Importance of Fluorine and Fluorocarbons in Medicinal Chemistry and Oncology
Received: 21-Mar-2013 / Accepted Date: 24-Apr-2013 / Published Date: 26-Apr-2013 DOI: 10.4172/2329-9053.1000104
Abstract
Carbon-Fluorine (C-F) can serve as a molecular tag for many applications in medicinal chemistry and oncology such as identification (i.e. screening), imaging (i.e. tracing) and analytical characterization. Thereby, fluorination, a chemical process to add a fluorine atom into a single molecule or a complex matrix materials (e.g. compounds) is largely used in the pharmaceutical field to confer some interesting properties to cancer drug compounds (e.g. enhancement of bioavailability). It is further more recently used for labelling some biological molecules of interest (i.e. peptides, nucleic acids) or nanomaterials (i.e. nanoparticles) which are of high importance for cancer chemoand biotherapy (e.g. immunotherapy) as well as for tumor (aka tumour)/cancer imaging (i.e. staging/prognosis, biodistribution, cancer diagnosis and therapy). Indeed, In addition to be easy-to-handle, efficient, soluble, smaller and cheaper, C-F bond is more stable than fluorescent dye, less toxic than fluorine radioisotopes, and less harmful than radio-waves. We have developed a patented technology called carbon-fluorine spectroscopy (CFS aka Spectro-Fluor®) along with methods and applications to not only specifically and sensitively detect C-F bonds in raw pure compound, complex materials but also to screen (e.g. drug discovery and drug security) as well as to trace F-molecules in vivo for improved medical care, particularly but not limited to the oncology sector (e.g. tumor/cancer imaging, development of new F- reagents, F-biomolecules, and anti-cancer agents). In this paper, we reviewed and discussed the major physical-chemical properties of C-F bond, the main applications of fluorocarbons as well as the state-of-art imaging technologies that use fluorine for clinical and research and development (R&D) oncology purposes (e.g. drug design, drug discovery, drug delivery and molecular imaging). An emphasis is put on the use of safer, unlabeled fluorinated molecules thanks to the emerging and promising CFS derived platform green technology that allows to reliably detecting unlabeled C-F molecules. Overall, we conclude that fluorine is a magical atom for molecular diagnosis and therapy that does not always need to be labelled.
Keywords: Fluorination; Carbone-Fluorine; Oncology; Nanomedicine; Pharmacy; Medicinal chemistry; Green chemistry; Green technology; Technological innovation; Carbon-fluorine spectroscopy; Nuclear magnetic resonance; Magnetic resonance imaging; Positron emission tomography
1538Abbreviations
ADCs: Antibody-Drug Conjugates; C-F: Carbone- Fluorine; C-T: Computed Tomography; CFS: Carbone Fluorine Spectroscopy; iRNA: Interference RiboNucleic Acid; miRNA: Micro iRNA; MRI: Magnetic Resonance Imaging; PCR: Polymerase Chain Reaction; ODN: Oligo Deoxy Nucleotide; PET: Positron Emission Tomography; NMR: Nuclear Magnetic Resonance; SiRNA: Small iRNA
Epistemology Of Fluorine: From The Atom To Fluorocarbons
Fluorine (name derived from Latin fleure, meaning to flow) is the lightest of the halogens, the most reactive of all the elements. In 1886, a French chemist, Ferdinand Frederic Henri Moissant (1852-1907), was the first to isolate fluorine [1]. He used platinum electrodes to produce fluorine from the electrolysis of potassium fluoride (KF), a hydrofluoric acid. In 1872, Sir James Crighton-Browne postulated that a deficiency of fluorine was responsible for higher incidency of dental carries [2]. In 1892, a Belgian chemist Frederic Jean Edmond Swarts discovered the Cl/F exchange chemistry of the inorganic antimony trifluoride (SbF3), a hydrofluoric acid (HF) widely used in dyeing and pottery [3]. The reaction, commonly called “Swarts reaction”, has since been improved to be an industrial process for the preparation of organofluorine compounds, such as for the synthesis of dimethyl and trimethyl chlorosilanes [4].
Although fluorine is the thirteenth most abundant element in the earth’s crust, fluoride concentrations in surface water are low and fluorinated metabolites are extremely rare [5]. Indeed, up-todate, only 13 naturally occurring fluorinated organic compounds are known. Among them, we can cite the bacterial fluorinating enzyme 5’-fluoro-5’-deoxyadenosine synthase used by Streptomyces cattleya to naturally catalyze a fluorination reaction [5]. This microorganism can form carbon-fluorine (C-F) bonds using aqueous fluoride through a nucleophilic substitution mechanism.
This particular rarity of natural fluorination is of high industrial importance, with applications in pharmaceutical, biomedical, agrochemical and materials products.
Carbon-Fluorine Properties And Effects
The C-F bond is the most polar bond in organic chemistry, and thus the bond has a relatively large dipole moment with a significant -ve charge density on the fluorine atom and correspondingly a +ve charge density on carbon [6]. Because the C-F bond has a much greater dipole moment than does the carbon-hydrogen (C-H) bond, a stronger binding with dipolar water might be expected [7]. The electrostatic nature of the C-F bond renders it the strongest one in organic chemistry [6]. Further, C-F displays isoelectronic effects to oxygen (-O) atom and hydroxyl (-OH) group, and the high electronegativity of fluorine (-F) frequently alerts chemical reactivity. However, the (-F) atom itself is almost non-polarizable, and thus, despite the charge localization on (-F), it is a poor hydrogen-bonding acceptor [6]. Although the polarizability of (-F) in the C-F bond is relatively low, considering its position in the periodic table, the dispersion interactions of C-F with water are reasonably expected to be more attractive than those of C-H with water [8]. Therefore, a fluorocarbon surface could be argued to be more hydrophilic than the corresponding hydrocarbon. A plausible resolution could be that the fluorocarbon with a molecular crosssection of 28.3 Å2 [9] occupies sufficiently more volume and surface area in water than the corresponding hydrocarbon with molecular crosssection of 18.9 Å2 [10]. It has recently be found that (poor) interaction of water with a hydrophobic solute/surface, such as fluorinated surfaces, is primarily a function of van der Waals interactions and is substantially independent of electrostatic interactions [7].
Overall, fluorination displays the following properties [11]: (i) enhancement of thermal stability (C-F: 107 Kcal/mol), (ii) increase of lipophilicity and hydrophobicity, (iii) improvement of the molecular bioavailability, and (iv) capability of mimicking enzyme substrate (comparable in size to H, 1.47Å versus 1.20Å).
Eventually, organofluorine affects nearly all physical, adsorption, distribution, metabolism, and excretion properties of a lead compound. Its inductive effects are relatively well understood (e.g. enhancement of bioavailability by reducing the basicity of neighboring amines). In contrast, exploration of the specific influence of C-F single bonds on docking interactions, whether through direct contact with the protein or through stereoelectronic effects on molecular conformation of the drug, remains poorly characterized and needs more attention.
Fundamental Applications Of Fluorocarbons In Oncology
The specific features of the fluorine in the formation of fluorocarbons make it attractive in the design of non-viscous but polar organic compounds, with a polarity limited to influencing the intramolecular nature of the molecule and intermolecular interactions with the immediate environment [6]. C-F bonds are unique in nature, more stable than fluorescent dyes due to their covalent interactions, much less toxic than radioisotopes, certainly less harmful at long-term exposure than radio-waves while inexpensively incorporated into molecules [12,13]. Therefore, C-F bond can generally be used as [12,14-18]: (i) a molecular security label to enhance the safety of an anti-cancer agent (i.e. useful within a prevention and banning program of counterfeited or substandard drugs), (ii) tracing an anti-cancer compound during the synthesis or the production cycle, providing necessary improvement in productivity and efficiency across various stages of the pharmaceutical development (i.e. discovery, synthesis and production cycles, clinical and post-approval stages), subsequently bringing the product faster to the market, (iii) internal (e.g. ex-vivo, in situ, in vivo) and/or external labels (e.g., surface, in vitro) of pharmaceutics (e.g. drugs) and/or biologics (i.e. peptides, proteins including antibodies, nucleic acids, cells) and/or various nanomaterials (e.g. gold nanoparticles, fullerenes including nanocarbon tubes, diamondoids, nanogels, nanoemulsions, nanoporous silica glasses, lipid-based nanopolymers) to characterize them (e.g. identification, screening, detection, assessment of biodistribution, metabolism, structure and function), as well as to validate or design new bio-therapeutics (e.g. F-nanoencapsulated anti-cancer drugs) with enhanced bioavailability or new tracers (i.e. unlabeled C-F), improved stability and less toxicity for long-term imaging and/or pharmacological studies (e.g. progressive/controlled drug release to targeted tumor).
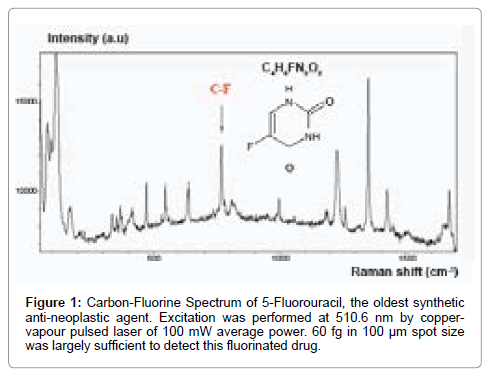
Thereby, fluorine substituents have become a wide spread and important drug component in pharmaceuticals, particularly for the pharmaceutical oncology segment. Thereby, thanks to the development of safe and selective fluorinating agents, more than 50% of the marketed drugs are fluorinated, including many anti-cancer agents such as fluorinated taxane anticancer agents and 5-Fluorouracil (5-FU) (Figure 1), this later being an anti-cancer compound employed for several decades since its discovery in 1957 by Charles Heidelberger et al. [12,13,19-22].
Also, fluorinated solvents and/or reagents for biological and clinical use in oncology are valuable. It is the case of perfluocarbons (e.g. perfluorodecalin, perfluorobron) which can serve as red cell substitutes (aka artificial blood substitutes) for untransfusable patients with severe cancer-induced anemia and hypoxia [12,13,23-25], and also as fluorine tumor imaging agent [26,27].
More recently, fluorinated analogues of the canonical α-Lamino acids have gained wide spread attention as building blocks that may endow peptides and proteins with advantageous biophysical, chemical and biological properties [28]. Recently, we developed a new molecular approach to label proteins and reliably detect them using non-labelled fluorinated aromatic amino-acids along with the use of the unique carbon-fluorine spectroscopy (CFS) [29]. Thereby, we were able to detect biomarkers of cancers as well as transcriptional targets of tumor suppressor genes (e.g. p21 Waf1/Cip1-downstream target of p53, the “guardian of the genome”) after ex vivo protein fluorination using cancer cells and an original established protocol involving only one unlabelled non-fluorinated antibody [29].
Further, the use of fluorinated-amino acids to increase the stability of oligomeric structures such for anti-cancer peptides is valuable, but still at its premisses. Indeed, peptide therapeutics is a promising field for emerging anti-cancer agents [12,30]. Benefits include the ease and rapid synthesis of peptides and capacity for modifications. Current research focuses on developing peptides that can (i) serve as tumor targeting moieties (i.e. tumor-homing peptides that can carry biologically active cargo to tumors or tumor vasculature), and (ii) permeabilize membranes with toxic consequences for tumor cells (i.e. apoptotic or necrotic cell death through disruption of cell or organelles membranes). For instance, amphiphilic peptides with clusters of hydrophobic and cationic residues showed considerable anticancer toxicity [30]. The main challenge still lies on improving delivery to tumors, minimizing non-specific toxic effects and discerning pharmacokinetic properties in order to produce a powerful therapeutic peptide for cancer treatment. Interestingly, a very recent study indicated that 18F-labeled peptides can be reproducibly prepared as stable aluminium-fluorine (Al-F) complexes with good radiochemical yield and high specific activity using a simple, one-step, lyophilized kit followed by a rapid purification through solid-phase extraction (SPE) then provided the 18F-peptide ready within 30 min for patient injection [31].
Besides, the development of F-antibodies (i.e. durable bi-functional antibody that can be used both as F-biodrugs and F-biotracers) is also highly desirable, in particular the monoclonal ones, which can be then both specific and sensitive and might overcome some important limitations such those associated with current antibody-drug conjugates (ADCs) for onco-therapy (e.g. aggregation, insolubility) and/or with cancer immunotherapy protocols (e.g. low sensitivity or specificity) [12].
Eventually, those applications using labelled fluorine are, for some of them, widely used following diverse protocols involving radioisotopes, fluorescent dyes. Nevertheless, there is still a paucity of reports regarding the alternative use of unlabeled C-F bond. Therefore, the use of C-F bond as an unlabeled tag remains undeniably challenging in oncology and cancer stem cells research since it has shown good promises for analytical characterization of F-molecules (e.g. F-drugs, F-biologics) and F-applications (e.g. compounds screening and tracking, metabolism-based assays, early imaging diagnosis, nanomaterials based (bio) drug delivery for efficient therapy) [12,13,29].
Nowadays, only carbon-fluorine spectroscopy (CFS) - later named Spectro-Fluor™ -, an innovative and powerful technology recently pioneering by our group and further developed for multiple uses (e.g. biomedical, pharmaceutical, food, material and environmental sciences), is really capable to qualitatively and quantitatively detecting unlabelled C-F bonds, and so enlarging applications in oncology (e.g. cancer drug discovery and delivery, cancer imaging) [12,13,29]. We practically expect from our previous in-vitro and ex-vivo studies many advantages over the conventional molecular imaging systems for in-vivo applications (e.g. in terms of safety, toxicity, comfort for the patients and small animals; reliability, cost-effectiveness, practical use for the practitioners and researchers).
Eventually, nucleic acid-based fluorinated derivatives such as nucleosides or oligonucleotides (aka bioprobes or primers) connected to highly fluorinated chains or labelled with one or more fluorine atoms have been developed [32,33]. They have been increasingly investigated due to their high potential for biomedical applications (e.g. radiofluorinated oligodeoxynucleotide (ODN) probe for use along with positron emission tomography (PET)) [33,34]. In oncology, this development has indeed its importance considering that some F-nucleic acids such as F-interference RNAs (e.g. siRNAs, miRNAs) can be used to more efficiently silent some genes and proteins of interest [35]. Also, F-oligonucleotides could be used to enhance the reliability of gene amplification by polymerase chain reaction (PCR). They have been recently used to image gene expression changes (i.e. uptake and distribution) in human tumor/cancer cells to monitor early tumor response to treatment using liposome-transfected 18F-labeled oligonucleotide probe [36].
Eventually, fluorination of cancer cells, cancer progenitor cells and “cancer stem cells” (aka initiating and/or propagating cells), using green approaches, shall provide further insights on carcinogenesis, cancer prognosis and cancer diagnosis.
Fluorous-Technologies For Clinical And R&D Oncology
Nuclear magnetic resonance (NMR)
Fluorine NMR-based spectroscopy has recently emerged as a versatile, reliable and efficient tool for performing binding and biochemical assays. Different libraries of fluorinated compounds, designed by maximizing the chemical space around the fluorine atom, are screened for identifying binding fragments and for detecting putative fluorophilic hot spots on the desired macromolecular target. A statistical analysis of the fluorine NMR chemical shift, which is both a marker of the fluorine local environment and X-ray structures of fluorinated molecules, has resulted in the development of the ‘rule of shielding’, useful method for ‘lead optimization’ and designing novel chemical scaffolds that recognize distinct (onco-) protein structural motifs [37]. Interestingly, recent studies [38,39] reported the usefulness of 19F-amino-acid (i.e. 5-fluoro-DL-tryptophan (5FW) or 3-fluoro-L-tyrosine (3FY)) as a dual NMR and fluorescent probe of α-synuclein, a molecule which plays a central role in Parkinson´s disease (PD). Indeed, analysis of such types of proteins (i.e. oncoproteins), highly proned to conformational changes (and aggregation), presented difficulties with conventional proton NMR, but according to the authors, it can be circumvented when non-native 19F nuclei are incorporated at specific sites within the amino-acid sequence [38]. In fine, 19F-NMR is then particularly suitable for characterization of unfolded structures because 19F chemical shifts are highly sensitive to local environments and conformations [38]. Subsequently, it could be used to monitor the insertion and conformation changes of a 19F-labeled cell penetrating peptide attached to the N-terminus of a protein (e.g. α-synuclein) upon interacting with the cellular plasma membrane, because of the 19F resonance decrease [39]. Furthermore, the incorporation of fluorine analogs of fluorescent amino acids (e.g. 5FW) allows for complementary studies of protein microenvironment via fluorescence spectroscopy [38] and protein sub-localization within the cell via confocal fluorescence microscopy [39].
Magnetic resonance imaging (MRI)
19F-MRI spectroscopy is a a promising tool for monitoring (cancer-) stem cell-based therapy, especially when those cells are labeled with perfluorocarbons (PFCs). Indeed, cells conventionally loaded with functionalized supramagnetic iron oxide nanoparticles (SPIONs) appear hypo-intense on MR images but the contrast generated by iron oxide labeled cells is neither specific due to ambiguous background nor quantitative. 19F-MRI can also be used for applications to assess the metabolism and/or biodistribution of F-drugs.
A recent study using human neural stem cells (NSCs) efficiently labeled with 19-perfluoropolyether (PFPE) with little effects on viability or proliferation and differentiation capacities, has shown for the first time that 19F-MRI can be utilized for tracking human NSCs in brain implantation studies, which ultimately could aim for restoring loss of function after acute and neurodegenerative disorders [40]. This study suggests that stem cells (SCs) involved in the initiation and/ or propagation of tumor cells can also be labeled and monitored by 19F-MRI, which is tremendously useful for better understanding the general oncogenesis/carcinogenesis process (e.g. cancer staging, dynamism of tumor microenvironment). Moreover, the labeling of stem cells can be useful to control in situ tissue engineering and so, become very useful in regenerative medicine. For instance, a very recent study reports the good performance of 19F-MRI to describe the association of the central zone with more aggressive prostate cancer [41]. Other studies used chemical-shift selective 19F-MRI to directly detect a specific intra-tumoral F-drug (e.g. 5-FU) trapping/retention (i.e. in solid tumors such hepatoma, in case of 5-FU), biodistribution (i.e. specific tissue uptake such as liver and kidneys, in case of 5-FU) and catabolism (i.e. major catabolite such as α-fluoro-β-alanine was detected in case of 5-FU) in tumor-bearing rats [42,43].
Besides, MRI can be coupled with other imaging technologies such as computed tomography (CT). As an example, 3D images and 2D models based on MRI/CT image fusion provided a powerful tool for the visualization of jaw tumors by defining the relationship between tumors and adjacent structures, thereby assisting the subjectspecific preoperative planning, surgical simulation, and intraoperative guidance for tumors [44]. MRI/CT also obtained a better estimation of the organ tumor size than CT alone, which tends to overestimate it, and is then a quite useful combination in ‘radiotherapy planning’ for localized cancers (e.g. rectal carcinoma, prostate carcinoma can be treated by more adapted radio-therapeutic doses consequently decreasing organ complications) [45,46].
Positron emission tomography (PET)
PET is a common and powerful analytical method for medical diagnosis, particularly in oncologic sector where it was extensively used for many years. Indeed, PET is a non-invasive imaging technique that provides functional or metabolic assessment of normal tissue or disease conditions and is playing an increasing role in cancer radiotherapy planning. The application of fluorinated radiotracers in PET studies for imaging in cancer patients, include 2-18F-fluoro-2-2-deoxy-D-glucose later referred to as 19FDG, fluorinated nucleosides, fluorinated aminoacids (e.g. 18F-fluoroethyl-l-tyrosine (FET), 18F-fluorothymidine (FLT), 18F-fluoro-α-methyltyrosine (FMT)) as well as fluorinated peptides among others. FDG is the « working horse » in oncology, including neuro-oncology, albeit FET is highly useful for glioma detection. FDG is a glucose analog that is taken by cells in a similar fashion as glucose, phosphorylated by hexokinase to 18F-FDG-6-phosphate but cannot undergo further glycolysis, and hence is trapped in the cell. In fact, more studies are required to really understand the full metabolism of 19FDG. 18F-FDG or 19F-FDG are the most often applied radiolabels in such studies because of [47]: (i) increased glucose metabolism in most types of tumors, (ii) their low positron energy with high abundance (96%) and a path length in tissue of approximately 0.1-0.2 cm, (iii) their relatively long half-time (110 min.) of the isotope, allowing extensive and complex imaging protocols including dynamic studies and investigations of slow metabolic processes, and (iv) their relatively good efficacy and safety. The low emitting energy results in a marginal risk for the patient because of the short range as well as the limited dose of emission.
Thereby, it has most recently shown that, in the evaluation of a regional lymph node based in primary Merkel cell cacinoma (MCC), a rare but agressive skin cancer, 18F-PET (aka PET-FDG) is significantly more sensitive and equally specific than traditional CT and, both techniques were more sensitive than clinical examination alone [48]. Interestingly, amino-acid analogs such 18F-choline and 18F-acetate can alternatively be used to FDG because of their higher specific to tumor cells, so they could play an important role both in differentiating cancers from benign conditions and in diagnosing cancers with either low FDG uptake or high background FDG uptake [49,50].
Further, the coupling PET/CT has been used in a most recent study to detect distant metastases (DMs) increase -especially in the lung (93%) and bone (43%) - with high sensitivity (86%), specificity (84%), accuracy (84%) in patients with recurrent head and neck squamous cell carcinoma (HNSCC) [51].
Interestingly, PET can also be coupled with MRI. Indeed, PET/ MRI fusion seems to be highly accurate in T(tumor)-staging of tumor entities for which MRI has traditionally been favored, (e.g. HNSCC). By adding functional MRI to PET, PET/MRI may improve diagnostic accuracy, owing to its capability to differentiate scar tissue from recurrence of tumors (e.g. rectal cancer) and so, play an important role in cancer staging. With regard to N(node)-staging, PET/MRI did not seem to provide considerable benefits as compared to PET/CT and provided similar N-staging accuracy when applied as a wholebody staging approach [52]. Besides, a recent study showed that PET/ MRI fusion is superior to PET/CT in characterizing pancreatic tumors, offering better mapping and fusion image quality [53]. Corroborating the importance of PET/MRI, another recent study that aimed to compared FDG PET/MRI, FDG PET/CT, MRI and CT imaging, showed that PET/MRI was the most reliable for focal invasion assessment and tumor size delineation in patients with advanced buccal squamous cell carcinoma (BSCC), while PET/CT had the lowest confidence level which may limit its use in the clinical setting [54]. Nevertheless, a very recent comparative study assessing the performance FDG PET/ MRI with FDG PET/CT as the reference standard in the staging of lung cancer (i.e. bronchial carcinoma) showed that PET/MRI had as good potential as PET/CT to characterize tumor lesion (i.e. pulmonary masses) and tumor stage with quality [55].
Eventually, advanced studies evaluating the reliability of FDGPET/ CT compared to 19F-MRI/CT in cancer patients are requested.
Carbon-Fluorine Spectroscopy (CFS)
As we could observe from conventional imaging techniques, quantitative and qualitative analyses of fluorinated molecules represent an important task. Nevertheless, the common molecular detection methods include molecular labelling using radioactive isotopes or fluorescent dyes. However, these types of detection require expensive safety and precautionary measures (e.g. especially with isotopes) or have limited sensitivity and stability (e.g. case of fluorophores aka fluorochromes). In this context, we have developed and validated CFS, a green technology derived from a non-competitive family of devices called PLIRFATM (“Pulsed Laser Isochronic Raman and Fluorescence Apparatus”) for multiple R&D applications across the biomedical, pharmaceutical and life sciences spectrum [12,13,29]. The key feature of the patented CFS is based on the discovery of a characteristic Raman signature of carbon-fluorine bond(s) in the fingerprint spectral area of 500 cm-1 and 800 cm-1 allowing detection, characterization, imaging, monitoring, screening and measurement of C-F utility, fluoro-nanomaterials, fluoroorganic compounds, fluoro-molecules, fluoroorganic impurities or fluoro-degradation products [12,13,29]. CFS not only represents an innovative, disruptive, green, affordable, cost-effective and flexible analytical tool of high resolution but also a reliable discovery tool notably in terms of reproducibility. Thereby, CFS represents a major advance over conventional Raman spectroscopy since it allows (i) highly specific and ultrasensitive detection (ppmppb level or
Although many studies have been performed in-vitro and exvivo to respectively characterize non-labelled C-F tagged molecules [12,13,29], ongoing promising studies are focused on molecular and tissue imaging in-vivo.
Conclusion
Despite recent advances in diagnostic and therapeutic modalities (aka theranostics modalities), cancer remains a major source of morbidity and mortality throughout the world. Moreover, the incidence of many cancers, including the skin, prostate, breast, and kidney cancers, continues to increase. The signature roles of fluorine in medicinal chemistry, diagnostic and therapy are now firmly established. The presence of fluorine in pharmaceuticals (i.e. radiopharmaceuticals, F-drugs) has had a major impact on a plethora of important medical applications, such as those cited above (e.g. treatment and imaging). The small attractive unlabelled C-F bond along with the innovative CFS technology, which aims to become the « gold standard technology » is offering huge green alternatives to the conventional technologies, and shall be undeniably an asset in the oncology and medicinal chemistry areas. Eventually, fluorine is a flourishing element that will very likely continue to contribute significantly in enhancing current and future medical advances (e.g. nanomedicine) by playing multifaceted roles.
Acknowledgments
We are grateful to Dr. Abder Menaa, MD for his pertinent review and suggestions of this manuscript.
References
- Tressaud A (2006) Henri Moissan: winner of the Nobel Prize for Chemistry 1906. Angew Chem Int Ed Engl. 2006, 45(41): 6792-6796.
- Crichton-Browne J (2004) An address on tooth culture. Am J Public Health 94: 722-725.
- Booth HS, Suttle JF (1946) IV. The Preparation and Fluorination of Dimethyl and Trimethyl Chlorosilanes. J Am Chem Soc 68: 2658-2660.
- Dong C, Huang F, Deng H, Schaffrath C, Spencer JB (2004) Crystal structure and mechanism of a bacterial fluorinating enzyme. Nature 427: 561-565.
- O'Hagan D (2012) Organofluorine chemistry: synthesis and conformation of vicinal fluoromethylene motifs. J Org Chem 77: 3689-3699.
- Dalvi VH, Rossky PJ (2010) Molecular origins of fluorocarbon hydrophobicity. Proc Natl Acad Sci U S A 107: 13603-13607.
- Miller TM (2010) In CRC Handbook of Chemistry and Physics, Atomic and molecular polarizabilities, ed Lide DR (CRC, Boca Raton, FL) Internet Version, 10193-10202.
- Broniatowski M, Dynarowicz-Latka P (2006) Iso-branched semifluorinated alkanes in Langmuir monolayers. J Colloid Interface Sci 299: 916-923.
- Fenter P, et al. (1997) Surface of n-octadecanethiol self-assembled on Ag(III) surface: An incommensurate monolayer. Langmuir 7: 2013-2016.
- Park BK, Kitteringham NR, O'Neill PM (2001) Metabolism of fluorine-containing drugs. Annu Rev Pharmacol Toxicol 41: 443-470.
- Menaa F, Menaa B, Sharts O (2011) Development of carbon-fluorine spectroscopy for pharmaceutical and biomedical applications. Faraday Discuss 149: 269-278.
- Menaa F, Menaa B, Sharts O (2012) Innovative Biotechnology: Carbon-fluorine Spectroscopy for a Broad Range of Applications. AAPS J 14(S1): 345.
- Song Z, Zhang Q (2011) Design, synthesis, and incorporation of fluorous 5-methylcytosines into oligonucleotides. J Org Chem 76: 10263-10268.
- Menaa B, Montoneri C, Menaa F, Montoneri E, Boffa V, et al. (2011) Protein helical structure enhancement in biocompatible fluoro-phosphonate based nanoporous silica glasses assessed by circular dichroism spectroscopy. Int J of Nanotechnology 8: 471-491.
- Wu D, Wan MA (2008) novel fluoride anion modified gelatin nanogel system for ultrasound-triggered drug release. J Pharm Pharm Sci 11: 32-45.
- Tan Y, Yang Z, Peng X, Xin F, Xu Y, et al. (2011) A novel bottom-up process to produce nanoparticles containing protein and peptide for suspension in hydrofluoroalkane propellants. Int J Pharm 413: 167-173.
- O'Hanlon CE, Amede KG, O'Hear MR, Janjic JM (2012) NIR-labeled perfluoropolyether nanoemulsions for drug delivery and imaging. J Fluor Chem 137: 27-33.
- Muller K, Faeh C, Diederich F (2007) Fluorine in pharmaceuticals: looking beyond intuition. Science 317: 1881-1886.
- Isanbor C, O’Hagan D (2006) Fluorine in medicinal chemistry: A review of anti-cancer agents. Journal of Fluorine Chemistry 127: 303-319.
- Ojima I (2004) Use of fluorine in the medicinal chemistry and chemical biology of bioactive compounds--a case study on fluorinated taxane anticancer agents. Chembiochem 5: 628-635.
- Heidelberger C, Chaudhuri NK, Danneberg P, Mooren D, Griesbach L, et al. (1957) Fluorinated pyrimidines, a new class of tumor-inhibitory compounds. Nature 179: 663-666.
- Lowe KC (2003) Engineering blood: synthetic substitutes from fluorinated compounds. Tissue Eng 9: 389-399.
- Klein HG (2002) Blood substitutes: how close to a solution? Oncology (Williston Park) 16: 147-151.
- Bauer J, Zahres M, Zellermann A, Kirsch M, Petrat F, et al. (2010) Perfluorocarbon-filled poly(lactide-co-gylcolide) nano- and microcapsules as artificial oxygen carriers for blood substitutes: a physico-chemical assessment. J Microencapsul 27: 122-132.
- Baete SH, Vandecasteele J, De Deene Y (2011) 19F MRI oximetry: simulation of perfluorocarbon distribution impact. Phys Med Biol 56: 2535-2557.
- Mason RP, Antich PP, Babcock EE, Gerberich JL, Nunnally RL (1989) Perfluorocarbon imaging in vivo: a 19F MRI study in tumor-bearing mice. Magn Reson Imaging 7: 475-485.
- Salwiczek M, Nyakatura EK, Gerling UI, Ye S, Koksch B (2012) Fluorinated amino acids: compatibility with native protein structures and effects on protein-protein interactions. Chem Soc Rev 41: 2135-2171.
- Menaa F, Boucharaba A, Menaa B, Guimaraes CA, Avakyants L, et al. (2009) Fluoro-Raman spectroscopy as a new analytical tool to detect ex-vivo proteins. AAPS J 11: 736.
- Boohaker RJ, Lee MW, Vishnubhotla P, Perez JM, Khaled AR (2012) The use of therapeutic peptides to target and to kill cancer cells. Curr Med Chem 19: 3794-3804.
- McBride WJ, D'Souza CA, Karacay H, Sharkey RM, Goldenberg DM (2012) New lyophilized kit for rapid radiofluorination of peptides. Bioconjug Chem 23: 538-547.
- Wilds CJ, Damha MJ (2000) 2´-Deoxy-2´-fluoro-beta-D-arabinonucleosides and oligonucleotides (2´F-ANA): synthesis and physicochemical studies. Nucleic Acids Res 28: 3625-3635.
- Pan D, Gambhir SS, Toyokuni T, Iyer MR, Acharya N, et al. (1998) Rapid synthesis of a 5'-fluorinated oligodeoxy-nucleotide: a model antisense probe for use in imaging with positron emission tomography (PET). Bioorg Med Chem Lett 8: 1317-1320.
- Dolain C, Patwa A, Godeau G, Barthelemy P (2012) Nucleic Acid Based Fluorinated Derivatives: New Tools for Biomedical Applications. Appl Sci 2: 245-259.
- Haas J, Mueller-Kuller T, Klein S, Engels JW (2007) RNAi activity of siRNAs modified with 2'-aminoalkyl-substituted fluorinated nucleobases. Nucleosides Nucleotides Nucleic Acids 26: 865-868.
- Koslowsky I, Shahhosseini S, Mirzayans R, Murray D, Mercer J (2011) Evaluation of an 18F-labeled oligonucleotide probe targeting p21(WAF1) transcriptional changes in human tumor cells. Oncol Res 19: 265-274.
- Vulpetti A, Dalvit C (2012) Fluorine local environment: from screening to drug design. Drug Discov Today 17: 890-897.
- Pfefferkorn CM, Lee JC (2012) 5-fluoro-D,L-tryptophan as a dual NMR and fluorescent probe of a-synuclein. Methods Mol Biol 895: 197-209.
- Zigoneanu IG, Pielak GJ (2012) Interaction of a-synuclein and a cell penetrating fusion peptide with higher eukaryotic cell membranes assessed by ¹?F NMR. Mol Pharm 9: 1024-1029.
- Boehm-Sturm P, Mengler L, Wecker S, Hoehn M, Kallur T (2011) In vivo tracking of human neural stem cells with 19F magnetic resonance imaging. PLoS One 6: e29040.
- Vargas HA, Akin O, Franiel T, Goldman DA, Udo K, et al. (2012) Normal central zone of the prostate and central zone involvement by prostate cancer: clinical and MR imaging implications. Radiology 262: 894-902.
- Brix G, Bellemann ME, Gerlach L, Haberkorn U (1999) Direct detection of intratumoral 5-fluorouracil trapping using metabolic 19F MR imaging. Magn Reson Imaging 17: 151-155.
- Brix G, Bellemann ME, Haberkorn U, Gerlach L, Bachert P, et al. (1995) Mapping the biodistribution and catabolism of 5-fluorouracil in tumor-bearing rats by chemical-shift selective 19F MR imaging. Magn Reson Med 34: 302-307.
- Dai J, Wang X, Dong Y, Yu H, Yang D, et al. (2012) Two- and three-dimensional models for the visualization of jaw tumors based on CT-MRI image fusion. J Craniofac Surg 23: 502-508.
- Dean CJ, Sykes JR, Cooper RA, Hatfield P, Carey B, et al. (2012) An evaluation of four CT-MRI co-registration techniques for radiotherapy treatment planning of prone rectal cancer patients. Br J Radiol 85: 61-68.
- Tanaka H, Hayashi S, Ohtakara K, Hoshi H, Iida T (2011) Usefulness of CT-MRI fusion in radiotherapy planning for localized prostate cancer. J Radiat Res 52: 782-788.
- Jaini S, Dadachova E (2012) FDG for therapy of metabolically active tumors. Semin Nucl Med 42:185-189.
- Colgan MB, Tarantola TI, Weaver AL, Wiseman GA, Roenigk RK, et al. (2012) The predictive value of imaging studies in evaluating regional lymph node involvement in Merkel cell carcinoma. J Am Acad Dermatol 67: 1250-1256.
- Zhu A, Marcus DM, Shu HK, Shim H (2012) Application of metabolic PET imaging in radiation oncology. Radiat Res 177: 436-448.
- Jadvar H (2011) Prostate cancer: PET with 18F-FDG, 18F- or 11C-acetate, and 18F- or 11C-choline. J Nucl Med 52: 81-89.
- Yi JS, Kim JS, Lee JH, Choi SH, Nam SY, et al. (2012) 18F-FDG PET/CT for detecting distant metastases in patients with recurrent head and neck squamous cell carcinoma. J Surg Oncol 106: 708-712.
- Buchbender C, Heusner TA, Lauenstein TC, Bockisch A, Antoch G (2012) Oncologic PET/MRI, part 1: tumors of the brain, head and neck, chest, abdomen, and pelvis. J Nucl Med 53: 928-938.
- Tatsumi M, Isohashi K, Onishi H, Hori M, Kim T, et al. (2011) 18F-FDG PET/MRI fusion in characterizing pancreatic tumors: comparison to PET/CT. Int J Clin Oncol 16: 408-415.
- Huang SH, Chien CY, Lin WC, Fang FM, Wang PW, et al. (2011) A comparative study of fused FDG PET/MRI, PET/CT, MRI, and CT imaging for assessing surrounding tissue invasion of advanced buccal squamous cell carcinoma. Clin Nucl Med 36: 518-525.
- Schwenzer NF, Schraml C, Muller M, Brendle C, Sauter A, et al. (2012) Pulmonary Lesion Assessment: Comparison of Whole-Body Hybrid MR/PET and PET/CT Imaging-Pilot Study. Radiology 264: 551-558.
Citation: Menaa F, Menaa B, Sharts ON (2013) Importance of Fluorine and Fluorocarbons in Medicinal Chemistry and Oncology. J Mol Pharm Org Process Res 1: 104. DOI: 10.4172/2329-9053.1000104
Copyright: ©2013 Menaa F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 21228
- [From(publication date): 10-2013 - Apr 01, 2025]
- Breakdown by view type
- HTML page views: 16222
- PDF downloads: 5006