Review Article Open Access
Improved Fetal Heart Rate Study Using Update Actocardiogram
Kazuo Maeda*
Department of Obstetrics and Gynecology, Tottori University Medical School, Japan
- *Corresponding Author:
- Kazuo Maeda
Department of Obstetrics and Gynecology
Tottori University Medical School, Yonago, Japan
Tel: 81-859-22-6856
E-mail: maedak@mocha.ocn.ne.jp
Received Date: June 20, 2016; Accepted Date: July 13, 2016; Published Date: July 21, 2016
Citation: Maeda K (2016) Improved Fetal Heart Rate Study Using Update Actocardiogram . Glob J Nurs Forensic Stud 1: 105. doi: 10.4172/2572-0899.1000105
Copyright: © 2016 Maeda K. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Global Journal of Nursing & Forensic Studies
Abstract
Aims: To precisely diagnose Fetal Heart Rate (FHR) with actocardiogram (ACG), which is the simultaneous record of FHR and movement signal spikes.
Method: Low frequency ultrasonic Doppler signal of fetal movement and high frequency Doppler of fetal heart beat were detected with single ultrasonic probe, and separated into two groups by a new electric circuit. FHR was recorded by autocorrelation heart rate meter, and fetal chest movement was changed to spikes, and their chart record was ACG.
Results: Whole body fetal movements were recorded, because fetal body movements were conducted to fetal chest and recoded. Movement spike amplitude was parallel to fetal movement. FHR increased if fetus moved with 7 sec delay. FHR acceleration was associated with fetal movement burst, moderate movement developed moderate FHR change, and FHR variability was provoked by minor fetal movements, where fetal brain responded fetal movements then produced FHR changes by integral function of fetal midbrain. Thus, FHR changes were lost, if fetal brain was damaged by hypoxia, i.e. FHR acceleration was lost in mild hypoxia preserving variability, then the variability was lost by severe hypoxia. Problems unsolved by the CTG were solved by ACG, e.g. physiologic sinusoidal heart rate was provoked by periodic changes of fetal movements to separate pathologic one in ACG. Fetal hiccupping was not recorded by CTG but recorded by ACG. Fetal behavior was classified by ACG. Outcome of fetal bradycardia was beneficial when FHR acceleration was provoked responding to fetal movements.
Conclusion: Actocardiogram is a useful new device to diagnose fetal brain function
Keywords
Actoardiogram; Cardiotocogram; Fetal heart rate; Fetal brain; Hypoxia; Ultrasound doppler
Introduction
Fetal abnormality was studied by Hon andCaldeyro-Barcia, detectingfetalbradycardia,latedecelerationsandlossofvariability [1]. However, developing mechanism of FHR changes was almost unknown, while subjective Fetal Heart Rate (FHR) patterns were discussed in clinical studies, then discrepancies between FHR deceleration and fetal outcome were discussed, and CTG was difficult to clarify fetal complications, though perinatal outcome was reported improved by fetal monitoring [2,3]. Fetal scalp lead ECG was used to record FHR, then changed to fetal heart sound by Hammaher [4] and Maeda [5], then further changed to ultrasonic Doppler fetal heart beat signals treated by autocorrelation heart rate meter [6], because the Doppler fetal heart signal was clearly detected even in the labor and autocorrelation heart rate meter recorded FHR as clearly as fetal scalp ECG. Ominous hypoxic signs were almost unknown, benign physiologic sinusoidal FHR was not separated from ominous pathologic sinusoidal one, fetal non-reactive state was diagnosed by maternal perception of fetal movement, and fetal hiccupping movement was unable to record on the CTG. Discrepancy was discussed between late decelerations and fetal outcome.
The author intended to compare the FHR to objective fetal movement record on the recording chart, and studied ultrasonic Doppler signals of fetal movement and fetal heart beat, then achieved to objectively differentiate their Doppler signals by their frequencies. Maternal motions developed large Doppler signals; however they were differentiated by their very low frequency [7,8].
Methods
Production of actocardiograph made by the author’s hand
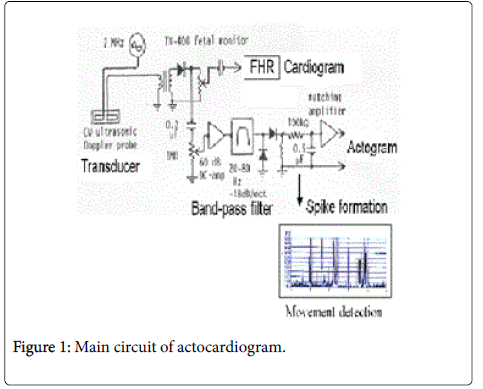
The author measured Doppler frequency of fetal movement by realtime FFT frequency analyzer listening noises of ultrasonic Doppler FHR monitor, fetal movement was identified by the observation of uterine wall movement, then detected fetal movement Doppler frequency to be 20 to 50 Hz when ultrasound was 2 MHz, while already we knew that Doppler frequency of fetal heart was 100 or more Hz in the analysis of Doppler fetal heart signal analysis, then it was possible to separate fetal heart signal from fetal movement one using ultrasound Doppler signals passing through a band pass filter, if the ultrasonic fetal monitor is used. Then TOITU TN400 ultrasound fetal CTG monitor (Tokyo) was remodeled attaching new electronic circuit (Figure 1) by the author’s hand, where maternal motion signals, of which frequency was 2 or lower Hz, were rejected by a CR high-pass filter, of which cut-off was 8 Hz, -6 dB/Oct to observe fetal Doppler signals with oscilloscope. FHR was recorded using original autocorrelation ultrasonic Doppler heart rate meter of TN400. The fetal movement Doppler signal was extracted by a band-pass filter, of which cut-off frequencies were 20 and 80 Hz, -18dB/Oct, in the prototype model. Movement spike signal was recorded on the channel which originally recorded uterine contraction; Commercial ACG models prepare 3 channels including FHR, fetal movement (associated a dot record) and proper channel to record uterine contraction to prepare CTG function.
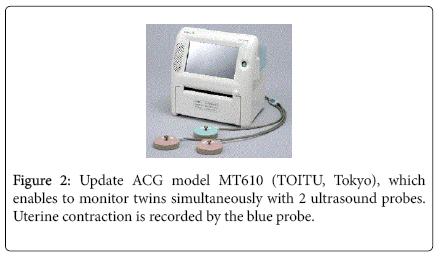
The proto-type originally produced by the author’s hand was changed to commercial models by the request of the author to TOITU (Tokyo) (Figure 2).
Production of prototype is about 50 pregnant women. Confirmation of the possibility of prototype is about 100 normal pregnant women. ACG function confirmation by world researchers is 100 pregnant women. Feta hiccupping movements is about 40 by the author and colleague. A/B ratio studies were carried out with about 50 normal and pathologic pregnancy cases. The fetus of central nervous system diseases is about 30 cases. Fetal disorders studied by the author are about 50 cases.
Recording method of ACG
The placement of ultrasound probe was the same as that of CTG, namely, the probe was placed on maternal abdomen via ultrasound jelly at the place of the most clearly listening fetal heart beat, corresponding fetal chest. Two probes were attached in case of twin pregnancy, where two heart beats were clearly listened; contraction probe was placed nearby uterine fundus. Each probe was fixed by belts.
The ACG should be recorded to include active and resting fetal states, of which duration may be 10-30 min each. Active state is characterized by frequent fetal movement bursts and responding FHR accelerations. Recording of no acceleration against fetal movement bursts is non-reactive FHR caused by mild hypoxia possibly followed by severe hypoxia, so that early delivery may be recommended in nonreactive FHR. FHR pattern was recognized by the CTG function.
Fetal resting state was characterized by neither FHR acceleration nor fetal movement spike was recorded, where baseline variability was preserved. It will be a dangerous situation when both variability and acceleration are lost, which meant severe fetal brain damage caused by heavy hypoxia.
Fetal highly active state was characterized by prolonged fetal movement burst and transient tachycardia, which were usually temporally and safe. Fetal hiccupping’s were repeated sharp spikes with 2-3 sec interval, where total hiccupping duration is 10 or more min. Some fetuses repeatedly develop repeated hiccupping movements twice in a daytime, while fetal hiccupping is physiologic and not hazardous.
Fetal hiccupping movements accompanied no FHR acceleration, because the hiccupping movements may be diaphragmatic convulsion which will not stimulate fetal brain then did not develop FHR acceleration, but it is not a pathologic phenomenon.
Results
More than 420 cases were studied and the resulted as follows:
Correct fetal movement spikes recorded on ACG chart
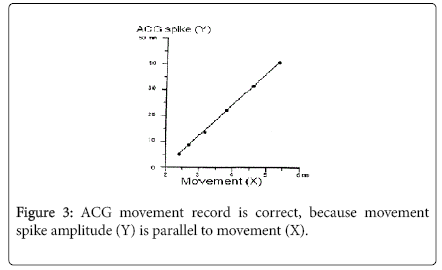
A steel ball was moved in water tank against the ACG probe. The steel ball movement was measured with a telescope. The relation of steel ball moving span and the spike amplitude was compared in the prototype model by the author, where the movement spike amplitude was completely parallel to the steel ball moving span (Figure 3). As steel ball motion represent fetal movement, therefore, movement spike recorded on the chart correctly represented fetal movement.
Earliest gestational weeks to record fetal movement
Fetal movement was recorded in 14 weeks of pregnancy with the ACG, while the FHR acceleration was associated in 20 or more weeks of pregnancy. Fetal brain will respond to fetal movement after 20 weeks of pregnancy.
ACG was checked by us and researchers in the world
The primary commercial ACG model was tested by world researchers, where almost all of fetal movements were recorded by the ACG to confirm the utility of ACG [9-11]. Fetal movements were visually checked on the monitor screen of real-time ultrasound Bmode imaging device by a colleague of the author and the movement event was marked on the ACG record by clicking a rapid Morse code key, where the event mark was fully corresponded to ACG spikes [12].
Fetal brain response to fetal movement
FHR increases when the fetus moves with the 7 sec time lag, which is the response of fetal brain function in 20 or later weeks of pregnancy, namely fetal movement burst (cluster of fetal movement) accompanies FHR acceleration, which is triangular shape that is formed by the integral function of midbrain, which is the location of the acceleration development [13], The time constant of the integral function is 7 sec, because the correlation coefficient of FHR and fetal movement was the largest when the FHR delayed for 7 sec [14].
Fetal hiccupping movement, which was not recorded by CTG, was clearly recorded by ACG as ca. 0.5 Hz repeated sharp spikes, while the hiccupping was not controlled by fetal brain, but it is convulsive diaphragmatic contraction, thus, it will not be used to the estimation of fetal brain function. However, sometimes fetal hiccupping was perceived by the mother as fetal movement without acceleration, and then erroneously thought to be non-reactive. Fetal Heart Rate (FHR), where the ACG is useful to correct diagnosis.
FHR changes in fetal growth restriction (FGR)
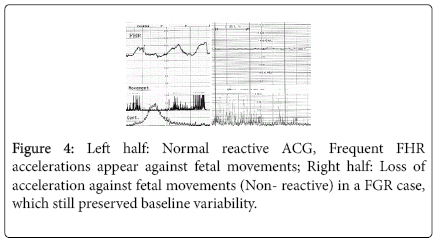
Fetal growth was restricted by the reduction of fetal nursing materials, which was caused by the damage of active transfer function of placental villi, but passive transfer of oxygen was also damaged after the reduction of active transfer function, namely, mild fetal hypoxia was shown by hypoxic FHR sign, which was the loss of acceleration against fetal movement burst (non-reactive FHR), which showed hypoxic suppression of fetal brain function, where FHR variability was still preserved (Figure 4).
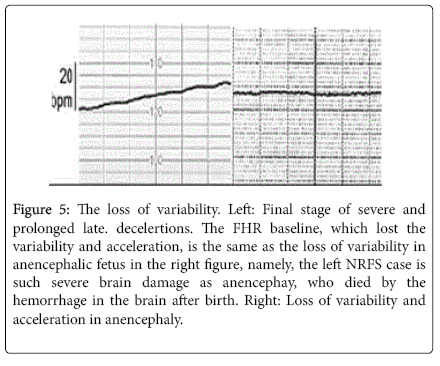
The most severe hypoxia attacks the fetus within 2 weeks after the loss of acceleration, where FHR variability was lost and bradycardia and late decelerations were accompanied. The NRFS fetus was delivered by emergency C-section, but the outcome was severe neonatal asphyxia, neonatal deaths due to fetal brain damage. Control group of no loss of acceleration showed favorable outcome after vaginal delivery and C-section of common grade fetal and neonatal asphyxia, without neonatal death or severe asphyxia [15]. The loss of acceleration alarms fetal brain damage and the loss of Fetal Heart Rate (FHR) variability, which is similar to anencephalic fetus, is the sign of severe brain damage (Figure 5).
Figure 5: The loss of variability. Left: Final stage of severe and prolonged late. decelertions. The FHR baseline, which lost the variability and acceleration, is the same as the loss of variability in anencephalic fetus in the right figure, namely, the left NRFS case is such severe brain damage as anencephay, who died by the hemorrhage in the brain after birth. Right: Loss of variability and acceleration in anencephaly.
The loss of FHR variability
FHR baseline variability develops as fetal brain response to the minor fetal movements and its disappearance was the most severe hypoxic brain damage similar to anencephalic fetus, which appears after the loss of FHR acceleration, therefore the fetus is recommended to deliver before the loss of variability to prevent such brain damage as cerebral palsy in postnatal life (Figure 5) [16].
Actually 2 cases of the loss of variability developed cerebral palsy or severe brain damage after the births, and cerebral palsy decreased in the cases who received C-section in severe FHR changes maybe before the loss of variability in the reports of fetal monitoring in old time [2,3]. Although the cerebral palsy was as rare as one in 5,000 term births, 200 babies will be damaged by cerebral palsy in one million births in Japan in a year, it is better to prevent developing the loss of variability.
How to avoid the birth after the loss of variability? Since FHR variability is lost after severe FHR changes such as severe bradycardia, severe late decelerations, repeated severe variable decelerations, truly ominous sinusoidal FHR, the loss of acceleration, the fetus is recommended to deliver by C-section at the appearance of these ominous FHR signs, not waiting the loss of variability. Another critical index of emergency C-section will be 20-24 of “hypoxia index”, which is the duration of fetal bradycardia (min) multiplied by 100, divided by the lowest FHR (bpm), since the hypoxia index was 25-26 in cases of the loss of variability, and 20-24 in severe FHR signs before the loss of variability.
Differentiation of physiologic sinusoidal FHR from pathologic sinusoidal one
Pathologic sinusoidal FHR was caused by severe fetal anemia of which Hb was 3-4 g/dl, where its outcome was ominous and frequently associated fetal death, therefore, pathologic sinusoidal should be cured by emergency C-section, while there was physiologic sinusoidal FHR, of which outcome was favorable without C-section, but it was impossible to differentiate from pathologic sinusoidal FHR with usual CTG fetal monitor. Although the differentiation was possible using frequency spectrum analysis of FHR baseline, it was necessary to study frequency spectrum of FHR record with special computer program [17].
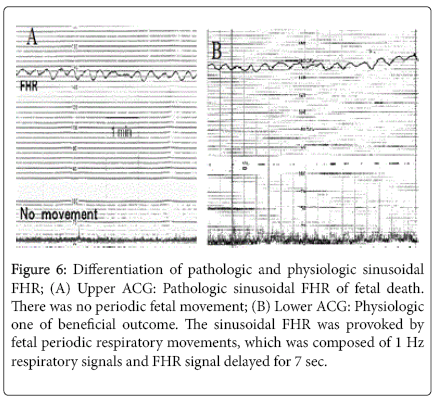
The differentiation was possible when fetal movements were simultaneously recorded with FHR in actocardiogram, namely, physiological sinusoidal FHR curve synchronized with periodic changes of fetal movement, which was periodic fetal respiration or fetal mouthing [18]. Therefore, physiologic sinusoidal is differentiated from pathologic sinusoidal FHR with the ACG which is simultaneous record of fetal movement and FHR (Figure 6).
Figure 6: Differentiation of pathologic and physiologic sinusoidal FHR; (A) Upper ACG: Pathologic sinusoidal FHR of fetal death. There was no periodic fetal movement; (B) Lower ACG: Physiologic one of beneficial outcome. The sinusoidal FHR was provoked by fetal periodic respiratory movements, which was composed of 1 Hz respiratory signals and FHR signal delayed for 7 sec.
Fetal outcome is favorable, if FHR develops acceleration against fetal movement in ACG, where fetal brain responds to fetal movement. In spite of expected neonatal depression after C-section due to ultrasonically diagnosed placental abruption and late deceleration of CTG, neonatal Apgar score was 9 point where no asphyxia was present, because of active fetal brain function estimated by the response of fetal brain with FHR acceleration against fetal movement in fetal ACG.
In spite of expected neonatal depression after C-section due to continuous fetal bradycardia of sick sinus syndrome, neonatal Apgar score was 9, and there was no asphyxia, because of normally active fetal brain estimated by its response with FHR acceleration against fetal movements in fetal ACG. Therefore, the discrepancy caused by pattern diagnosis of CTG was solved by the diagnosis of active fetal brain response to fetal movements in fetal ACG study.
Conclusion
The actocardiogram, created to simultaneously record fetal movement and fetal movement on the chart. Fetal heart rate changed as the response of fetal brain to fetal movement, recorded by ultrasonic Doppler fetal movement signal. Various problems which were controversial in the cardiotocogram were solved by the studies on fetal brain response to fetal movements, thus, FHR changes should be studied with fetal movements in the actocardiogram.
References
- Hon EH (1968) An Atlas of Fetal Heart Rate Patterns. HartyPresss 1968. New Haven, Connecticat.
- Takeshita K, Ando Y, Ohtani K, Takashima S (1989) Cerebral palsy in Tottori, Japan. Neuroepidemiology8: 184-192.
- Tsuzaki T, Sekijima A, Morishita K, Takeuchi Y, Mizuta M,et al. (1990) The survey on the perinatal variables and the incidence of cerebral palsy for 12 years before and after the application of the fetal monitoring system. Nihon SankaFujinkaGakkaiZasshi 42:99-105.
- Hammacher K (1976)Einf√?¬?hrung in die Cardiotokograohie. Die SchweizerHebamme74: 6-12.
- Maeda K (1965) Studies on the cardiotachography during labor, Digest of the 6th International Conference on Medical Electronics and Biological Engineering, Tokyo, 514-515.
- Takeuchi Y, Hogaki M(1977) Auto Correlation Method for Fetal Heart rate Measurement from Ultrasonic Doppler Fetal Signal. Ultrasound in Medicine Volume 3B Engineering Aspects, Plenum pp: 1327-1331.
- Maeda K (1984) Studies on new ultrasonic Doppler fetal actocardiography and continuous recording of fetal movement. ActaObstetGynecolJpn36: 280-288.
- Maeda K (2016) Invention of ultrasonic Doppler fetalactocardiograph and continuous recording of fetal movements. J ObstetGynaecol Res 42: 5-10.
- DiPietro JA, Costigan KA, Pressman EK (1999) Fetal movement detection: comparison of the Toituactogram with ultrasound from 20 weeks gestation. J Matern Fetal Med8:237-242.
- Schwöbel E, Fallenstein F, Huch R, Huch A, Rooth G (1987) Combined electronic fetal heart rate and fetal movement monitor – a preliminary report. J Perin Med15: 174-184.
- Wheeler T, Robertz K, Peters J, Murrills A (1987) Detection of fetal movement using ultrasonic Doppler ultrasound. ObstetGynecol 70: 251-254.
- Maeda K, Tatsumura M, Utsu M (1999) Analysis of fetal movementsby Doppler actocardiogram and fetal B-mode imaging. ClinPerinatol26: 829-851.
- Terao T, Kawashima Y, Noto H, Inamoto Y, Lin TY et al. (1984) Neurological control of fetal heart rate in 20 cases of anencephalic fetuses. Am J ObstetGynecol140:201-208.
- Takahashi√£¬?¬?H (1990) Studies on cross correlation coefficient of fetal heart rate and fetal movement signals detected by ultrasonic Doppler fetal actocardiogram. ActaObstetGynecolJpn 42: 443-449.
- Tesima N (1993) Non-reactive pattern diagnosed by ultrasonic Doppler fetal actocardiogram and outcome of the fetuses with non-reactive pattern. Nihon SankaFujinkaGakkaiZasshi 45: 423-430.
- Maeda K (2014) Modalities of fetal evaluation to detect fetal compromise prior to the development of significant neurological damage. J ObstetGynaecol Res 40:2089-2094.
- Maeda K, Nagsawa T (2005) Automatic computerized diagnosis of fetal sinusoidal heart rate. Fetal DiagTher20: 328-334.
- Ito T, Maeda K, Takahashi H, Nagata N, Nakajima K, et al. (1994) Diffrentiation between physiologic and pathologic sinusoidal FHR pattern by fetal actocardiogram. J Perinat Med 22: 39-43.
Relevant Topics
- Adult Protective Care Unit
- Correctional Nursing
- Critical Care Nursing
- Emergency and Acute Care Setting
- Forensic and Victimology
- Forensic Mental disorder
- Forensic Mental Health Nursing
- Forensic Mental Illness
- Forensic Nursing
- Forensic Nursing Care
- Forensic Nursing Clinical Practice
- Forensic Nursing Science
- Healthcare Management
- Interpersonal Violence
- Intimate Partner Violence
- Nursing research
- Post Exposure Prophylaxis
- Psychosocial Intervention
- Trauma Nursing
Recommended Journals
Article Tools
Article Usage
- Total views: 12298
- [From(publication date):
September-2016 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 11353
- PDF downloads : 945