Improvement of Visceral Adiposity and Intracellular Fluid in Weight Loss Participants Taking Anti-Diabetic Medication
Received: 23-Jul-2021 / Accepted Date: 18-Nov-2021 / Published Date: 25-Nov-2021
Abstract
Background: Obesity and diabetes are a worldwide epidemic, and their complex physiological interactions contribute to an increase in morbidity and mortality. Previous studies have shown that the use of diabetes medications could affect the response to weight loss interventions. However, the results are still scarce and contradictory. Therefore, the aim of this study was to investigate whether body composition improvements of participants in a comprehensive weight loss program focused on reducing visceral adiposity were affected by prescription diabetes medications.
Methods: This retrospective study analyzed from 2,200 subjects who completed a commercially available expert supervised program including ~6 weeks of a structured, nutritionally complete low/very low-calorie diet followed by a ~3-week structured transition back to a normal dietary intake. Overall, 33% of the subjects reported taking at least one prescription diabetes medication. Endpoints assessed included weight, body mass index, body fat percentage, intracellular fluid, and visceral adiposity.
Results: Our data show participants in both groups (+/- diabetes drugs) achieved clinically relevant and statistically significant improvements in standard measures of weight loss and outcomes known to be directly related to inflammation and diabetes (intracellular fluid, visceral adiposity).
Conclusions: A non-pharmacologic, non-surgical low/very low calorie-based weight loss and metabolic health program is a therapeutic approach capable of producing clinically significant improvements in body composition and physiological outcomes, including those linked to diabetes, cardiovascular disease, and inflammation. Additionally, this approach is equally effective for adults taking prescription diabetes medications, as well as for participants who are not.
Keywords: Body composition; Diabetes; Intracellular Fluid; Very Low-Calorie Diet; Visceral Adiposity
Introduction
According to the United States Center for Disease Control’s (CDC) most recent data of 18, approximately 42.5% of adults the United States are obese [1], and statistics show over 122 million Americans are currently diagnosed with type 2 diabetes (T2D), insulin resistance, or prediabetes [2]. The presence of obesity increases the risk of a wide range of disorders including T2D and Cardiovascular Disease (CVD), as well as metabolic and inflammatory disturbances [3-6], and it is known that complex physiological interactions contribute to and perpetuate a heightened morbidity and mortality in this population [7]. The interrelated mechanisms have been directly or indirectly linked to development and progression of insulin resistance, hyperglycemia, endothelial dysfunction, as well as increased risk of hypertension, hyperlipidemia, inflammation, and production of adipokines and oxidative stress products [6-9]. Several pharmacological treatments are used for both diabetes and obesity, aiming to control weight gain and hyperglycemia [7-10]. However, the effects on weight gain and weight maintenance vary among the classes of diabetes medications and, in fact, may vary somewhat within each class. In this sense, metformin has been reported as having a small but favorable effect on weight maintenance, while insulin, sulfonylurea, and thiazolidinedione were previously associated with variable weight gain [10-13]. Considering this evidence, in the present study, we asked whether diabetes medications affect the ability of participants to achieve the same level of body composition improvements as participants who were not taking anti-diabetic medication. We assessed changes in body weight, Body Mass Index (BMI), Visceral Adiposity (VA), body fat percentage, and Intracellular Fluid (ICF) to provide a clear picture of outcomes in participants with or without prescription diabetes medication in an effort to identify any advantage or disadvantage. We have found that prescription treatment for diabetes does not negatively affect body weight or BMI reduction of male and female participants in a two-month intensive weight reduction program focused on lowering subcutaneous and visceral adiposity and that both groups achieved similar, meaningful improvements in VA and ICF, which are linked to cardiovascular and metabolic disease risk, inflammation, and overall health.
Methods
Subject and Program Overview
This was a retrospective review of data from 2200 participants of the 20Lighter program (20L, Cheyenne, WY) [14]. This study was conducted with informed consent under a protocol reviewed and approved by a third-party Institutional Review Board (Asentral, Inc. Institutional Review Board approval received on November 1, 2016 for protocol # 2016-443A) and in accordance with the 1964 Declaration of Helsinki and its subsequent amendments. Briefly, 20L is a commercially available, expert supervised 3-phase program included a loading day followed by ~6 weeks of a proprietary structured, nutritionally complete, low calorie/very low calorie diet (LC/VLCD, 510-1000 kcal/ day), and a ~3 week structured customized transition back to a normal dietary intake [15–17]. Participants engaged in once daily home weighins, daily communication with the supervising provider, proprietary vitamin/mineral supplementation, daily journaling, and at least three body composition analyses (initial baseline, ~day [18-22], ~day 36-40, and ~day 60-65) using a bioelectrical impedance device (see below). Participants were encouraged to engage in light physical activity (e.g., walking) but to avoid beginning highly strenuous exercise until they completed the structured LC/VLCD and had begun the third phase (dietary transition period) of the program (weeks 6-9). Management of prescription medications was handled by each participant’s primary care physician (PCP). Clinical trial registration number: NCT04807959Arabia.
Body Composition Analysis: 20L measured body composition via an FDA-cleared Class 2 medical device with Bioelectrical Impedance Analysis (BIA) via bipolar foot electrodes (Tanita Corporation) to monitor participant progress. Endpoints of interest assessed included BMI, visceral adiposity (VA), body fat % and intracellular fluid (ICF). The measure of VA is calculated by a proprietary Tanita Corporation algorithm as a visceral fat rating (VFR, range 1-59 points; where a rating above 12 is considered abnormal). These endpoints were calculated using the proprietary Health Edge Software (Tanita Corporation criteria).
Comparison of Groups & Statistical Analysis
20L measured body composition via an FDA-cleared Class 2 medical device with Bioelectrical Impedance Analysis (BIA) via bipolar foot electrodes (Tanita Corporation) to monitor participant progress [18,19]. Endpoints of interest assessed included BMI, visceral adiposity (VA), body fat % and intracellular fluid (ICF) [20]. The measure of VA is calculated by a proprietary Tanita Corporation algorithm as a visceral fat rating (VFR, range 1-59 points; where a rating above 12 is considered abnormal). These endpoints were calculated using the proprietary Health Edge Software (Tanita Corporation). Baseline demographic and characteristic values are described in Table 1. Fisher’s exact test was used to test significance of categorical data. To assess for significance of each outcome from baseline to 60 days, a Wilcoxon matched-pairs signed rank test was employed. A comparison between the groups (participants taking prescription Diabetes Medication (DIA) and those who were not (NON-DIA)) of change in outcomes from baseline to 60 days was assessed as a percentage of improvement (change/baseline value *100). To assess significance of mean changes between groups, a D’Agostino & Pearson normality test (DAP) was used to show presence of normality in the population of means. If the population of means passed the DAP normality test (parametric), subsequent statistical analysis was done via an unpaired T-Test with Welch’s Correction. If the means failed the DAP Normality Test (nonparametric), subsequent statistical analysis was done via a Mann- Whitney U-Test. Baseline demographic values (age, BMI) are reported as median ± SD. All outcome data is shown as mean ± SEM. In all cases, the statistical significance threshold was p<0.05.
| Age, years Gender male, n (%) BMI |
54.4±4.6 1062 (55.0) 33.9±5.7 |
53.5±9.7 207 (76.7) 36.3±6.2 |
| Prescription Medications by Health Condition, n (%) | ||
| Hypertension | 576 (29.8) | 150 (55.6) |
| Dyslipidemia | 376 (19.5) | 218 (80.7) |
| Diabetes | 0 (0) | 270 (100) |
| Type 1 | 0 (0) | 5 (1.85) |
| Type 2 | 0 (0) | 265 (98.15) |
| Depression | 467 (24.1) | 47 (17.4) |
| Gout | 108 (5.6) | 31 (11.5) |
| Arthritis | 57 (3.0) | 22 (8.1) |
| Other | 343 (17.8) | 81 (30) |
| Comorbidities | ||
| ≥3 | 184 (9.5) | 219 (81.1) |
| 2 | 439 (22.7) | 47 (17.4) |
| 1 | 786 (40.7) | 4 (1.5) |
| 0 | 521 (27) | 0 (0.0) |
| Data presented as median ± SD or number (%). Significant differences between groups include gender, hypertension medication, dyslipidemia medication, other prescription medication, and 1 comorbidity (**** = <0.0001), gout medication and arthritis medication (*** = <0.001), and depression medication (* = <0.05). | ||
Table 1. Baseline characteristics.
Results
Baseline Demographics: Age, BMI, comorbidities, history and prescription medications of 2,200 participants completing a 20Lighter program are presented in Table 1. Of the 2,200 participants, 270 (12.3%) reported taking at least one prescription diabetes medication, an additional 334 (15.2%) reported they declined to begin prescription diabetes medication, or their PCP had indicated they were prediabetic or if body composition and/or HbA1c, and/or blood glucose levels did not improve medication would be prescribed. Comorbidities included dyslipidemia or triglyceridemia, T2D, hypertension, depression, previous treatment for cancer, at least one previous heart attack, fatty liver disease, joint replacement or reconstructive surgery, arthritis, gout, epilepsy, angina, atrial fibrillation, sleep apnea requiring a CPAP machine, among others. Participants taking diabetes medication were slightly younger (median age: 52.1 ± 9.7 DIA, 55.2 ± 4.6, NON-DIA) and had a higher BMI (median BMI: 37.0 ± 6.2 DIA, 35.6 ± 5.7, NONDIA) than those who were not, but the differences were not statistically different. Significant differences in characteristics of the DIA and NON-DIA groups are noted in Table 1.
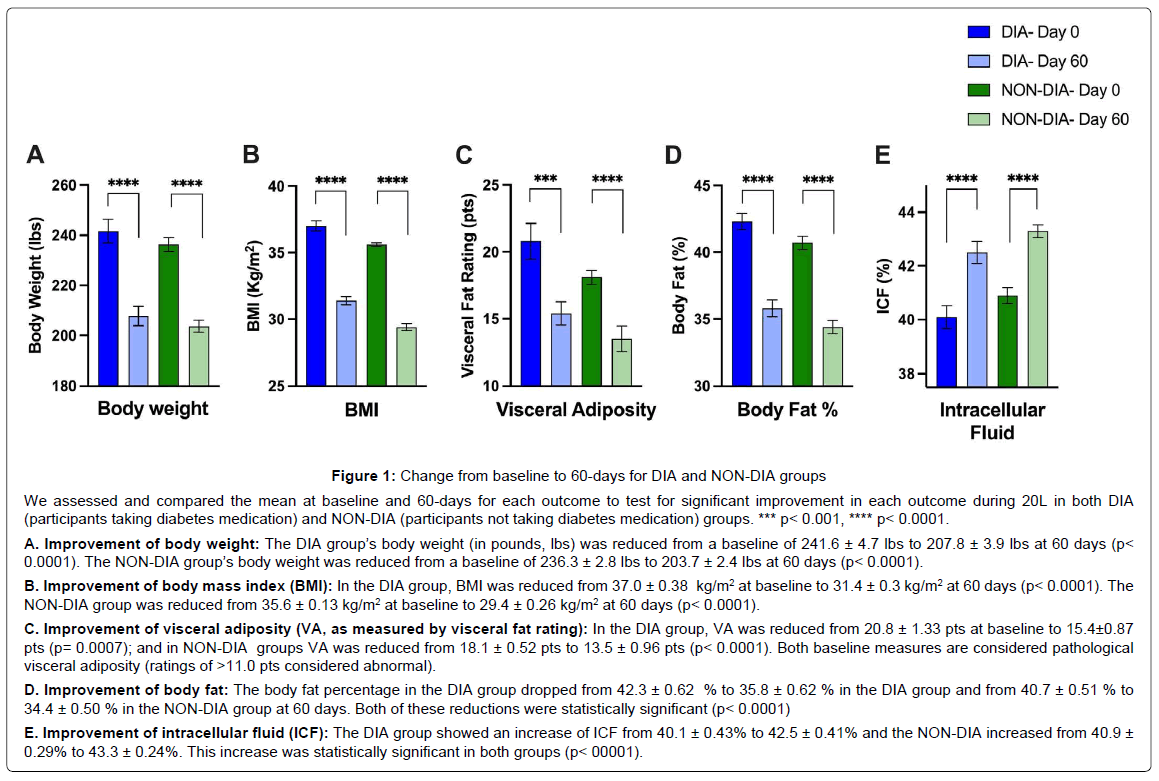
Improvement of Outcomes within DIA & NON-DIA: Groups From baseline to 60 days both DIA and NON-DIA groups showed similar clinically relevant and statistically significant changes in body weight, the most basic weight loss outcome measurement. The DIA group’s body weight was reduced from a mean baseline of 241.6 ± 4.7 lbs to 207.8 ± 3.9 lbs at 60 days (p<0.0001). The NON-DIA group’s body weight was reduced from a mean baseline of 236.3 ± 2.8 lbs to 203.7 ± 2.4 lbs at 60 days (p<0.0001, Figure 1A). Mean BMI, a standardized measure of height and weight, was reduced for both groups with the DIA group 37.0 ± 0.38 kg/m2 at baseline and 31.4 ± 0.3 kg/m2 at 60 days (p<0.0001). The NON-DIA group was reduced from 35.6 ± 0.13 kg/m2 at baseline to 29.4 ± 0.26 kg/m2 at 60 days (p<0.0001), as shown in Figure 1B. As we looked into more complex body composition changes over 60 days, we continued to see significant improvements. As shown in Figure 1C, in DIA group, the VA was reduced from a mean of 20.8 ± 1.33 pts at baseline to 15.4 ± 0.87 pts (p=0.0007); and in NON-DIA groups VA was reduced from a mean of 18.1 ± 0.52 pts to 13.5 ± 0.96 pts (p<0.0001, Figure 1C). The body fat percentage in the DIA group dropped from a mean of 42.3 ± 0.62 % to 35.8 ± 0.62% in the DIA group and from a mean of 40.7 ± 0.51 % to 34.4 ± 0.50 % in the NON-DIA group at 60 days. Both of these reductions were statistically significant (p<0.0001, Figure 1D). More nuanced and less often reported body water percentage represents intracellular fluid20. The DIA group showed an increase of ICF from a mean of 40.1 ± 0.43% to 42.5 ± 0.41% and the NON-DIA increased from a mean of 40.9 ± 0.29% to 43.3 ± 0.24%. This increase was statistically significant in both groups (p<0.0001, Figure 1E).
Figure 1: Showing the test for significant improvement in each outcome during 20L in both DIA.
We assessed and compared the mean at baseline and 60-days for each outcome to test for significant improvement in each outcome during 20L in both DIA (participants taking diabetes medication) and NON-DIA (participants not taking diabetes medication) groups. *** p< 0.001, **** p< 0.0001.
A. Improvement of body weight: The DIA group’s body weight (in pounds, lbs) was reduced from a baseline of 241.6 ± 4.7 lbs to 207.8 ± 3.9 lbs at 60 days (p< 0.0001). The NON-DIA group’s body weight was reduced from a baseline of 236.3 ± 2.8 lbs to 203.7 ± 2.4 lbs at 60 days (p< 0.0001).
B. Improvement of body mass index (BMI): In the DIA group, BMI was reduced from 37.0 ± 0.38 kg/m2 at baseline to 31.4 ± 0.3 kg/m2 at 60 days (p< 0.0001). The NON-DIA group was reduced from 35.6 ± 0.13 kg/m2 at baseline to 29.4 ± 0.26 kg/m2 at 60 days (p< 0.0001).
C. Improvement of visceral adiposity (VA, as measured by visceral fat rating): In the DIA group, VA was reduced from 20.8 ± 1.33 pts at baseline to 15.4±0.87 pts (p= 0.0007); and in NON-DIA groups VA was reduced from 18.1 ± 0.52 pts to 13.5 ± 0.96 pts (p< 0.0001). Both baseline measures are considered pathological visceral adiposity (ratings of >11.0 pts considered abnormal).
D. Improvement of body fat: The body fat percentage in the DIA group dropped from 42.3 ± 0.62 % to 35.8 ± 0.62 % in the DIA group and from 40.7 ± 0.51 % to 34.4 ± 0.50 % in the NON-DIA group at 60 days. Both of these reductions were statistically significant (p< 0.0001)
E. Improvement of intracellular fluid (ICF): The DIA group showed an increase of ICF from 40.1 ± 0.43% to 42.5 ± 0.41% and the NON-DIA increased from 40.9 ± 0.29% to 43.3 ± 0.24%. This increase was statistically significant in both groups (p< 00001).
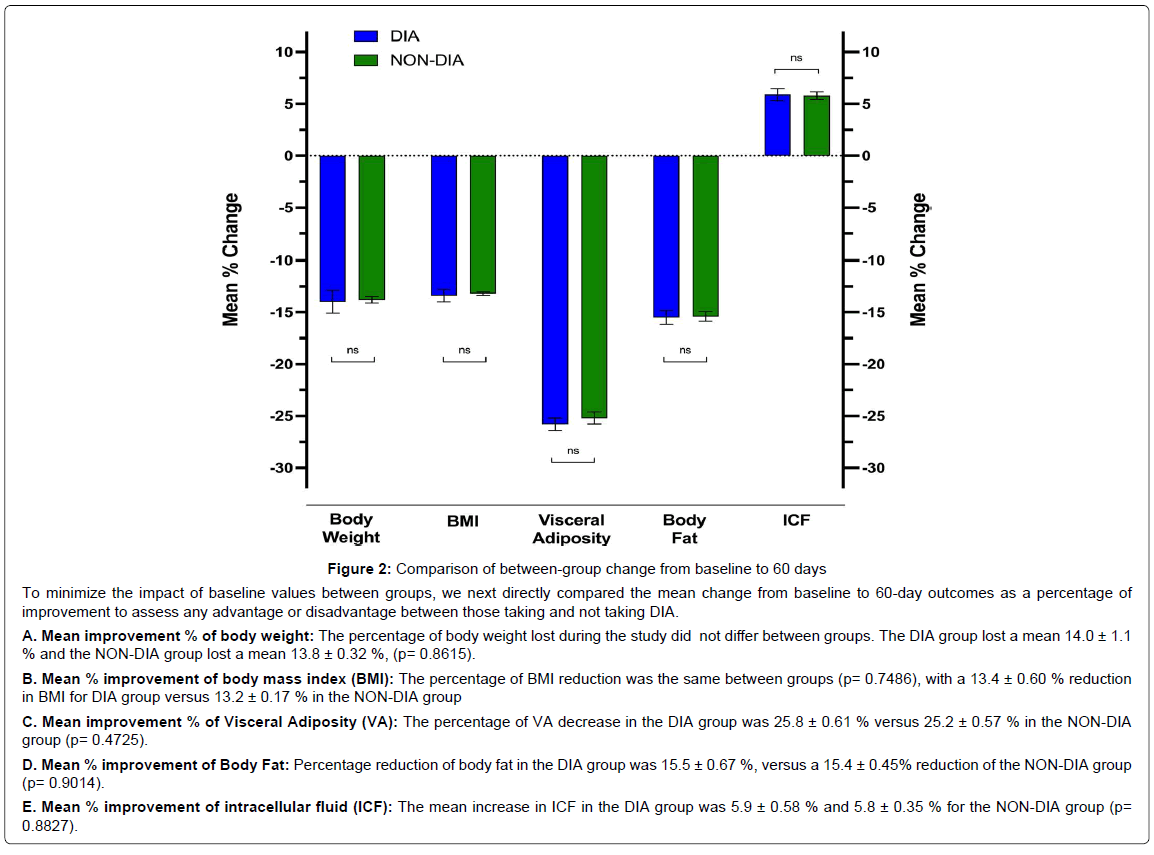
Comparison of DIA and NON-DIA Groups: To compare the improvements between groups to assess for any advantage or disadvantage in either group from baseline to 60 days, we compared the mean percentage change in body weight, BMI, VA, body fat, and ICF (Figure 2). The percentage of body weight lost during the study did not differ between groups. The DIA group lost a mean 14.0 ± 1.1% and the NON-DIA group lost a mean 13.8 ± 0.32%, (p=0.8615). The percentage of BMI reduction was the same between groups (p=0.7486), with a 13.4 ± 0.60% reduction in BMI for DIA group versus 13.2 ± 0.17% in the NON-DIA group. Similar to body weight and BMI, VA (as measured by VFR) and body fat reductions did not differ between groups. The percentage of VA decrease in the DIA group was 25.8 ± 0.61% versus 25.2 ± 0.57% in the NON-DIA group (p=0.4725). Percentage reduction of body fat in the DIA group was 15.5 ± 0.67%, versus a 15.4 ± 0.45% reduction of the NON-DIA group (p=0.9014). Lastly, no statistical significance was found between the groups for percentage improvement in ICF. The mean increase in ICF in the DIA group was 5.9 ± 0.58% and 5.8 ± 0.35.
Figure 2: To minimize the impact of baseline values between groups, we next directly compared the mean change from baseline to 60-day outcomes as a percentage of improvement.
To minimize the impact of baseline values between groups, we next directly compared the mean change from baseline to 60-day outcomes as a percentage of improvement to assess any advantage or disadvantage between those taking and not taking DIA.
A. Mean improvement % of body weight: The percentage of body weight lost during the study did not differ between groups. The DIA group lost a mean 14.0 ± 1.1 % and the NON-DIA group lost a mean 13.8 ± 0.32 %, (p= 0.8615).
B. Mean % improvement of body mass index (BMI): The percentage of BMI reduction was the same between groups (p= 0.7486), with a 13.4 ± 0.60 % reduction in BMI for DIA group versus 13.2 ± 0.17 % in the NON-DIA group
C. Mean improvement % of Visceral Adiposity (VA): The percentage of VA decrease in the DIA group was 25.8 ± 0.61 % versus 25.2 ± 0.57 % in the NON-DIA group (p= 0.4725).
D. Mean % improvement of Body Fat: Percentage reduction of body fat in the DIA group was 15.5 ± 0.67 %, versus a 15.4 ± 0.45% reduction of the NON-DIA group (p= 0.9014).
E. Mean % improvement of intracellular fluid (ICF): The mean increase in ICF in the DIA group was 5.9 ± 0.58 % and 5.8 ± 0.35 % for the NON-DIA group (p= 0.8827).
Discussion
Obesity is more often than not, comorbidity for dyslipidemia [3,21], CVD [5,22] and T2D. We have previously shown that the 20L LC/VLCD-based program is associated with significant and meaningfully improvements of BMI, VA, ICF, and % body fat in participants including those taking prescription hypertension, thyroid replacement, and depression medications14,16,17. Importantly, in a case report this level of reduction in body weight and VA improvement was associated with the cessation of hypertension, lipid-lowering, and T2D medication [15]. Here we investigated how body composition improvements of participants with T2D (the DIA group, defined by prescribed use of medication for T2D) compared to non-diabetic participants. This group started with a higher body weight, BMI, VA and % body fat and lower ICF than other participants. Despite this disadvantage, participants on T2D medication achieved improvements in body weight, BMI, VA, ICF, and % body fat on par with other participants. To our knowledge, this is the first large-scale study assessing VA and ICF changes between weight loss participants taking and not taking T2D medications. T2D is associated with numerous macrovascular and microvascular complications [5-8,22], and as such, effective options to reduce its severity and provide a path towards remission is crucial for reducing risk of morbidity and mortality. Anti-diabetic drugs exert different, class-dependent actions on weight. Although, sulphonylureas and glitazones are associated with weight gain, the more commonly prescribed metformin and dipeptidyl peptidase (DPP)-4 inhibitors have been suggested to slightly aid weight loss [12,13] and no studies have assessed the impact of the different classes of diabetes medication on body fat %, VA, or ICF. We do not know the DIA participants exact breakdown of anti-diabetic therapy, but assuming normal prescribing patterns, metformin is likely be the most commonly used in this group with more than 50% taking it at least as a monotherapy [23]. T2D and obesity are associated with multiple pro-inflammatory pathways that benefit from weight loss interventions [7,10]. The link between systemic inflammation and the development of obesity and T2D is widely accepted [24,25] with VA a major source of inflammatory molecules and markers [4,26]. VA is highly metabolically active, releasing significant amounts of free fatty acids into the general and portal circulation [3]. In addition, VA also accumulates around the vascular wall and heart tissue [26], the primary sites for CVD development. VA produces cytokines and several other bioactive substances involved in inflammatory pathways: tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-6, IL-10, leptin, adiponectin, monocyte chemoattractant protein1 (MCP-1), angiotensinogen, resistin, chemokines, serum amyloid protein, and many others adipokines [3,4,6,27,28]. Furthermore, visceral adipose tissue is associated with infiltrated pro-inflammatory M1 macrophages and immune cells (B cells and T cells) which trigger local and systemic chronic low-grade inflammation, producing more cytokines and chemokines that serve as a pathologic link between obesity, insulin resistance, and diabetes [25,28]. Indeed, there is positive association between VA and pro-inflammatory IL-6, and resistin levels and a negative correlation between VA and the anti-inflammatory adipokine adiponectin in subjects with obesity [28-30]. It is of significant interest to identify weight loss interventions that reduce VA and its endocrine, metabolic, and immunological functions [3,4,24]. VA contributes to several metabolic outcomes, including hyperinsulinemia, hyperglycemia, systemic inflammation, dyslipidemia, hypertension, and non-alcoholic fatty liver disease [4,5,24,26,31]. In addition, VA is associated with a number of cancers [32-34]. The ability to significantly reduce VA and body fat in all participants shows the 20L LC/VLCD-based program reduces factors associated with developing CVD and metabolic syndrome features, independent of the presence of type 2 diabetes and /or use of diabetes medications. In addition to improvements in VA and total adiposity, we also observed an increase in ICF levels. This increase in ICF is linked to a decrease in adiposity, as adipose tissue proportionally contains less water than most other tissue with the exception of bone. It is also tempting tospeculate whether this increase in ICF also is an indication of stabilized osmoregulation and reduction of chronic inflammation. ICF can directly affect cell metabolism and physiological function and it is considered as a major player in osmotic stress balance that is integrally tied to both acute and chronic inflammation [35]. Of note, the amount of intracellular water is directly related to the reduction of the inflammatory state, while the extracellular fluid to a greater inflammatory state [36,37]. Changes in fluid osmolarity can contribute to the initiation and development of both local and systemic disorders, including diabetes, obesity, and CVD [7,35-38]. A vicious pro-inflammatory cycle has also been described, where chronic disease can induce osmotic stress, which in turn leads to further inflammation and chronic disease [35]. Moreover, diseases such as T2D or chronic kidney disease that can cause, and be affected by, changes in fluid osmolarity [39,40] are strongly associated with pro-inflammation cytokine secretion, including TNF-α, IL-1β, IL- 6, IL-8 and, IL [35,37]. Of importance, both T2D and obesity have several pro-inflammatory pathways that are reduced following weight loss intervention [7,10], however more work needs to be done to establish the relationship between ICF, dysfunctional osmoregulation and the subsequent inflammation that drives the pathologies linked to obesity.
Conclusion
In conclusion, this study demonstrates that a safe nonpharmacologic, non-surgical complementary therapeutic approach is capable of producing clinically significant improvements in body composition outcomes (body weight and BMI) and markers associated physiological function, osmotic stress balance, acute and chronic inflammation, and morbidity and mortality (body fat, VA & ICF) in all participants, being equally effective for adults with T2D as it is for those participants.
Acknowledgement
The authors thank Linda Tighe, Maria Lee, Cindy Tervalon, and Krista Curry for their help in study data collection, and Dr. David Bishop-Bailey for assistance with manuscript preparation. The results and views of the current study do not constitute endorsement by the Journal of Diabetes & Clinical Practice.
References
- Fryar CD, Carroll MD, Afful J (2020) Prevalence of overweight, obesity, and severe obesity among adults aged 20 and over: United States, 1960–1962 through 2017–2018. NCHS Health E-Stats. 1:1-6.
- Centers for Disease Control and Prevention (2020). National diabetes statistics report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services. 6:12-15.
- Tchernof A, Després JP (2013) Pathophysiology of human visceral obesity: an update. Physiol rev 93: 359-404.
- Piché ME, Tchernof A, Despres JP (2020) Obesity phenotypes, diabetes, and cardiovascular diseases. J Cir res 126:1477-1500.
- Mathieu P, Poirier P, Pibarot P, Lemieux I, Despre´s JP (2009) Visceral obesity: the link among inflammation, hypertension, and cardiovascular disease. J Hyper 53: 577-84.
- Ellulu MS, Patimah I, Khazai H, Rahmat A, Abed Y (2017) Obesity and inflammation: the linking mechanism and the complications. Arch med sci AMS 13: 851-63.
- Al-Goblan AS, Al-Alfi MA, Khan MZ (2014) Mechanism linking diabetes mellitus and obesity. Diabetes, metabolic syndrome and obesity. J targ ther 7: 587-90.
- DeMarco VG, Aroor AR, Sowers JR (2014) The pathophysiology of hypertension in patients with obesity. Nature Rev Endo 10: 364-76.
- Mathieu P, Poirier P, Pibarot P, Lemieux I (2009) Visceral obesity: the link among inflammation, hypertension, and cardiovascular disease. J Hyper 53: 577-84.
- Bramante CT, Lee CJ, Gudzune KA (2017) Treatment of obesity in patients with diabetes. J Diab Spec 30: 237-43.
- Mitri, J, Hamdy O (2009) Diabetes medications and body weight. J Expert opinion on drug saf 8: 573-84.
- Apovian CM, Okemah J, O’Neil PM (2019) Body weight considerations in the management of type 2 diabetes. J Adv ther 36: 44-58.
- Lau DC, Teoh H (2015) Impact of current and emerging glucose-lowering drugs on body weight in type 2 diabetes. Can j diab 39: S148-S154.
- Dembrowski G, Barnes J (2019) SAT-LB019 Improvement of Visceral Fat and Body Composition Endpoints in Participants on Prescription Thyroid Replacement with a Doctor-Supervised Weight Loss Program. J Endo Soc 3: 10-19.
- Dembrowski GC, Barnes J W (2020) Resolution of Metabolic syndrome with reduction of visceral adipose tissue in a 47 year old patient with Type 2 Diabetes Mellitus. Diabetes and Metabolic Syndrome: J Clin Res and Rev 14: 1001-4.
- Dembrowski GC, Barnes JW (2020) Body composition outcomes and visceral fat reduction in weight loss program participants taking antidepressant medication. J Obes Med 20: 100291.
- Dembrowski GC, Barnes J W (2021) Visceral fat reduction and increase of intracellular fluid in weight loss participants on antihypertension medication. Cardiovasc. Endocrinol. Metab 10: 31-6.
- Fernandes RA, Rosa CS, Buonani C, Oliveira ARD, Freitas Júnior I F (2007) The use of bioelectrical impedance to detect excess visceral and subcutaneous fat. J Pediatr 83: 529-34.
- Unno M, Furusyo, N, Mukae H, Koga T, Eiraku K et al. (2012) The utility of visceral fat level by bioelectrical impedance analysis in the screening of metabolic syndrome-the results of the Kyushu and Okinawa Population Study (KOPS). J atheroscler throm.
- Earthman, C, Traughber D, Dobratz J, Howell W (2007) Bioimpedance spectroscopy for clinical assessment of fluid distribution and body cell mass. Nutr Clin Pract 22: 389-405.
- Jung UJ, Choi MS (2014) Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci 15: 6184-223.
- Petrie JR, Guzik TJ, Touyz RM (2018) Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Canadian J Card 34: 575-84.
- Engler C, Leo M, Pfeifer B, Juchum M, Chen-Koenig D, etal. (2020) Long-term trends in the prescription of antidiabetic drugs: real-world evidence from the Diabetes Registry Tyrol 2012–2018. BMJ Open Diabetes Res Care 8: e001279.
- . Chait, A, den Hartigh LJ (2020) Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front Cardiovasc Med 7:22.
- . Chait, A, den Hartigh LJ (2020) Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front Cardiovasc Med 7:22.
- Nikolajczyk BS, Jagannathan-Bogdan M, Shin H, Gyurko R (2011) State of the union between metabolism and the immune system in type 2 diabetes. Genes Immun 12: 239-50.
- Alexopoulos N, Katritsis D, Raggi P (2014) Visceral adipose tissue as a source of inflammation and promoter of atherosclerosis. Atherosclerosis 233: 104-12.
- Shoelson SE, Herrero L, Naaz A (2007) Obesity, inflammation, and insulin resistance. Gastroenterology 132: 2169-80.
- Jonas MI, Kurylowicz A, Bartoszewicz Z, Lisik W, Jonas M etal. (2016) Adiponectin/resistin interplay in serum and in adipose tissue of obese and normal-weight individuals. Diabetol Metab Syndr 9: 1-9.
- Li S, Shin HJ, Ding EL, van Dam RM (2009) Adiponectin levels and risk of type 2 diabetes: asystematic review and meta-analysis. JAMA 302: 179-88.
- Toney GM, Stocker SD (2010) Hyperosmotic activation of CNS sympathetic drive: implications for cardiovascular disease. J Physiol 588: 3375-84.
- Yki-Jarvinen H (2014) Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2: 901-10.
- Silveira EA, Kliemann N, Noll M, Sarrafzadegan N, Oliveira C (2021) Visceral obesity andincident cancer and cardiovascular disease: An integrative review of the epidemiologicalevidence. Obes Rev 22: e13088.
- Deng T, Lyon CJ, Bergin S, Caligiuri MA, Hsueh WA (2016) Obesity, Inflammation, and Cancer. Annu Rev Pathol Mech Dis 11: 421-49.
- Brocker C, Thompson DC, Vasiliou V (2012) The role of hyperosmotic stress in inflammationand disease. Biomol Concepts. 3: 345-64.
- Bhave G, Neilson EG (2011) Body fluid dynamics: back to the future. J Am Soc Nephrol 22: 2166-81.
- Schwartz L, Guais A, Pooya M, Abolhassani M (2009) Is inflammation a consequence of extracellular hyperosmolarity? J Inflamm 6: 21-8.
- Stookey JD, Barclay D, Arieff A, Popkin BM (2007) The altered fluid distribution in obesitymay reflect plasma hypertonicity. Eur J Clin Nutr 61: 190-99.
- Liamis G, Liberopoulos E, Barkas F, Elisaf M (2014) Diabetes mellitus and electrolyte disorders. World J Clin Cases 2: 488-96.
- Roncal-Jimenez C, Lanaspa MA, Jensen T, Sanchez-Lozada LG, Johnson RJ (2015) Mechanisms by Which Dehydration May Lead to Chronic Kidney Disease. Ann Nutr Metab 66: 10-13.
Citation: Dembrowski GC, Barnes JW (2021) Improvement of Visceral Adiposity and Intracellular Fluid in Weight Loss Participants Taking Anti-Diabetic Medication. J Diabetes Clin Prac 4: 138.
Copyright: © 2021 Dembrowski GC. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 2796
- [From(publication date): 0-2021 - Nov 19, 2025]
- Breakdown by view type
- HTML page views: 2162
- PDF downloads: 634