of the aqueous and hydro-ethanolic extracts of three leafy vegetables commonly used in traditional medicine to treat infectious diarrhoeas. To this end, the extracts were tested on 12 clinical bacteria isolates obtained from diarrheal faeces samples and on 4 reference strains.
S. aureus ATCC 25923 was the most susceptible reference strain to the extracts of C. adansonii (aqueous extract: MIC=1.25 mg/ml; hydro-ethanolic extract: MIC=0.625 mg/ml). However, a total absence of MBC was recorded with the aqueous extract of C. adansonii at all the considered concentrations. Nevertheless, its hydro-ethanolic extract showed a bacteriostatic effect on S. aureus ATCC 25923 with a MBC of 2.5 mg/ml. Moreover, no MBC was induced by the aqueous extracts of C. adansonii on the clinical isolates. However, V. cholerae and P. vulgaris were susceptible to its hydro-ethanolic extract with a bacteriostatic power on these two isolates. In addition, S. aureus (CMI = 1.25 mg/ ml) and Shigella spp (1.25 mg/ml) were also susceptible to this extract though without a MBC. These results are better than those reported by Lagnika et al. [19] on S. aureus isolated from Thryonomys swinderianus (aqueous extract of the leaves of Crateva religiosa: MIC = 10 mg/ml; ethanolic extract of the leaves of Crateva religiosa: MIC=2.5 mg/ml). This discrepancy could be explained by the fact that the tested bacteria strains were not from the same origin and the two studies were not conducted in the same area. Furthermore, the findings of the current study are better than those obtained by Ayodeji et al. [20] on E. coli (12.5 mg/ml), S. Typhi (12.5 mg/ml), S. aureus (12.5 mg/ml) and K. pneumoniae (25 mg/ml). Agboke Ayodeji et al. actually carried out their study on the methanolic extract of the leaves of C. adansonii and the reported MIC was beyond the one of this study. This is probably due to the difference of extract used.
All used reference and clinical strains were resistant to the extracts of the leaves of V. amygdalina, except S. aureus ATCC and the isolated S. aureus that were susceptible to the hydro-ethanolic extract of this vegetable (MIC = 2.5 mg/ml; MIC = 5 mg/ml). However, this extract has no antibiotic power (absence of MBC). These results are similar to those of Anibijuwon et al. [21] and Ogundare [22]. Anibijuwon et al. [21] reported that S. aureus isolated from saliva sample were sensitive to the aqueous and ethanolic extracts of the leaves of V. amygdalina with MIC of 45 mg/ml and 60 mg/ml, respectively. The same situation is noted with the results of Ogundare [22] who reported that S. Typhi were resistant to the ethanolic extract of the leaves of V. amygdalina while S. aureus were sensitive to this same extract with a MIC of 25 mg/ml. The results of this study are also constant with those of Adetunji et al. [23], whereby there was a total absence of MBC with the hydro-ethanolic extract of V. amygdalina on P. aeruginosa, E. coli and S. aureus.
All the reference strains were susceptible to the extracts of S. radiatum with MICs from 0.3125 to 5 mg/ml. The most susceptible strains to the hydro-ethanolic extract were S. aureus ATCC 25923 (0.3125 mg/ml), E. coli ATCC 25922 (0.625 mg/ml), P. mirabilis A 24974 (0.625 mg/ml) and P. aeruginosa ATCC 27853 (1.25 mg/ml). The MBC values of the hydro-ethanolic extract of the leaves of S. radiatum on these reference strains range from 0.625 to 10 mg/ml, which confer to this extract its bactericidal power on S. aureus ATCC 25923 and P. mirabilis A 24974 and a bacteriostatic power on E. coli ATCC 25922 and P. aeruginosa ATCC 27853. The only sensitive strain to the aqueous extract of the leaves of S. radiatum was S. aureus ATCC 25923 with a MBC of 2.5 mg/ml, demonstrating the bacteriostatic power of this extract on the strain. Some similar results were reported by Seukep et al. [24], in which E. coli ATCC 8739 and P. aeruginosa ATCC 29916 were sensitive to the methanolic extract of the leaves of S. radiatum (MIC=1.024 mg/ml). Those results are better than those obtained with the hydro-ethanolic and ethanol extracts of Solanum incanum and Asparagus africanus (absence of MIC on S. aureus ATCC 25923, E. coli ATCC 25922 and P. aeruginosa ATCC 27853); Jatropha curcas, Khaya senegalensis (absence of antibiotic power on S. aureus ATCC 25923 and E. coli ATCC 25922); Clerodendrum splendens (bacteriostatic effect on E. coli ATCC 25922) and Moringa oleifera (absence of MIC on E. coli ATCC 25922) [14,25-27].
Among the clinical isolates used in this study, only S. aureus (0.3125 mg/ml), E. coli (1.25 mg/ml), V. cholerae (0.625 mg/ml), S. Typhi (0.625 mg/ml) and P. vulgaris (0.625 mg/ml) were susceptible to the hydroethanolic extract of S. radiatum with MBCs from 0.3125 to 5 mg/ml. Besides, S. aureus, V. cholerae, S. Typhi and P. vulgaris were susceptible to the aqueous extract of S. radiatum with the same MIC of 0.625 mg/ml and MBCs ranging between 1.25 and 5 mg/ml. The antibiotic powers of the hydro-ethanolic extract of S. radiatum show that it has a stronger antibacterial activity on the clinical isolates than the aqueous extract. These results are however better than those of Shittu et al. [28], who reported that S. aureus was resistant to the ethanolic extract of S. radiatum. This could be attributed to the difference in plant varieties because Shittu and his collaborators carried out their study in Lagos, Nigeria.
Many bacteria were susceptible to the hydro-ethanolic extract of C. adansonii (S.aureus ATCC 25923, V. cholera and P. vulgaris) while a total absence of inhibition zone diameters was observed with the aqueous extract on the studied strains. It can therefore be concluded that the hydro-ethanolic extract of C. adansonii has a better antibacterial activity over its aqueous extract. These results are constant with those reported by Gowsaya and Saravanababu in 2013 [29], where 50 μl of the ethanolic extract of Crateva religiosa did not induce any inhibition zones on E. coli and S. aureus isolates.
Furthermore, all the studied bacteria strains (references and clinical isolates) did not show any sensitivity towards the extracts of the leaves of V. amygdalina. This is explained by the absence of inhibition zones. Opara et al. [30] reported similar results with an absence of inhibition zones by the aqueous and ethanolic extracts on E. coli, S. aureus and P. aeruginosa isolates.
S. aureus ATCC 25923, S. aureus, V. cholerae, S. Typhi and P. vulgaris were susceptible to the aqueous and hydro-ethanolic extracts of S. radiatum, with inhibition zone diameters varying from 9.67 ± 0.57 to 18.67 ± 0.57 mm. A comparison of the Means of inhibition zone diameters between these two extracts revealed a significant difference (p < 0.05). This confirms that the hydro-ethanolic extract of the leaves of S. radiatum has a stronger antibacterial activity than its aqueous extract. Moreover, E. coli ATCC 25922 (13 ± 1 mm), P. mirabilis A 24974 (09 ± 1 mm), P. aeruginosa ATCC 27853 (11 ± 1 mm) and E. coli (9.67 ± 0.57 mm) were also susceptible to the hydro-ethanolic extract of S. radiatum. Osibote et al. (2009) reported similar results that E. coli ATCC 25922, E. coli, P. aeruginosa and S. aureus were susceptible to the essential oil of the stem of S. radiatum, with inhibition zone diameters from 10 to 19 mm. However, they also reported that Citrobacter spp. and P. mirabilis were sensitive to the essential oil of the leaves of S. radiatum while S. aureus ATCC 25923 were resistant to it. This dissimilarity could be explained by the differences in the parts of the plant used in the two studies and the type of extracts. Nevertheless, the results of this study are better than those reported using the hydro-ethanolic extract of the leaves of Argemone mexicana on S. aureus (7 ± 0 mm), E. coli (8 ± 1 mm), S. Typhi (11 ± 2 mm) and K. pneumoniae (8 ± 1 mm); Pupalia lappacea on E. coli and S. Typhi (a bacteriostatic activity) and Dalechampia clematidifolia on S. Typhi (6 mm) and S. aureus (14.5 mm) [17,31,32]. This confirms the antibacterial activity of the hydro-ethanolic extract of S. radiatum on bacterial strains responsible for diarrheal diseases and infections. Such antibacterial activity is stronger on reference strains than on clinical strains and can be explained by the fact that reference strains are sensitive strains which helped to validate the methodology and to confirm the results of the clinical strains.
A two by two comparison of the Means of inhibition zone diameters of the aqueous extracts showed that there is a significant difference (p < 0.05) between the aqueous extracts of the leaves of S. radiatum and the one of C. adansonii and V. amygdalina. The aqueous extract of the leaves of S. radiatum therefore has a better antibacterial activity than those of the other vegetables. Additionally, a significant difference (p< 0.05) was recorded between the means of the inhibition zone diameters of the hydro-ethanolic extract of the leaves of S. radiatum and the one of the leaves of C. adansonii and V. amygdalina. This shows that the hydroethanolic extract of the leaves of S. radiatum has a stronger antibacterial activity than the one of the leaves of C. adansonii. Moreover, a significant difference was observed between the hydro-ethanolic extract of the leaves of S. radiatum and the aqueous extract of the same leaves. It can be concluded that out of the six studied vegetable extracts, the most active one is the hydro-ethanolic extract of the leaves of S. radiatum followed by its aqueous extract and the hydro-ethanolic extract of the leaves of C. adansonii. S. radiatum possess a strong antibacterial activity and the hydro-ethanolic extract of its leaves have broad spectrum antibacterial effects, capable of killing a number of enteric bacteria responsible for diarrheal infections. Therefore this extract can be recommended in the treatment of infectious diarrhoeas, food poisoning or infections due to enteric bacteria.
Chloramphenicol is commonly used as a reference antibiotic in studies involving antibacterial assessment of plant extracts on pathogens [22,24,33]. Ciprofloxacin belongs to the family of Fluoroquinolones, and often used in the treatment of acute bacterial diarrhoeas [34]. Cotrimoxazole is also an antibiotic used in the treatment of acute bacterial diarrhoeas, especially in children [22]. These three antibiotics (Chloramphenicol, Ciprofloxacin and Cotrimoxazole) were used as reference antibiotics in this study. The comparison of the Means of inhibition zone diameters of each antibiotic with those of the active extracts (hydro-ethanolic extract of the leaves of S. radiatum, aqueous extract of the leaves of S. radiatum and the hydro-ethanolic extract of the leaves of C. adansonii) on most of the studied strains, showed a significant difference (p< 0.05). The reference antibiotics have stronger antibacterial activities than those of the active plant extracts. Even at a higher load (1 mg), the active plant extracts induced significantly lower inhibition zone diameters than those recorded with reference antibiotics at smaller load (25 in 30 μg). This is attributable to the fact that reference antibiotics are made of chemical, synthetic and pure compounds, while plant extracts are mixtures of non-purified active principles (secondary metabolism compounds). Nevertheless, V. cholerae was resistant to Cotrimoxazole whereas this same bacterium was susceptible to the three active plant extracts. Although antibiotics are more effective in the treatment of acute bacterial diarrhoeas, the antibacterial activity of plant extracts should not be undermined especially to overcome the increasing challenges of bacteria’s resistance to antibiotics.
Conclusion
This study revealed that among the six studied vegetable extracts, the hydro-ethanolic extract of the leaves of Sesamum radiatum possess the best antibacterial activity on the used bacterial strains followed by its aqueous extract and the hydro-ethanolic extract of the leaves of C. adansonii. These extracts are large spectrum products. They are active against a wide range of bacterial species responsible for diarrheal infections or diseases, especially those belonging to the family of Enterobacteriacea. Probably, they can be used to treat diarrhoeas and food poisoning cases, as well as or enteric bacterial infections.
References
- OMS (2011) Statistiquessanitairesmondiales. OMS 171.
- Soro D, Koné MW, Kamanzi AK (2010) Evaluation de l’activitéantibactérienne et anti-radicalelibres de quelquestaxonsbioactifs de Côte d’Ivoire. J Sci Res 40: 307-317.
- Akoua KC, Guessend N, Gbonon V, Faye-kette AY, Dosso M (2004) Methicillini-resistant of S. aureus activity in Abidjan (1998-2001): A new hospital problem. Medecines Maladies Infectieuses 34: 132-136.
- Guillemot D, Maugendre P, Vhauvin, Sermé TC (2004) Consommation des antibiotiquesen France. BEH 3233 :141-147.
- Mangambu M, Kamabu V, Bola MF (2008) Les plantesmédicinalesutiliséesdans le traitement de l’asthme à Kisangani et ses environs (Province Orientale, R.D.Congo). Annales des Sciences, UniversitéOfficielle de Bukavu 1 : 63-68.
- Mangambu M, Noiha NV, Zapfack L, Sonké B (2010) Etude phytosociologique du groupement à piper capensis (RD Congo). International journal of environmental studies 67: 417-430.
- OMS (2012) Stratégie pour la médecinetraditionnelle 2007–2011. Genève.
- Kolling M, Winkley K, Von Deden M (2010) “For someone who’s rich, it’s not a problem.” Insights from Tanzania on diabetes health-seeking and medical pluralism among Dar es Salam’s urban poor. Globalization and Health 6: 8.
- Mangambu J (2013) Taxonomie, biogéographie et écologie des Ptéridophytes de l’écosystèmeforestier des montagnes du Parc National de Kahuzi-Biega à l’Est de la R.D. Congo. Thèse de doctorat, Universitéd’Anvers/Belgique 463.
- MangambuMokoso JD, Van Diggelen R, Jean-Claude MM, Ntahobavuka H, Malaisse F, Robbrecht E (2012) Etude ethnoptéridologique, évaluation des risquesd’extinction et stratégies de conservation aux alentours du Parc National de KahuziBiegaen RD Congo. Geo-Eco-Trop 36: 137-158.
- Mensah JK, Ihenyen JO, Okhiure MO (2013) Nutritional, phytochemical and antimicrobial properties of two wild aromatic vegetables from Edo State. J Nat Prod Plant Resour 3: 8-14.
- Agbankpé AJ, Dougnon TV, Bankolé HS, Yèhouénou B, Yédomonhan H, Lègonou M, et al. (2014) Etude ethnobotanique des légumesfeuillesthérapeutiquesutilisésdans le traitement des diarrhées au sud-Bénin (Afrique de l’Ouest), Int J BiolChem Sci. 8: 1784-1795.
- Agbankpé AJ, Bankolé SH, Dougnon TJ, Yèhouénou B, Hounmanou YM, et al. (2015) Comparison of Nutritional Values of Vernoniaamygdalina, Cratevaadansonii and Sesamumradiatum: Three main vegetables used in traditional medicine for the treatment of bacterial diarrhea in Southern Benin (West Africa). Food and Public Health 5: 144-149.
- Houngbeme AG, Gandonou C, Yehouenou B, Kpoviessi SD, Sohounhloue D, et al. (2014) Phytochemical analysis, toxicity and antibacterial activity of benin medicinal plants extracts used in the treatment of sexually transmitted infections associated with hiv/aids. Int J Pharm Sci Res. 5: 1739-1745.
- Comité de l’Antibiogramme de la SociétéFrançaise de Microbiologie. (2012).
- Oke MA, Bello AB, Odebisi MB, Ahmed El-Imam AM, Kazeem MO (2013) Evaluation of antibacterial efficacy of some alcohol-based hand sanitizers sold in ilorin (north-central Nigeria). Ife Journal of Science 15: 111-117.
- Tsirinirindravo L, Andrianarisoa B (2009) Activitésantibactériennes de l’extrait des feuilles de Dalechampiaclematidifolia (Euphorbiaceae). Int J BiolChem Sci. 3: 1198-1202.
- OMS (2002) L’utilisation des antimicrobiensendehors de la médecinehumaine et les résistances qui enrésultent chez l’homme. OMS Aide-Mémoire N°268, Genève.
- Selvamohan T, Ramadas V, ShibilaSelva KS (2012) Antimicrobial activity of selected medicinal plants against some selected human pathogenic bacteria. Advances in Applied Science Research 3: 3374-3381.
- Dibong SD, MpondoMpondo E, Ngoye A, Kwin MF, Betti JL (2011) Ethnobotanique et phytomédecine des plantesmédicinales de Douala. J ApplBiosci 37: 2496-2507.
- Lagnika L, Anago E, Atindehou M, Adjahoutonon B, Dramane K, et al. (2011) Antimicrobial activity of CrataevareligiosaForst against bacteria isolated from ThryonomysswinderianusTemminck. African Journal of Biotechnology 10: 10034-10039.
- AgbokeAyodeji A, Attama Anthony A, MomohMumuni A (2011) Evaluation of the antimicrobial activities of crude extract of Cryptolepissanguinolenta and Cratevaadansonii leaves and their interactions. Journal of Applied Pharmaceutical Science 1: 85-89.
- Anibijuwon II, Oladejo BO, Adetitun DO, Kolawole OM (2012) Antimicrobial Activities of Vernoniaamygdalina Against Oral Microbes. Global Journal of Pharmacology 6: 178-185.
- Ogundare AO (2011) Antibacterial properties of the leaf extracts of Vernoniaamygdalina, Ocimumgratissimum, Corchorousolitorius and Manihot palmate. Journal of Microbiology and Antimicrobials 3: 77-86.
- Adetunji CO, Olaniyi OO, Ogunkunle AT (2013) Bacterial activity of crude extracts of Vernoniaamygdalina on clinical isolates. Journal of Microbiology and Antimicrobials 5: 60-64.
- Seukep JA, Fankam AG, Djeussi DE, Voukeng IK, Tankeo SB, et al. (2013). Antibacterial activities of the methanol extracts of seven Cameroonian dietary plants against bacteria expressing MDR phenotypes. Springerplus. 2: 363.
- Teka A, Rondevaldova J, Asfaw Z, Demissew S, Van-Damme P, et al. (2015) In vitro antimicrobial activity of plants used in traditional medicine in Gurage and Silti Zones, south central Ethiopia. BMC Complementary and Alternative Medicine. 15: 286.
- Konan Kouadio F, Guessennd NK, Karamoko O, Bahi C, Adama C, et al. (2013) Action antibactérienne de l’extraitéthanolique 70% de Clerodendrumsplendens (G. Don) (Verbenacae) sur des souchesbactériennesisolées de selles chez des enfantsdiarrhéiques. Int J BiolChemSci 7: 1332-1337.
- Millogo-Koné H, Kini BF, Yougbaré Z, Yaro MB, Sawadogo M (2012) Etudes de la phytochimie et de l’activitéantimicrobienne in vitro des feuilles de Moringaoleifera (Moringaceae). Revue CAMES, Serie Pharm Med TradAfr 6.
- Shittu LA, Bankole MA, Ahmed T, Bankole MN, Shittu RK, et al. (2007) Antibacterial and Antifungal Activities of Essential Oils of Crude Extracts of Sesame Radiatum against Some Common Pathogenic Micro-Organisms. Iranian Journal of Pharmacology and Therapeutics. 6: 165-170.
- Gowsalya P and Saravanababu (2013) Phytochemical and Antimicrobial Activity of Selected Microorganism of Bark Extract of the Plant Crataevareligiosa. International Journal of Pharmaceutical & Biological Archives 1: 179-181.
- Opara AU, Egbuobi RC, Dike Ndudim JN, Onyewuchi CE, Nnodim JK (2014) Antibacterial Activity of Ocimumgratissimum (Nchu-Anwu) and Vernoniaamygdalina (Bitter-Leaf) Antibacterial Activity of Ocimumgratissimum (Nchu-Anwu) and Vernoniaamygdalina (Bitter-Leaf). British Biotechnology Journal 4: 1115-1122.
- Osibote EA, Ogunlesi M, Okiei W, Asekun T, Famoloni OB (2009) Assessment of antimicrobial activity of the essential oil from the stem powder of Cissuspopulnea and the leaves of Sesamumradiatum, herbal medications for male infertility factor. Research Journal of Medicinal Plant.
- Sourabie TS, Nikiema JB, Lega I, Nacoulma OG, Guissou IP (2010) Etude in vitro de l’activitéantibactérienned'extraitsd’uneplante de la pharmacopéeburkinabé: casd’Argemonemexicana L. (Papaveraceae). Int J BiolChemSci 4: 2009-2016.
- Hoekou YP, Batawila K, Gbogbo KA, Karou DS, Ameyapoh Y, et al. (2012) Evaluation des propriétésantimicrobiennes de quatreplantes de la floretogolaiseutiliséesenmédecinetraditionnelledans le traitement des diarrhéesinfantiles. Int J BiolChemSci 6: 3089-3097.
- Djeussi DE, Noumedem JA, Seukep JA, Fankam AG, Voukeng IK, et al. (2013) Antibacterial activities of selected edible plants extracts against multidrug-resistant Gram-negative bacteria. BMC Complementary and Alternative Medicine 13: 164.
- Carre D, Coton T, Delpy R, Guisset M, Debonne JM (2001) Diarrhéesaiguësinfectieuses : Traitementactuel et perspectives. Med Trop 61: 521-528.
Relevant Topics
- Advanced Therapies
- Chicken Pox
- Ciprofloxacin
- Colon Infection
- Conjunctivitis
- Herpes Virus
- HIV and AIDS Research
- Human Papilloma Virus
- Infection
- Infection in Blood
- Infections Prevention
- Infectious Diseases in Children
- Influenza
- Liver Diseases
- Respiratory Tract Infections
- T Cell Lymphomatic Virus
- Treatment for Infectious Diseases
- Viral Encephalitis
- Yeast Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 16471
- [From(publication date):
June-2016 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 15626
- PDF downloads : 845