Research Article Open Access
In Vitro and In Vivo Effects of Aqueous Extract of Rosmarinus officinalis L. (Rosemary) in The Control of Late Blight Disease of Potato Caused by Phytophthora Infestans Mont. De Bary
| Messgo Moumene S1*, Olubunmi OF2, Laidani M1, Saddek D1, Houmani Z1 and Bouznad Z3 | |
| 1Laboratory for Research on Medicinal and Aromatic plants, Science and life Faculty, University of Blida1, BP. 270, Soumaa road, 09100, Blida, Algeria | |
| 2Department of Crop Protection and Environmental Biology, University of Ibadan, Nigeria | |
| 3Laboratory of Phytopathology and Molecular Biology, National graduate school of Agronomy El Harrach, Algeria | |
| Corresponding Author : | Messgo-Moumene Saida Laboratory for Research on Medicinal and Aromatic plants Science and life Faculty, University of Blida1 BP. 270, Soumaa road, 09100, Blida, Algeria Tel: +39-0332-789-7 E-mail: moumene_saida@yahoo.fr |
| Received January 23, 2015; Accepted July 10, 2015; Published July 17, 2015 | |
| Citation: Moumene MS, Olubunmi OF, Laidani M, Saddek D, Houmani Z, et al. (2015) In Vitro and In Vivo Effects of Aqueous Extract of Rosmarinus officinalis L. (Rosemary) in The Control of Late Blight Disease of Potato Caused by Phytophthora Infestans Mont. De Bary. in Algeria. Adv Crop Sci Tech 3:177. doi:10.4172/2329-8863.1000177 | |
| Copyright: © 2015 Moumene MS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Advances in Crop Science and Technology
Abstract
The fungus Phytophthora infestans is known to develop resistance against the metalaxyl (fungicide), commonly used in the control of potato mildew disease. There is therefore urgent need to explore the potentials of alternative fungicides which are potent, affordable, readily available, easy to prepare and environment friendly. The study was carried out to test the fungicidal potential of aqueous extracts of Rosmarinusofficinalis (Rosemary), in vitro and in vivo on two isolates of P. infestans collected from two potato producing Algerian areas: Bourkika (Tipaza City) and El Abbadia (Aindefla City). Various concentrations of crude extracts of Rosmarinus officinalis applied by direct contact in the following dilutions: 5%, 10% and 20% on medium with pea-agar (PPA), allowed the inhibition of mycelial growth of P. infestans isolates. The observed rates of inhibition exceeded 85% and the inhibitive minimal concentration (CMI) was 5%. Parallel structural modifications, caused by mycelial lyses, as well as the deformation or, and the digestion of the contents of sporangia affected the morphology of both strains from the lowest concentration. The sporulation and the germination were inhibited by this aqueous extract (100%). Also, the absence of resumption of mycelial growth on medium PPA and absence of the mildew symptoms on detached Spunta potato leaves confirmed the fungicidal effect of the Rosemary aqueous extract. This also translated in vivo as significant reduction of the disease was observed. Disease reduction was recorded for the preventive application modes by spraying with the crude aqueous extract (86.2%) and by watering, while for the curative mode with crude extract (81%). On the other hand, Spunta variety was more marked for preventive mode by watering (85%) and the curative one (90%) also, A2 isolate was more inhibited for the application of R. officinalis aqueous extract by curative (83%), spraying mode (86%) and watering modes. Besides, treatments made in preventive modes by spraying and watering showed a total inhibition of the sporulation (100%), exceeding 85% in Spunta variety and 96% for A1 isolate was observed in the curative mode of application. This study thus confirms the antifungal potential of aqueous extract of Rosmarinus officinalis on P. infestans isolates. It is thus recommended for use as bio-fungicide in the management of potato mildew disease.
| Keywords |
| Rosmarinus officinalis; Phytophthora infestan; Fungicide; Solanum tuberosum |
| Introduction |
| Late blight potato, caused by Phytophthora infestans is one of the most destructive diseases of potato. Until now, chemical control remains the most important control against the disease. However, the use of pesticides has several constraints such as high cost of fungicides, negative effects on the environment and the health of the consumers [1]. Also, the appearance of aggressive isolates of this fungus, mostly resistant to the current synthetic fungicides, has created new challenges for potato growers [1]. |
| Various reports have highlighted the action of certain plant extracts and some essential oils against the phyto pathogenic agent of potato mildew disease [2].The experiment, therefore aimed at evaluation of the antifungal activities of crude aqueous extract of Rosmarinus officinalis on Phytophthora infestans isolates, while determining in vitro the effect of the extract on the mycelial growth, sporulation and germination, the determination of the minimal and lethal inhibitory concentrations, as well as the inhibition of their survivability after treatments. Also, observations were made in vivo on detached potato’s leaves, its effect on the symptoms appearance period, disease reduction and sporulation inhibition. |
| Materials and Methods |
| Plant material: The plant material used includes the aerial parts composed of stalks, leaves and flowers of rosemary (Rosmarinus officinalis L.) as well as seed potato tubers. The collection of plants was made in May, 2011 in Medea city in M’sallah locality. After the harvest, the plant material was cleaned with tap water to clear it of fragments of soil, then it was left to dry away from direct sunlight, at ambient temperature and in open air. |
| Two approved varieties of potato, certified and widely cultivated in Algeria, Spunta and Kondor were collected and retained for in vivo study. Seed tubers were supplied by the National Center of Control and Certification of seeds and seedlings (C.N.C.C) of EL Harrach, Algeria. |
| Fungal isolates: Two purified fungal isolates of Phytophthora infestans, identified respectively as A1 and A2, were selected for this study. The latter was taken from potato producing areas: El abadia of Ain defla city and Bourkika of Tipaza city. They were maintained by transplanting on pea- agar medium and incubated at 18°C during 20 days [3]. |
| Preparation of rosemary aqueous extract: The aqueous extract was obtained by decoction of 100 g from dried plants in 1 L of distilled water and heated in the autoclave at 100°C for 30 minutes in well closed vials to avoid contamination. |
| The extract was filtered using Whatman’s sterile filter paper in the laboratory. The obtained filtrate was collected in sterile glass vials hermetically closed and stored in a refrigerator at 4°C until its use in the following dilutions: 5, 10, 20 and 100% [4,5]. |
| Potato varieties cultivation: Pre-germinated potato tubers were planted in pots previously prepared (at a rate of one tuber per pot) and depth of 4 to 5 cm, the substrate constituted a mixture of 2/3 of unused soil and 1/3 of peat [6]. |
| Planting was done in 12 pots among which 6 were reserved as controls. The planting was replicated thrice for each variety. Aqueous extract were applied to the soil in 6 pots, to field capacity, every three days at a dilution of 20% from date of planting to pre-flowering. The control experiment was irrigated with clean tap water. |
| In vitro study: This part of study was based on inhibition of mycelial growth, sporulation and germination of both isolates of P. infestans. |
| Mycelial growth inhibition: Four concentrations: 5%, 10%, 20% and 100% of R. officinalis aqueous extract were treatments used for this study, and correspond respectively to treatments D1, D2, D3 and D4.Mycelial growth inhibition was based on direct contact method, described by Mishra and Dubey [7].The microbiological procedures and the minimum inhibitory concentration (MIC) of the aqueous extract were determined according to Paranagama et al. [8] method For each treatment, 5 ml of plant extract was poured into Petri dishes of the same diameter (90 mm) using micropipettes. The Pea- agar medium was maintained in surfusion (45°C) then poured into Petri dishes containing aqueous extract. The latter were slightly shaken to homogenize the medium. Plant aqueous extract in the control experiment was substituted by sterile distilled water. Treatments were replicated five times for each P. infestans isolate. |
| Using sterile Pasteur pipettes, a disk of 50 mm in diameter of inoculum, for each isolate was taken and inoculated at the center of Petri dishes. Incubation of plated dishes in the hot air oven was done at a temperature of 18°C to evaluate the mycelial growth, which was daily observed for a period of 15 days. Readings were taken by calculating the average of two diameters measured on two perpendicular axes drawn on the reverse side of the plated petri dishes. |
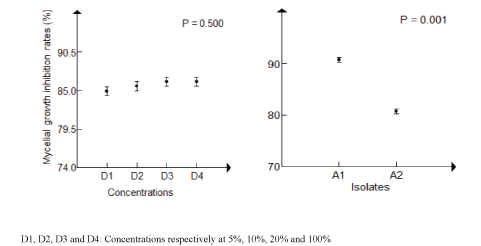
| The minimum inhibitory concentration of mycelial growth (MIC) was determined for each isolate. |
| Mycelial growth inhibition rate was determined for each P. infestans isolate according to the formula described by Rollan et al. in Ibarra- Medina et al. [9]. |
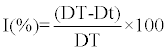 |
| Where I is a percentage Inhibition rate of mycelial growth of P.infestans isolate, |
| DT is a Mycelial growth (mm) of P. infestans isolates in control and |
| Dt is a Mycelial growth (mm) of P. infestans isolates developed in the medium in the presence of R. officinalis aqueous extract. |
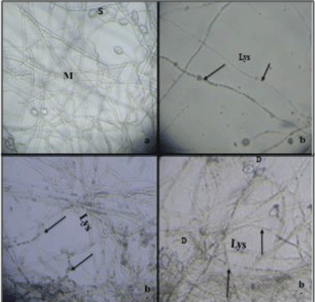
| Antifungal activity of rosemary aqueous extract on morphology of P. infestans isolates: In evaluating the effects of rosemary aqueous extract on the phyto pathogenic isolates, a morphological description was done after 15 days of incubation, by direct observation of the treated cultures and controls of A1 and A2 of P. infestans isolates under photonic microscope at magnification (X125). |
| Sporulation and germination inhibition: After incubation for 21 days, at 18°C, each plated petri dish was collected, and 15 ml of sterile distilled water poured in, and then scraped with sterile Pasteur pipette to recover separately sporangial suspensions in sterilized test tubes. These were agitated using an agitator of tubes vortex. The sporangial suspensions prepared for A1 and A2 isolates were observed to determine the concentration of spores using a hemacytometer under optical microscope. Five repetitions were carried out for each fungal isolate, and each concentration to calculate sporulation inhibition rates according to the formula of Hibar et al. [10]. |
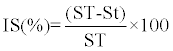 |
| Where IS is a percentage Inhibition rate of P. infestans sporulation, |
| ST is a concentration in sporangia of control P. infestans isolates (sporangia.ml-1) and |
| St is a concentration in sporangia of P. infestans isolates developed in the medium in the presence of R. officinalis aqueous extract (sporangia. ml-1). |
| Parallel, germination inhibition rate (IG%) was calculated for each isolate, according to the formula described by Hill and Nelson [11]. |
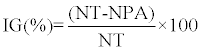 |
| Where IG is a percentage germination inhibition rate of P. infestans strain, |
| NT is a concentration of P. infestans sporangia germinated in control (sporangia.ml-1) and |
| NPA is a concentration of P. infestans sporangia developed in medium in presence of R. officinalis aqueous extract (sporangia.ml-1). |
| Survivability of treated P. infestans isolate: To evaluate the fungistatic and fungicidal effects of R. officinalis aqueous extract, in vitro and in vivo survivability of P. infestans isolates previously treated were monitored respectively on PPA medium and on leaf disks of Spunta potato’s cultivar. |
| In vitro survivability study was based on the technique modified by Mahanta et al. [12].The test was based on the resumption or the absence of mycelial growth on the isolates inhibited by R. officinalis aqueous extract. Explants were transplanted on fresh Pea- agar medium under conditions of incubation previously mentioned. Four explants of each P. infestans isolate were transferred to Petri dishes, with four replicates. Treatments were administered and observations made in comparison with the control. Readings were taken daily, for 7 days. |
| The lethal inhibitory concentrations (CIL) were estimated at the end of the experiment. The CIL was determined from the smallest concentration for which no mycelial growth and no resumption of the explant was observed on the PPA medium in the term of 7 days of incubation [8]. |
| Besides, the in vivo survivability of P. infestans isolates beforehand treated with the plant extract at different concentrations was realized according to the method of Klarfeld et al. [13]. |
| Healthy detached Spunta potato leaves having a diameter greater or equal to 50 mm were chosen and collected from healthy plants in Tipaza city. The leaves were cut to uniform disks using a punch, they were washed with clean tap water then disinfected in 2% of Sodium hypochlorite solution for 3 minutes, then rinsed in 3 changes of sterile distilled water. |
| Sterile filter paper moistened with sterile distilled water was deposited in transparent plastic and sterile boxes, a plastic mesh was also placed in, then 5 potato leaf disks were placed in the box, and the explants of isolates were introduced. Previously treated leaves along with the controls were also observed. |
| Incidence of the disease was defined by the number of leaf disks presenting typical symptoms of mildew, while disease severity was represented by expression of the symptoms in terms of percentage of surface infected by the mildew. Disease reduction rate was calculated using the formula proposed by Hill and Nelson [11]. |
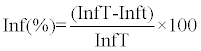 |
| Where Inf is a percentage infection rate of detached potato leaf disks, |
| Inf T is a% infection rate of positive controls detached potato leaf disks and |
| Inf t is a% infection rate of detached potato leaf disks treated by aqueous extract. |
| In vivo antifungal potential of R. officinalis aqueous extract: In vivo antifungal potential evaluation was done by the application of potato leaf disks with treatments in vivo as well as the leaf disks controls inoculated by A1 and A2 of P. infestans isolates. Various modes of treatment were used for this study: |
| • Preventive application through spraying potato leaf disks with rosemary aqueous extract at concentration of 20% for few minutes. 24 hours after the treatment, 100 μl of sporangial suspension of 105 sporangia.ml-1 were deposited by means of a micropipette on the lower surface of potato leaf disk at 5 replications per fungal isolate. |
| • Curative application through the inoculation of potato leaf disks by depositing 100 μl of sporangial suspension on the lower leaf surface, then after 24 hours, application of droplets of 50 μl crude aqueous extract of R. officinalis at 20% concentration. |
| • Disks of detached potato leaves earlier treated with R. officinalis aqueous extract diluted at 20% were inoculated with 100 μl of sporangial suspension of P. infestans and incubated at 18°C for 10 days in the sterile transparent boxes. |
| The frequency of attacks was estimated two to four days later. |
| Both negative and positive controls were observed. Negative control, in which the disks of detached potato leaves were treated with sterile distilled water, and positive controls, where the detached leaf discs were inoculated with A1 and A2 of P. infestans isolates [14,15]. In vivo antifungal potential evaluation of R. officinalis aqueous extract on P. infestans isolates was done using the following parameters. |
| Period of appearance of the symptoms: It is the necessary time for the appearance of the infection by the phyto pathogenic agent on the inoculated foliar tissue. |
| Reduction of late blight disease: The reduction of the disease or (%DR) was translated by the product of the incidence of the disease (number of infected leaf disks) by the scale attributed to the infected foliar surface. It is determined by the formula proposed by Hill and Nelson [11]. |
| RM (%)= CIP- CIPE/ CIP x 100 |
| Where CIP is a coefficient of infection of controls (detached potato leaves inoculated with P. infestans isolates.), |
| CIPE is a coefficient of infection of treated detached potato leaves inoculated with the phyto pathogenic isolates. |
| Inhibition of the sporulation: After 10 days of incubation, the infected disks of detached potato leaves were carefully dipped into sterile tubes containing 10 ml of sterile distilled water then subjected to agitation by means of an agitator of tubes vortex to release the sporangia produced. The content of each tube was observed to determine the concentration of spores by means of a Hemacytometer under optical microscope at magnification (X125). |
| Sporangial production inhibition rate or IPC was calculated using the formula proposed by Hill and Nelson [11]. |
| IPC (%)= NCP- NCPE/ NCP x 100 |
| Where NCP is a number of sporangia produced on surface of detached potato’s leaf disk inoculated by P. infestans isolates, |
| NCPE is a number of sporangia produced on surface of detached potato leaf disk treated with R. officinalis aqueous extract and inoculated with P. infestans isolates. |
| Statistical analysis: Data obtained was analyzed using Analysis of variance, ANOVA SYSTAT vers.7, variance calculated using the GLM (Generalized Linear Model), the differences were considered significant for P < 0.05. |
| Results and Discussion |
| In vitro antifungal potential |
| Evaluation of mycelial growth inhibition: The analysis of variance of mycelial growth inhibition showed statistically significant differences between the two isolates, but no significant difference between the various R. officinalis aqueous extract studied at different concentrations (Table 1). In GLM, the latter exceeded 85% for 5% concentration to evolve slightly to 20% concentration where, it was more pronounced on A1 isolate. Therefore, 5% concentration represents the minimum inhibitory concentration (CMI) of R. officinalis aqueous extract (Figures 1 and 2). |
| Antifungal effects of R. officinalis aqueous extract on P. infestans isolates: The inhibition of the mycelial growth of P. infestans isolate could have resulted from the effect of the extract causing lysis and vesiculation of the mycelium, as well as the deformation of sporangium and the digestion of their contents. These morphological modifications were also observed from the lowest concentration of this tested plant extract (Figure 3). |
| Sporulation and germination inhibition of P. infestans isolates: The sporulation, as well as the germination of the studied isolates were affected by aqueous extract of R. officinalis at 5%, concentration, where 100% inhibition was observed. |
| Effect of Rosemary aqueous extract on the survivability of P. infestans isolates: Concerning the in vitro study, the isolate A1 was more sensitive to the treatment and, the inhibition evolved with aqueous extract tested at different concentrations. Also, inhibition was observed in vivo for both isolates and four concentrations of the R. officinalis aqueous extract. However, the lethal inhibitory concentration (CIL) of the isolate A1 was higher than that of the A2 isolate. |
| In vivo antifungal potential |
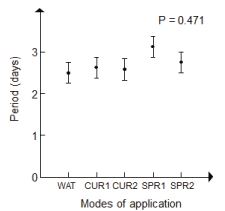
| Determination of the symptoms appearance period: The analysis of variance of the symptoms appearance period did not show significant differences between the treatments application rates, potato varieties, P. infestans isolates and R. officinalis aqueous extract (Table 2). |
| Variability in the period of symptoms appearance was observed among the treatments. It was more in crude than in diluted extracts. However, it was approximately similar for both concentrations of aqueous extract applied with respect to the curative rate (2.6 days). |
| On the other hand, the periods of symptoms appearance by activity of both isolates of P. infestans showed that A1 isolate reproduced the symptoms more quickly than A2 isolate for various treatment application rates. |
| The symptoms appearance period extended till the 3rd day. The shortest period was marked for the preventive mode by watering while the mode of spraying with the crude aqueous extract showed the longest period. Therefore, the classification of the various application rates was established in the following decreasing order: preventive by spraying with the crude extract (SPR1: 3 days) and, in diluted extract to 20% (SPR2: 2.8 days), curative by use of crude and diluted extracts (CUR1, CUR2: 2.7 days), preventive by watering (WAT: 2.6 days) (Figure 4). |
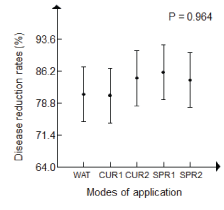
| Antifungal potential of rosemary aqueous extract on disease reduction: The analysis of variance of disease reduction rates did not show significant difference between the application rates, on both isolates of P. infestans, concentrations of rosemary aqueous extract applied for all application rates and potato varieties for the preventive mode by spraying and watering (Table 3). |
| However, a significant difference was noticed between potato’s varieties according to the curative application mode (F=10.662, P=0.031) (Table 3). |
| In GLM, the highest disease reduction was observed for the preventive mode in treatment with the crude extract (SPR1: 86.2%), while the lowest rate was observed for the preventive treatment by watering and curative with crude extract (WAT, CUR1: 81%). |
| Therefore, the classification of application rates was established in decreasing order. |
| • Preventive mode by spraying potato leaf disks with crude aqueous extract (SPR1: 86.2%) |
| • Preventive mode by spraying potato leaf disks with diluted aqueous extract at 20% and curative mode with diluted aqueous extract at 20% (SPR2, CUR2: 84%) |
| • Preventive mode of watering and curative mode with crude rosemary aqueous extract (WAT, CUR1: 81%). |
| On the other hand, the disease reduction rates registered for all treatment application modes were (up to 70%) on both varieties, but the Spunta variety showed a more significant disease reduction for preventive mode treatment by watering (85%) and curative mode (90%) while it was more marked on kondor variety for the preventive mode by spraying (88%) (Figure 5). |
| Besides, it was important also for both isolates of P. infestans exceeding 75% for the various treatment application rates. On the other hand, the reduction affected much more A2 isolate for treatment application rates by spraying (86%), watering and curative (83%) (Figure 5). |
| This also confirms that the reduction of the disease was slightly higher in application of the crude treatments than with treatments diluted at 20% for both modes of application: preventive by spraying and curative. |
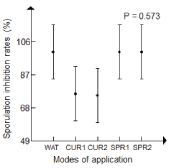
| Antifungal potential of R. officinalis aqueous extract on sporulation inhibition of P. infestans: The variance analysis of sporulation inhibition rates did not show significant difference between treatment application modes, potato’s varieties, P. infestans isolates and, R. officinalis aqueous extract concentrations (Table 4). |
| In GLM, all the sporulation inhibition rates registered showed antifungal effect against P. infestans sporulation (rate exceeding 75% and reaching 100%) (Figure 6). |
| • Classification of treatments application rates was established in the following decreasing order. |
| • Watering by the crude aqueous extract and spraying with crude and diluted at 20% of rosemary aqueous extract (100%). |
| • Curative mode with a crude and diluted at (20%) of rosemary aqueous extract (77%). |
| The inhibition of sporulation was recorded on both varieties in curative application. Spunta variety showed higher inhibition (over 85%) than Kondor (bordering 55%). |
| The latter was variable on both isolates of Phytophtora infestans. Sporulation of A1 isolate was higher than A2 isolate for curative mode (over 96%), while the rate registered for A2 isolate borders 45%. |
| On the other hand, a slight variation of sporulation inhibition rates was noticed between both concentrations of aqueous extract applied for the curative mode (77%) for the crude extract and (68%) for the diluted extract at concentration of 20%. |
| It is very important to indicate that the treatments used in preventive mode by spraying and watering showed a complete inhibition of the sporulation (100%) on P. infestans isolates and potato’s varieties. |
| Discussion |
| Plants are able to produce various compounds. Besides the classic primary metabolites, they synthetize and accumulate secondary metabolites which the physiological function is not always obvious but represents a wide range of exploitable molecules in agriculture within the framework of phyto-protection. |
| R. officinalis antibacterial and antifungal activities can be summarized by the oil composition of these extracts [16]. The study revealed the phenolic compounds such as the terpenes, which include borneol, camphore, 1,8 cineole, pinene camphone, verbenonone and bornyl acetate [17]. |
| This study as well as previous reports confirms the efficiency of certain extracts of plants in the control of potato mildew [18]. |
| Several authors have also asserted that R. officinalis aqueous extract has a powerful antioxidant activity, associated with the presence of several di-terpenes phenolic as the carnosique acid, carnosol, rosmanol, rosmariquinone and rosmaridiphenol [19]. |
| Besides, some reported that a number of compounds contained in the extracts of R. officinalis revealed antibacterial and antifungal properties [20,21]. |
| So, the antifungal activity of Rosmarinus officinalis L. extracts was estimated against the fungal infections of wheat. Their inhibitory effect on the mycelial growth asserted that their use in low concentrations could have a significant potential for the biological control of phytopathogen fungi such as Alternaria alternata, Botrytis cinerea and Fusarium oxysporum [22]. |
| On the other hand, Goussous et al. [23] reported important in vitro inhibitory effects of the crude extracts of various concentrations of R. officinalis against Alternaria solani. In addition, the microscopic observations of the fungal isolates treated by the extracts of R. officinalis revealed structural modifications leading to the lysis of the mycelium and, the digestion of sporangia contents, there by confirming the fungicidal effect of the plant extract. It is the toxic effect of its components on the structure and the physiology of the cellular membrane that is responsible for the antifungal effect [24,25]. In this sense, Omidbeygi et al. [26] suggested that the components of essential oil and extracts of plants cross cell wall and interact with enzymes and proteins, it is the production of a flux of protons towards the exterior cell which incites cellular changes and after all, the death of the microorganism. Cristani et al. [27] suggested that the antimicrobial activity is connected to the capacity of terpenes to affect not only the permeability but also the other functions of cell wall. |
| Our results revealed that all the extracts of R. officinalis even in the lowest concentration (5%), were excellent inhibitors of P. infestans sporulation and germination. Their inhibition rates were as high as 100%. This could have been caused by the deformation of sporangia and the lysis of the contents by the bioactive molecules contained in this aqueous extract. |
| It has further proved that the extracts of plants are rich in phenols also showed, an inhibitory activity particularly raised against the sporulation of fungi [28]. |
| Duru et al. [29] confirmed that the antifungal effects of several extracts of plants seem to have a correlation with the incomplete development of conidiophores and morphological modifications of Aspergillus fumigatus. |
| In the same context, Blaeser and Steiner [30] showed that the extracts of various plants prevent the germination and affect the release and the mobility of P. infestans zoospores, in agreement with this study. |
| In vitro and in vivo survivability of P. infestans isolates showed a fungistatic effect of R. officinalis with low concentrations and a fungicidal effect at increasing concentrations. The latter was able to prevent completely the appearance of the late blight symptoms on leaf disk of potato. The CIL value allowed us to know from which concentration the extract of the rosemary becomes fungicidal. Based on the scale of Koba et al and Webster et al. [31,32], we can deduce that R. officinalis extract has an interesting inhibitory power for A1 isolate because the A2 CIL exceeds 70%. |
| These results could be connected to the phytochemical composition of the plant. They suggest that it contains molecules with fungicidal activity. This hypothesis coincides with several studies reported by the bibliography. Indeed, Banso et al. [33] revealed fungistatic antifungal substances in the extracts of plants in low concentrations but, which become fungicidal in higher concentrations. |
| Conclusion |
| Results from this study as certain that aqueous extract of R. officinalis powder was an excellent inhibitor of P. infestans mycelial growth, sporulation and germination in the lowest dilution (5%). This could have resulted from the action of bioactive molecules contained in the rosemary aqueous extract, that causes the lysis of the mycelia and sporangia of the fungus. The fungicidal effects increased with increasing concentrations of this aqueous extract. |
| On the other hand, the aqueous extract of R. officinalis led to a reduction of the disease on leaf disk of potato. Important disease reduction rates (over 70%) were registered in both varieties and for both isolates of P. infestans exceeding 75%, while Spunta variety showed a more important reduction for treatment preventive application modes by watering (85%) and for the curative mode (90%). Also, the A2 isolate was greatly inhibited by treatments application rates of spraying (86%), watering and curative (83%). The sporulation inhibition was very pronounced in vivo (75% and 100%) and as the rates of preventive treatments made by spraying and watering. This present work thus confirms the bio-fungicidal potentialities of aqueous extract of R. officinalis on P. infestans isolates with the aim of its use in the bio control of late blight potato. |
| Acknowledgements |
| Authors are very grateful to National Plant Protection Institute (INPV) for providing necessary facilities. |
| References |
References
- value="1" id="Reference_Titile_Link">Andrivon D, Lebreton L (1997)Mildiou de la pomme de terre, ou en sommes-nous après 150 ans.Phytoma 494: 24-27.
- value="2" id="Reference_Titile_Link">Rashid A, Ahmad I Iram S, Mirza JI, Rauf CA (2004) Efficiency of Different Neem (AzadirachtaindicaA. Juss) Products against Various Life Stages of Phytophthorainfestans (Mont.) de BaryPak J Bot 36:881-886.
- value="3" id="Reference_Titile_Link">Gallegly ME, Galindo J (1958) Mating types and oospores of Phytophthorainfestans in nature in Mexico. Phytopathology48: 274-277.
- value="4" id="Reference_Titile_Link">Grainge M, Ahmed S (1988) Handbook of plant with pest control properties. Wiley, New York 2nd edition 470.
- value="5" id="Reference_Titile_Link">Krebs H, Dornand B, Forrer HR (2006) Fight against blight of potato with herbal preparations. Swiss magazine Agric 38:203-207.
- value="6" id="Reference_Titile_Link">Compobello EWA, Drenth HH, Leifrink RS(2002) Professional culture of Potato, Plantation, 2nd edition, NIVVA, Dutch Institute for the promotion of markets for agricultural products 22.
- value="7" id="Reference_Titile_Link">Mishra AK, Dubey NK (1994) Evaluation of some essential oils for their toxicity against fungi causing stored deterioration of food commodities. Applied and environmental microbiology 60:1101-1105.
- value="8" id="Reference_Titile_Link">Paranagama PA, Abeysekera KHT, Abeywickrama K, Nugaliyadde L (2003) Fungicidal and anti-aflatoxigenic effects of the essential oil of Cymbopogoncitratus(DC.) Stapf. (lemon grass) against AspergillusflavusLink. isolated from stored rice. Letter in Applied Microbiology 37:86 - 90.
- value="9" id="Reference_Titile_Link">Ibarra-Medina VA, Ferrera-Cerrato R, Alarcón A, Lara-Hernández ME, Valdez-Carrasco JM (2010) Isolation and screening of Trichodermastrains antagonistic to Sclerotiniasclerotiorum and Sclerotinia minor.Rev MexMic 31:53-63.
- value="10" id="Reference_Titile_Link">Hibar K, Daami-Remadi M, Khiareddine H, MahjoubMEl (2005) In vitro and in vivo inhibitor effect of Trichodermaharzianum against Fusariumoxysporumf.sp. radicis-lycopersici. BiotechnolAgronSoc Environ9:163-171.
- value="11" id="Reference_Titile_Link">Hill JP, Nelson RR (1983) Genetic control of two parasitic fitness attribuates of Helminthosporiummaydis race T Phytopathology 73: 455-457.
- value="12" id="Reference_Titile_Link">Mahanta JJ, Chutia M, Bordoi M, Pathak MG, Adhikary RK, et al.(2007) CymbopogoncitratusL. essential oil as a potential antifungal agent against key weed moulds of Pleurotusspp. Spawns. Flavour Fragrance Journal 22: 525-530
- value="13" id="Reference_Titile_Link">Klarfeld S, Rubin AE, Cohen Y(2009) Pathogenic Fitness of Oosporic Progeny Isolates of Phytophthorainfestanson Late-Blight-Resistant Tomato Lines. Plant Disease 93: 947-953.
- value="14" id="Reference_Titile_Link">Fontem DA, Olanya OM, Tsopmbeng GR, Owona MAP (2005) Pathogenicity and metalaxyl sensitivity of Phytophthorainfestans isolates obtained from garden huckle berry, potato and tomato in Cameroon. Crop Protection Journal 24:449-456.
- value="15" id="Reference_Titile_Link">Abd- El- Khair H, Haggag WM (2007) Application of Some Egyptian Medicinal Plant Extracts Against Potato Late and Early Blights. Res JAgricBiolSci3:166-175.
- value="16" id="Reference_Titile_Link">Pinto E, Vaz CP, Salgueiro L, Goncalves MJ, Costa-de-Oliveira SC, et al.(2006) Antifungal activity of the essential oil of Thymus pulegioides on Candida, Aspergillus and dermatophyte species. Journal of Medical Microbiology 55: 1367-1373.
- value="17" id="Reference_Titile_Link">Makhloufi A, Moussaoui A , Lazouni HA, Hasnat N, Abdelouahid DE (2011) Antifungal activity of essential oil of Rosmarinusofficinalis L. and its impact on the conservation of a local variety of dates during storage. Medicinal Plants- International Journal of Phytomedicines and Related Industries 3:129-134.
- value="18" id="Reference_Titile_Link">Ashrafuzzaman MH, Khan AR, Howlide AR (1990) In vitro effect of lemon grass oil and crude extracts of some higher plants on Rhizoctoniasolani. Bangladesh. JPlant Pathol 6: L 17-18.
- value="19" id="Reference_Titile_Link">Georgantelis D, Ambrosiadis I, Katikou P, Blekas G, Georgakis SA (2007) Effect of rosemaryextract, chitosan and a-tocopherol on microbiologicalparameters and lipidoxidation of freshporksausagesstoredat 4°C. MeatSci76:172-181.
- value="20" id="Reference_Titile_Link">Del Campo J, Amiot MJ, Nguyen C (2000) Antimicrobial effect of rosemary extracts. Journal of Food Protection, 63: 1359-1368.
- value="21" id="Reference_Titile_Link">Djenane D, Sánchez-Escalante A, Bel-trán JA, Roncalés P (2002) Ability of a-tocopherol, taurine and rosemary, in combination with vitamin C, to increase the oxidative stability of beef steaks packaged in modified atmosphere. Food Chemistry 76: 407-415.
- value="22" id="Reference_Titile_Link">Centeno S, Calvo MA, Adelantado C, Figueroa S (2010) Antifungal activity of extracts of Rosmarinusofficinalis and Thymus vulgaris against Aspergillusflavus and A. ochraceus. Pakistan. Journal of Biological Sciences 13: 452-455.
- value="23" id="Reference_Titile_Link">Goussous SJ, Abu-El-Samen FM, Mas’adb IS, Tahhan RA (2013) In vitro inhibitory effects of rosemary and sage extracts on mycelial growth and sclerotial formation and germination of Sclerotiniasclerotiorum. Archives of Phytopathology and Plant Protection 46: 1745-1757.
- value="24" id="Reference_Titile_Link">Bouchra C, Achouri M, Hassani LMI, Hmamouchi M (2003) Chemical composition and anti-fungal activity of essential oils of seven Moroccan Labiatae against Botrytis cinereaPers: FrJEthnopharmacol89:165-169.
- value="25" id="Reference_Titile_Link">Yoshimura H, Sawai Y, Tamotsu S, Sakai A (2011)1,8-cineole inhibits both proliferation and elongation of BY-2 cultured tobacco cells. Journal of Chemical Ecology 37: 320-328.
- value="26" id="Reference_Titile_Link">Omidbeygi M, Barzegar M, Hamidi Z, Naghdibadi H (2007) Antifungal activity of thyme, summer savory and clove essential oils against Aspergillusflavus in liquid medium and tomato paste. Food control 18: 1518-1523.
- value="27" id="Reference_Titile_Link">Cristani M, Arrigo MD, Mandalari G, Castelli F, Sarpietro MG,et al.(2007) Interaction of four monoterpenes contained in essential oils with models membranes: application for their antibacterial activity. JAgricFood Chem55: 6300-6308.
- value="28" id="Reference_Titile_Link">Inouye S, Watanabe M, Nishiyama Y, Takeo K, Akao M, et al. (1998) Anti-sporulating and respiration-inhibitory effects of essential oils on filamentous fungi. Mycoses 41:403-410.
- value="29" id="Reference_Titile_Link">Duru ME, Cakir A, Kordali S, Zengin H, Harmandar M, et al.(2003) Chemical composition and anti-fungal properties of essential oils of three Pistaciaspecies. Fitoterapia74:170-176.
- value="30" id="Reference_Titile_Link">Blaeser P, Steiner U(1999) Antifungal activity of plant pathology extracts against potato late blight (Phytophthorainfestans), In: Lyr H, Russel PE, Dehne HW, Sisler HD (eds.). Book Modern Fungicides and Antifungal Compounds II, Thuringia, Germany 491-499.
- value="31" id="Reference_Titile_Link">Koba K, Sanda K, Raynaud C, Nenonene Y A, Millet J, et al. (2004) Activités antimicrobiennes d’huiles essentielles de trois Cymbopogonsp. africains vis-à-vis de germes pathogènes d’animaux de compagnie. Annales de Médecine Vétérinaire 148: 202-206.
- value="32" id="Reference_Titile_Link">Webster D, Taschereau P, Belland RJ, Rennie RP (2008) Antifungal activity of medicinal plant extracts; preliminary screening studies. Journal of Ethno pharmacology 115: 140-146.
- value="33" id="Reference_Titile_Link">Banso ASO, Adeyemo, Jeremiah P (1999) Antimicrobial properties of Vernoniaamygdalinaextract. J Appl Sci Manage 3: 9-11.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
 |
 |
 |
| Figure 4 | Figure 5 | Figure 6 |
Relevant Topics
- Agricultural science
- Agronomy
- Climate impact on crops
- Crop Productivity
- Crop Sciences
- Crop Technology
- Field Crops Research
- Hybrid Seed Technology
- Irrigation Technology
- Organic Cover Crops
- Organic Crops
- Pest Management
- Plant Genetics
- Plant Breeding
- Plant Nutrition
- Seed Production
- Seed Science and Technology
- Soil Fertility
- Weed Control
Recommended Journals
Article Tools
Article Usage
- Total views: 14155
- [From(publication date):
August-2015 - Apr 17, 2025] - Breakdown by view type
- HTML page views : 9603
- PDF downloads : 4552
Peer Reviewed Journals
Make the best use of Scientific Research and information from our 700 + peer reviewed, Open Access Journals
