Implication of Quinazoline-4(3H)-ones in Medicinal Chemistry: A Brief Review
Received: 16-Oct-2015 / Accepted Date: 15-Nov-2015 / Published Date: 22-Nov-2015 DOI: 10.4172/2572-0406.1000104
Abstract
Quinazoline, a heterocyclic compound, has been extensively studied and used in certain specific biological activities. The quinazoline-4(3H)-one and its derivatives constitute an important class of fused heterocycles that are found in more than 200 naturally occurring alkaloids. With passage of time, newer and more complex variants of the quinazolinone structures are being discovered. The stability of the quinazolinone nucleus has inspired researchers to introduce many bioactive moieties to this nucleus to create new potential medicinal agents. With a view to explore the versatile lead molecule 4(3H)-quinazolinones, a series of novel 2-methyl-3-(1’3’4-thiadiazole-2- yl)-4-(3H) quinazolinones have been synthesized by reacting 2-amino-5-aryl/alkyl-1’3’4’-thiadiazoyl with 2-substituted benzoxazin-2-one. The designed compounds have shown antibacterial activity on Staphylococcus aureus, Bacillus subtilis and Escherichia coli. In the journey of a compound to be established as a lead and finally to a drug, the problem of solubility is a major challenge for medicinal chemists and formulation scientists. The present review has touched all these issues with a hope that some of the quinazoline derivative with sufficient bioavailability could help us to counter the menace of antibiotic resistance.
Keywords: Quinazoline-4-(3H)-ones; Heterocycles; Antibiotic resistant; ADMET
9588Introduction
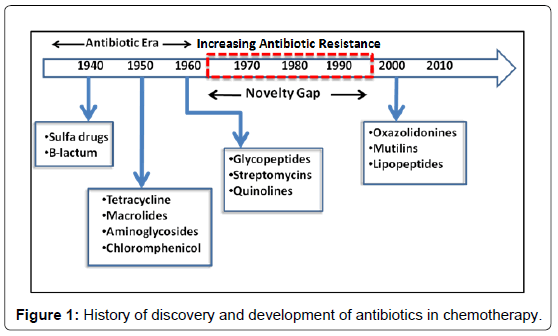
In the early 1900s, Paul Ehrlich, the legendary German chemist, initiated the use of drugs for infectious diseases. He developed methods for screening a series of chemicals for their potential activity against diseases. The term ‘‘chemotherapy’’, which means the use of chemicals to treat disease, was also coined by him [1]. The synthetic drugs were hugely used in early twentieth century (1900-1930s). But the use of synthetic drugs for treating microbial diseases reduced after the discovery and development of antibiotics. A paradigm shift in therapeutics for treating bacterial diseases took place after the industrial production of penicillin and succeeding development of other antibiotics. There was extraordinary decline in encumber of disease due to large-scale use of these antibiotics [2]. Hence, a general opinion was generated among citizens and policy-makers that infectious diseases would not produce significant problem in the future. But to everyone’s surprise, in the last few decades the historical statement, made by the surgeon-general William H. Stewart in the US Congress (1969)- “It is time to close the book on infectious diseases”, has not only been reversed but left least possibility of the closure of the said book [3]. With progress in time, the effect of antibiotics is getting drastically reduced in treatment of bacterial infection. A great constraint arose with the emergence of antibiotic resistant variants of the earlier sensitive bacteria and the emergence vis-à-vis development of new infectious diseases. A timeline representing the discovery and development of antibiotics and the emergence of antibiotic resistant bacteria is shown in Figure 1. It is true that the pressure currently imposed on pharmaceutical companies to deliver novel antimicrobials more rapidly and at lower cost will coerce innovation and discovery to enable many new methods. To tackle the problem of emergence of resistant bacteria against the newly discovered antibiotics, happening in a very short period of time from the date of release in the market, it has now become imperative to return back to the classical approach of drug design where substrate analogues gain inspiration from existing natural ligands. In this context, heterocycles are good targets and are,found abundantly in natural products. Heterocyclic compounds have already provided a platform for the rapid swap of research in the areas of organic, pharmaceutical, analytical, and medicinal chemistry. In the pharmaceutical industry, among the top two hundred branded drugs, more than 75% have heterocyclic fragments in their structures [4]. Most importantly, we find in the literature that 4(3H)-quinazolinones with 3-substitution has been associated with antimicrobial properties [5]. Hence we have attempted to review the implications of heterocycles with special emphasis on quinazoline-4-(3H)-ones in medicinal chemistry and also hint upon the prospect of developing antibacterial compounds.
Quinazoline-4-(3H)-Ones Are Basically Heterocyclic Compounds
A cyclic organic compound containing all carbon atoms in ring formation is designated as a carbocyclic compound, while the cyclic compounds consisting of at least one hetero (i.e., noncarbon) atom in the ring are known as heterocyclic compounds [6]. The most common heteroatoms are oxygen, nitrogen, and sulphur but heterocyclic ring containing other hetero atoms (such as phosphorus) are also widely known [7]. Heterocyclic chemistry comprises at least half of all organic chemistry research worldwide and are also known as the largest of classical organic synthesis [8]. They play important role in processes and industries, as well. It is well known that more than 90% of new drugs consist of heterocyclic, which play a vital role as interface between chemistry and biology [9]. Most of the discovery and development of new scientific insight consists of heterocyclic compounds. Quinazolines are heterocyclic compounds that are of considerable interest because of the diverse range of their biological activities [10].
The quinazoline-4(3H)-one and its derivatives constitute an important class of fused heterocycles that are found in more than 200 naturally occurring alkaloids. This fused bicyclic compound was earlier known as benzo-1,3-diazine. The name quinazoline (German: Chinazolin) was first proposed for this compound by Weddige, on observing that this was isomeric with the compounds cinnoline and quinoxaline [11]. A brief history of development of quinazoline moiety has been provided in Table 1. Though quinazoline had been synthesized in good yield by oxidation of 3,4 -dihydroquinazoline with alkaline potassium ferricyanide as early as in 1903, [12] it was only half a century later, from 1950 onwards, medicinal chemists started to take interest in the moiety after the elucidation of a quinazolinone alkaloid, 3-[β-keto-g-(3-hydroxy-2-piperidyl)-propyl]-4-quinazolone. This quinazolinone derivative was isolated from the traditional Chinese herb Dichroa febrifuga, which was found to be effective against malaria [13].
| Year | Discovery | Number of Quinazoline compounds known till date |
|---|---|---|
| 1869 | Griess prepared the first quinazoline derivative, 2-cyano-3,4-dihydro-4-oxoquinazoline |
>300000 quinazoline as a substructure compounds available in SciFinder. Interestingly, nearly 40000compounds were found to be biologically active |
| 1887 | The name quinazoline (German: Chinazolin) was first proposed for this compound by Weddige | |
| 1889 | Paal and Bush suggested the numbering of quinazoline ring system | |
| 1903 | More satisfactory synthesis of quinazoline was subsequently devised | |
| 1951 | The first renowned quinazoline marketed drug – Methaqualone is used for its sedative–hypnotic effects | |
| 1957 | Chemistry of quinazoline was reviewed by Williamson | |
| 1959 | Chemistry of quinazoline was further reviewed by Lindquist | |
| 1963 | Brought up to date by Armarego in 1963 | |
| 1960-2010 | More than hundred drugs containing Quinazoline moieties have made their way to the market |
Table 1: Timeline representing the development of quinazoline scaffold.
Substitutions in quinazoline scaffold
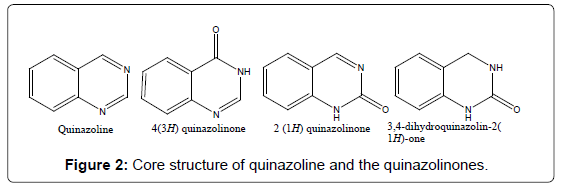
The most important class of compounds containing the quinazoline nucleus is composed of those compounds, which have hydroxyl group in the 2 or 4 positions in the quinazoline ring, adjacent to a heterocyclic nitrogen atom (Figure 2). Also considered in this class are those compounds having a functional group, which is easily derived on and converted to hydroxyl group like alkoxy, aryloxy, chloro, amino, thioethers and seleno etc. Depending upon the position of the keto or oxo group, these compounds may be classified into two types: 2-(1H) quinazolinones and 4-(3H) quinazolinones [14]. Thus 4-hydroxyquinazoline, tautomeric with 4-keto-3,4- dihydroquinazoline, is commonly named 4(3H)-quinazolone, or simply 4-quinazoline. The major subclasses of quinazolinones based on the substituents [11] present on different positions are as follows.
• 2-Substituted-4(3H)-quinazolinones
• 3-Substituted-4(3H)-quinazolinones
• 4-Substituted-quinazolines
• 2,3-Disubstituted-4(3H)-quinazolinones
Among the four quinazoline structures, 4(3H)-quinazolinones are most prevalent, either as intermediates or as natural products in many proposed biosynthetic pathways. This is partly due to the structure being derived from the anthranilates while the 2(1H)-quinazolinone is predominantly a product of anthranilonitrile, or benzamide with nitriles. Through the process of auto-oxidation quinazoline precursors can be converted to the corresponding 4(3H)-quinazolinone.
Methods of synthesis of 4-(3H) quinazolinones
The first reported synthesis of a quinazolinone occurred in 1869, which was prepared from anthranilic acid and cyanide in ethanol, creating 2-ethoxy-4(3H)-quinazolinone [15,16]. Most of the methods used for the synthesis of 4-(3H)-quinazolinones make use of anthranilic acid or one of their functional derivatives as the preparatory materials. Quinazolin-4-one is synthesized when the keto group is introduced in the pyrimidine ring of quinazoline. Based on this factor, the general methods of synthesis are listed as follows:
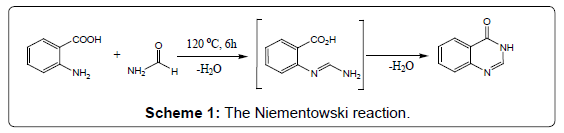
Condensation of anthranilic acid with acid amides: A simple and easy conversion to 4-(3H) quinazolinones can be achieved when anthranilic acid is heated in an open container with excess of formamide at 120°C. The reaction involves elimination of water and proceeds via an o-amidobenzamide intermediate (Scheme 1) [16]. The method is commonly known as the Niementowski synthesis. However, Besson et al. modified Niementowski synthesis to improve the yield and reaction time by using microwave irradiation techniques.
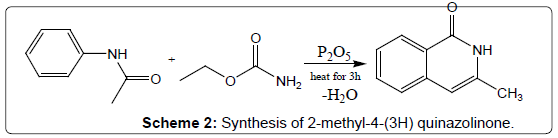
Condensation of acetanilides with urethanes: Another effective conversion is to condense a urethane derivative with aniline to give 4-(3H) quinazolinone. 2-methyl-4-(3H) quinazolinone was synthesized by heating urethane and acetanilide for 3 hours with phosphorus pentoxide in toluene (Scheme 2). Quinazolinones may also be synthesized directly from the corresponding N-acylanthranilic acid by heating with ammonia or substituted amines. 2-methyl-3- alkyl-6-nitro-4-(3H) quinazolinones have been prepared from N-acyl-5- nitroanthranilic acid and a variety of primary amines via this method [16].
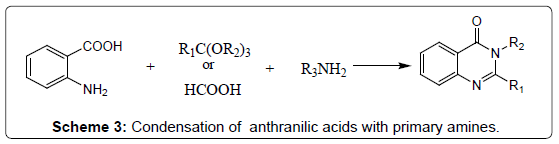
Condensation of N-acylanthranilic acids with primary amines: A survey of the literature suggests that 4-(3H) Quinazolinones may also be synthesized directly from the corresponding N-acylanthranilic acid by heating with ammonia or substituted amines (Scheme 3). Therefore, a variety of primary amines and N-acyl-5-nitroanthranilic acid have been condensed to synthesize 2-methyl-3-alkyl-6-nitro-4- (3H) quinazolinones in good yields [17].
Chemistry and chemical properties
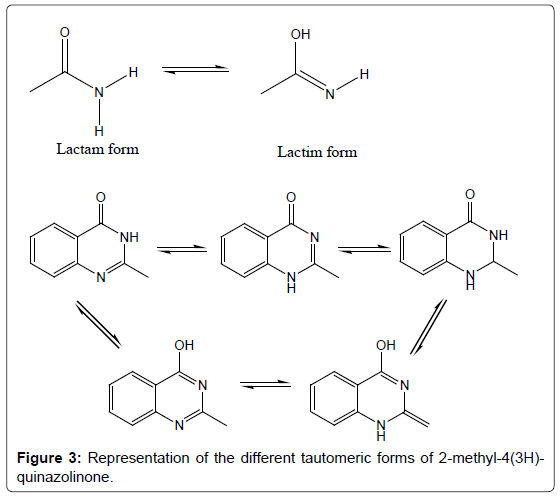
Despite quinazolinone chemistry being considered as an established area, day by day newer and more complex variants of the quinazolinone structures are still being discovered [18]. A strong lactam-lactim tautomeric interaction is observed in quinazolinones (Figure 3) [19]. This tautomericinteraction can also be observed when a 4(3H)-quinazolinone containing a methyl in the 3-position is subjected to chlorination with POCl3, the methyl group is lost and chlorination proceeds [20] and furthermore, when the methyl group is present in the 2-position, the tautomeric effect is extended generating an exo methylene carbon. As a result of these extended tautomeric effects, the reactivity of the substituted-4-(3H)-quinazolinones is increased [21]. Hence, the quinazolinones are regarded to be a “privileged structure” for drug development and discovery [22,23]. Moreover, an assortment of literature, including structure activity relationship studies, of quinazolinone ring system revealed that the positions 2, 6 and 8 of the ring system are very much important for structure activity studies. It is also suggested that chemotherapeutic activity could be increased by the inclusion of different heterocyclic moieties to the position 3 of the quinazolinone ring system.
Stability of the ring system: It has already been found and reported that the quinazolinone ring is quite stable towards oxidation, reduction and hydrolysis reactions. No reactions of ring degradation via simple chemical oxidation have been cited till date [16].
Biological importance of 4(3H)-quinazolinones
Pharmacologically quinazoline, particularly quinazolin-4-one or quinazolinone are among the most important classes of heterocyclic compounds. The stability of the quinazolinone nucleus has inspired medicinal chemists to introduce many bioactive moieties to this nucleus to synthesize new potential medicinal agents. The quinazolinone skeleton is a frequently encountered heterocycle in medicinal chemistry literature with applications including antibacterial, analgesic, antiinflammatory, antifungal, antimalarial, antihypertensive, CNS depressant, anticonvulsant, antihistaminic, antiparkinsonism, antiviral and anticancer activities [24-30].
Antileishmanial agents: Chauhan and co-workers reported four novel series of quinazolinone hybrids bearing interesting bioactive scaffolds (pyrimidine, triazine, tetrazole, and peptide). Most of the synthesized analogues exhibited potent leishmanicidal activity against intracellular amastigotes. The SAR analysis revealed that among the synthesized quinazolinone hybrids, quinazolinone pyrimidine, triazine, and ferrocene containing quinazolinone peptide displayed potent antileishmanial activity [31].
Anticonvulsant agents: Gupta and co-workers [32] have reported a new series of 2-phenyl-3-(3-(substituted-benzylideneamino))- quinazolin-4(3H)-one derivatives and screened for their anticonvulsant activity against standard models MES (maximal electroshock seizure test) for their ability to reduce seizure spread. Zheng and co-workers [33] described the syntheses and anticonvulsant activity evaluation of 5-phenyl [1,2,4]triazolo[4,3-c] quinazolin-3-amine derivatives. El- Azab and co-workers [34] designed and synthesized a new series of quinazoline analogues and evaluated for their anticonvulsant activity. Khan and Malik [35] reported a new synthesis of quinazolin-4(3H)- one substituted 1H and 2H-tetrazole derivatives and evaluated for anticonvulsant screening based on the NIH anticonvulsant drug development (ADD) program protocol. Zayed and co-workers [36] synthesized some novel derivatives of 6,8-diiodo-2-methyl- 3-substituted-quinazolin-4(3H)-ones and evaluated for their anticonvulsant activity by the maximal electroshock-induced seizure and subcutaneous pentylenetetrazole tests. The neurotoxicity was assessed using rotorod test. All the tested compounds showed considerable anticonvulsant activity in at least one of the anticonvulsant tests.
Anti-inflammatory agents: Hussein [37] reported the synthesis of 2,3-dihydro-2-(3,4-dihydroxyphenyl) pyrazolo [5,1-b] quinazolin- 9(1H)-one and tested for their anti-inflammatory activity. Eweas and co-workers [38] designed and synthesized some novel 2-pyridyl (3H)- quinazolin-4-one derivatives and evaluated for their anti-inflammatory activity. All the tested compounds showed good anti-inflammatory activity. Saravanan and co-workers [39] synthesized a new series of novel quinazolin-4(3H)-one derivative and tested for their antiinflammatory activity. A series of novel 2-(2,4-disubstituted-thiazole- 5-yl)-3-aryl-3H-quinazoline-4-one derivatives were found to be good inhibitors of NFκB and AP-1 mediated transcription activation [25]. Zayed and Hassan synthesized some novel 6,8-diiodo-2-methyl-3- substituted-quinazolin-4(3H)-ones bearing sulfonamide derivatives and evaluated for their anti-inflammatory activity by carrageenaninduced hind paw edema test using ibuprofen as a standard drug. Among the screened compounds, aliphatic side chain bearing compounds were found to be more active than those with aromatic ones [36].
Antitumor Activity: Quinazoline scaffold resembles both the purine nucleus as well as the pteridine one. As a consequence, some compounds which are able to inhibit the purinic [40] or the folic acid [41-44] metabolic pathways have been discovered. Structure modification of folic acid has also led to the discovery of a number of antifolates as efficient anticancer agents [45]. In an effort to look for the possible non-classical antifolates acting as antitumor agents, Cao et al. incorporated the dithiocarbamate moiety with 4(3H)-quinazolinone. Thus, a series of 4(3H)-quinazolinone derivatives with dithiocarbamate side chains were synthesized and tested for their in vitro antitumor activity against human myelogenous leukemia K562 cells by MTT(3- (4,5-dimethylthiazol-2-yl)-2 , 5-diphenyl-tetrazolium bromide) assay. Among them, (3,4-dihydro-2-methyl-4-oxoquinazolin-6-yl)-methyl- 4-(4-fluorophenyl)-piperazine-1-carbodithioate showed higher inhibitory activity against K562 cells with IC50 value of 0.5 μM [24].Quinazoline scaffold resembles both the purine nucleus as well as the pteridine one. As a consequence, some compounds which are able to inhibit the purinic [40] or the folic acid [41-44] metabolic pathways have been discovered. Structure modification of folic acid has also led to the discovery of a number of antifolates as efficient anticancer agents [45]. In an effort to look for the possible non-classical antifolates acting as antitumor agents, Cao et al. incorporated the dithiocarbamate moiety with 4(3H)-quinazolinone. Thus, a series of 4(3H)-quinazolinone derivatives with dithiocarbamate side chains were synthesized and tested for their in vitro antitumor activity against human myelogenous leukemia K562 cells by MTT(3- (4,5-dimethylthiazol-2-yl)-2 , 5-diphenyl-tetrazolium bromide) assay. Among them, (3,4-dihydro-2-methyl-4-oxoquinazolin-6-yl)-methyl- 4-(4-fluorophenyl)-piperazine-1-carbodithioate showed higher inhibitory activity against K562 cells with IC50 value of 0.5 μM [24].
Antimicrobial activity: It is well documented that 4(3H)- Quinazolinones with 3-substitution has been associated with antimicrobial properties [5]. Various substituted phenyl ring moieties, bridged phenyl rings, heterocyclic rings and aliphatic systems have been incorporated as substituent in the 3-substituted quinazolinones. It is also reported that hydrazine derived Schiff’s bases have potential antibacterial activity. In our previous study, we have synthesized 3-(Arylideneamino)-2-phenylquinazoline-4(3H)-ones by placing two potential bio-active sites, a quinazolone moiety as well as a Schiff’s base in the system to increase biological activity. The compounds were found to inhibit the growth of both Gram-positive (Staphylococcus aureus 6571 and Bacillus subtilis) and Gram-negative bacteria (Escherichia coli K12 and Shigella dysenteriae). We have proposed the promising effect of such compounds against Multiple Antibiotic Resistant Gramnegative enteric bacteria which could lead to the development of new drugs [46]. Recently, Bouley et al (2015), [47] have discovered E)-3- (3-carboxyphenyl)-2-(4-cyanostyryl)quinazolin-4(3H)-one as an antibiotic, effective in vivo against methicillin-resistant Staphylococcus aureus (MRSA). They also found that this antibiotic damages cell-wall biosynthesis by binding penicillin-binding protein (PBP) 2a. They
A new series of novel 2-methyl-3-(1,3,4-thiadiazol-2-yl)-4-(3H) quinazoline have been synthesized by reacting 2-amino-5-aryl/alkyl-1,3,4-thiadiazoyl with 2-substituted benzoxazin-2-one [27]. These compounds have been found to possess antibacterial activity against Staphylococcus aureus, Bacillus subtilis and Escherichia coli. Antifungal activity was screened against Candida albicans, Aspergillus niger and Curvularia lunata. Interestingly, the synthesized compounds have shown both antibacterial and antifungal activity.
Quinazolinone drugs available in the market
The limitation of the drug agents is not only the rapidly emergence of drug resistance but also the drug side effects. This fact creates a crisis in the usage of antimicrobial drugs. The unsatisfactory status of the present drugs has forced scientists to develop new antibacterial agents having broad antimicrobial spectrum. Moreover, in the present scenario, it has become imperative to resolve the setback of emergence of microbial resistance towards conventional antimicrobial agents and also to minimize the side effects of existing drugs.
It is well known that quinazolinone skeleton containing drugs have been considered as very important class of therapeutic agents, hence large number of quinazoline compounds have been synthesized and evaluated for their different biological activities. This rapid development indicates that there will be more quinazoline derivatives in clinical trials in the near future. These compounds are likely to surpass the available organic based pharmaceuticals in the very near future. The first renowned quinazoline marketed drug – Methaqualone is used for its sedative–hypnotic effects since 1951 [48]. Presently, a large number of quinazoline derivatives are patented and available in the market as potential drugs for various diseases. The following table lists out a few marketed quinazoline drugs used for treatment of various diseases (Table 2).
| S.no | Drug | IUPAC Name | Activity | References |
|---|---|---|---|---|
| 1 | Afloqualone | 6-amino-2(fluoomethyl)-3-(2-methylphenyl) quinazolin-4-one | Sedative, Hypnotic, Anticancer, Anti-Anxiety Agents | [49-50] |
| 2 | Albaconazole | 7-chloro-3-[(2R,3R)-3-(2,4-difluorophenyl)-3-hydroxy-4-(1,2,4-triazol-1-yl)butan-2-yl]quinazolin-4-one | Antifungal | [51] |
| 3 | Balaglitazone | 5-[[4-[(3,4-dihydro-3-methyl-4-oxo-2-quinazolinyl) methoxy]phenyl]methyl]-2,4-thiazolidinedione | Peroxisome proliferator-activated receptor (PPAR) gamma agonist , Antidiabetic | [52] |
| 4 | Cloroqualone | 3-(2,6-Dichlorophenyl)-2-ethyl-4-quinazolinone | Sedative, Antitussive | [49-50] |
| 5 | Diproqualone | 3-(2,3-dihydroxypropyl)-2-methyl-quinazolin-4-one | Anxiolytic,Analgesic, Antihistamine, RheumatoidArthritis | [53] |
| 6 | Etaqualone | 3-(2-ethylphenyl)-2-methyl-quinazolin-4-one | nervous system depressant properties | [54] |
| 7 | Fluproquazone | 4-(4-fluorophenyl)-7-methyl-1-propan-2-ylquinazolin-2-one | Antipyretic activity, NSAID | [55-56] |
| 8 | Halofuginone | 7-Bromo-6 chloro-3-[3-[(2S,3R)-3-hydroxy-2-piperidinyl]-2-oxopropyl]-4-quinazolinone | Coccidiostat, Antitumor, Autoimmunedisorders | [57] |
| 10 | Isaindigotone | 3-[(3,5-dimethoxy-4-oxocyclohexa-2,5-dien-1-ylidene)methyl]-2,4-dihydro-1H-pyrrolo[2,1-b]quinazolin-9-one | Acetylcholinesterase and butyrylcholinesterase | [58] |
| 11 | Ispinesib | N-(3-aminopropyl)-N- [(1R)-1-[7-chloro-3,4-dihydro-4-oxo-3- (phenylmethyl)-2-quinazolinyl]-2-methylpropyl]- 4-methyl-benzamide |
Anticancer | [59] |
| 12 | Methaqualone | 2-methyl-3-o-tolyl-4(3H)-quinazolinone | Hypnotic | [60] |
| 13 | Nolatrexed | 2-Amino-6-methyl-5-(4-pyridylthio)-1H-quinazolin-4-one | Thymidylate synthase inhibitor, Anticancer | [61] |
| 14 | Piriqualone | 3-(2-methylphenyl)-2-[(E)-2-pyridin-2-ylethenyl]quinazolin-4-one | Anticonvulsant | [62] |
| 15 | Quinethazone | 7-chloro-2-ethyl-4-oxo-1,2,3,4-tetrahydroquinazoline-6-sulfonamide | Antihypertensive | [63] |
| 16 | Raltitrexed | N-[(5-{methyl[(2-methyl-4-oxo-1,4-dihydroquinazolin-6-yl)methyl]amino}-2-thienyl)carbonyl]-L-glutamic acid | Anticancer | [64] |
| 17 | Tempostatin | 7-bromo-6-chloro-3-[3-[(2R,3S)-3-hydroxy-2-piperidyl]-2-oxo-propyl]quinazolin-4-one | inhibiting the deposition of collagen | [10] |
| 18 | Tiacrilast | (E)-3-[6-(Methylthio)-4-oxoquinazolin-3(4H)-yl]propenoic acid | Antiallergic | [65] |
| 19 | Rutaecarpine | 8,13-Dihydroindolo[2',3':3,4]pyrido[2,1-b]quinazolin-5(7H)-one | Alzheimer’s disease | [66] |
| 20 | Proquazone | 1-Isopropyl-7-methyl-4-phenyl-2(1H)-quinazolinone | non-steroidal anti-inflammatory potential | [67] |
Table 2: Some marketed available drugs contain quinazolinone moiety.
In addition to these, the other quinazoline marketed drugs are Gefitinib, Erlotinib, Trimetrexate, Vandetanib, Evodiamine, Dacomitinib, Barasertib, Cediranib, Elinogrel, Letermovir, Milciclib, Sotrastaurin, Tandutinib, Varlitinib, etc. [68].
Evolution and Prospect of Quinazolinone Chemistry
Although, recent developments in quinazoline based drugs indicate that the quinazoline and quinazolinone based pharmaceuticals will be created on a large scale by different research development processes and will become more available commercially for therapeutic uses but the mission of medicinal chemists is to design and discover hits that can be improved to leads, leads that can be optimized to candidates and candidates that will become valuable drugs. In other words, the strategy of medicinal chemists in their research is to discover new chemical entities which maximally resemble existing drugs with respect to key physicochemical and biological properties, with the knowledge that the quest for ‘drug-like’ properties may indeed help achieve good pharmacokinetic and pharmacodynamic properties [69]. In this regard, Computer-aided drug design plays a vital role in drug discovery and development and has become an indispensable tool in the pharmaceutical industry. Computational medicinal chemists can take advantage of all kinds of software and resources in the computeraided drug design field for the purposes of discovering and optimizing biologically active compounds [70].
In sum, these drug-likeness filters based on physicochemical properties and/or structural features are too simple. Moreover, these rules were inferred from the limited known drugs or drug-like molecules and may not cover the chemical space outside the data for training. It has been found that different classes of drugs focus on different chemical space and then the drug-likeness criterion may be different correspondingly [71]. Therefore, these drug-likeness filters or rules may be useful in the early stage of drug discovery to some extent while they should be used cautiously. It should however be mentioned that the process of discovering a drug is quite timely and costly. One of the current approaches for shortening the time required and cutting down the cost for the discovery of lead compounds which potentially inhibit or modulate known drug targets is to incorporate computerbased methods like docking techniques, pharmacophore-based searches and neural networking [72]. Computer-based methods have also been incorporated in the prediction of likely metabolic pathways of drug molecules, as well as predict their pharmacokinetic profiles [73- 75]. The absorption, distribution, metabolism, excretion and toxicity (ADMET) profile of a potential drug molecule should be known if it has to stand the chances of entering the market. Hence, assessing such information for lead compounds early enough would help eliminate molecules with predicted uninteresting profiles and eventually cut down the price of drug discovery [72]. Therefore, these drug-likeness filters or rules may be useful in the early stage of drug discovery to some extent while they should be used cautiously.
In the journey of a compound to get established as a lead and finally to a drug, the problem of solubility is a major challenge for medicinal chemists and formulation scientist. Literature supports that more than 40% of new chemical entities developed in pharmaceutical industry are practically insoluble in water. These poorly water soluble drugs having slow drug absorption leads to inadequate bioavailability [76]. Hence, drug solubility is the most important consideration to reach their desired concentration in systemic circulation for pharmacological activity. Essentially in pharmaceutical industry the impact of poor water solubility, or inadequate dissolution rate, on product discovery are just considered as the tip of the iceberg [77].
Therefore, the improvement of drug solubility and thereby its oral bio-availability remains one of the most challenging aspects of the drug development process especially for oral drug delivery systems. Proper selection of solubility enhancement method is the key to ensure the goals of a good formulation like good oral bioavailability, reduce frequency of dosing and better patient compliance combined with a low cost of production.
Conclusion
Quinazoline derivatives are the emerging pharmacophore which has drawn a growing interest in the arena of drug designing. In last year, about hundred publications were recorded on the quinazolone derivatives only. This article will contribute to the analog design strategies based on the pharmacophore quinazoline-4(3H)-one.
References
- DeVitaJr VT, Chu E (2008) A history of cancer chemotherapy. Cancer Res 68: 8643–8653.
- Davies J, Davies D (2010) Origins and Evolution of Antibiotic Resistance.MicrobiolMolBiol Rev 74: 417–433.
- Spellberg B, Taylor-Blake B (2013) On the exoneration of Dr. William H. Stewart: debunking an urban legend.Infect Dis Poverty 2: 3.
- Joule JA,Mills K (2007) Heterocyclic Chemistry at a Glance. Blackwell Publishing, Oxford, UK.
- Nagarajan G,KavimaniS(2010) Synthesis and in vitro antibacterial studies of some novel 3-(5-amino-6(2, 3-dichlorophenyl)-1, 2, 4-triazin-3-yl)-2-aryl quinazoline-4(3H)-one. Der Pharmacia Sinica 1: 109-116.
- Katritzky AR (1985) Handbook of Heterocyclic Chemistry, Pergamon Press,New York.
- Gomtsyan A (2012) Heterocycles in drugs and drug discovery. ChemHeterocyclCompd48: 7–10.
- Joule JA, Mills K (2000) Heterocyclic Chemistry(4th edn) Blackwell, Oxford, UK.
- DuaRS,Shrivastava SK,Sonwane SK, Shrivastava S (2011) Pharmacological significance of synthetic heterocycles scaffold: a review. AdvBiol Res 5: 120-144.
- Asif M (2014) Chemical characteristics, synthetic methods, and biological potential of quinazoline and quinazolinone derivatives.Int J Med Chem.2014: 395637.
- Rajput R,Mishra AP (2012) A Review on BiologicalActivity of Quinazolinones.Int J Pharm PharmSci 4: 66-70.
- Fry D, Kendall JD, Morgan AJ (1960)The reactivity of the alkylthio-group in nitrogen ring compounds. Part IV. Quaternary salts of 4-substituted quinazolines. J OrgChem5062–5069.
- Koepfli JB, Mead JF, Brockman JA Jr(1947) An alkaloid with high antimalarial activity from Dichroafebrifuga.J Am Chem Soc.69: 1837-1841.
- Mhaske SB, Argade NM (2006) The chemistry of recently isolated naturally occurring quinazolinone alkaloids. Tetrahedron 62:9787–9826.
- Mahato AK, Srivastava B, Nithya S (2011) Chemistry Structure activity relationship and Biological activity of Quinazoline -4 (3H)-one derivatives. Inventi Rapid Medicinal Chemistry 2(1).
- Bogert MY, Steiner SH (1905) The Synthesis of 7-Nitro-2-Alkyl-4-Ketodihydroquinazolines from 4-Nitroacet anthranilic acid, and from 4-Nitroacetanthranil. J Am ChemSoc 27: 1327-1331.
- Shobha RS, Raju B (2015) Review on QuinazolineDeirivatives, Journal of Global Trends in Pharmaceutical Sciences 6: 2510-2527.
- Weber C, Demeter A, Szendrie G, Freiner I (2003) Solid-phase synthesis of 2,6-and 2,7-diamino-4(3H)-quinazolinones via palladium-catalyzed amination.Tetrahedron let 44: 7533-7536.
- Bogert MY, Seil HA(1907) Researches on Quinazolines (eighteenth paper), on 2, 3-dialkyl-4-quinazolones and the products obtained by alkylating 2-Alkyl-4-Quinazolones (2-Alkyl-4-Hydroxy Quinazolones).J Am ChemSoc 29:517–536.
- Marr EB, Bogert MT (1935) Researches on Quinazolines. XXXIX. The Synthesis of quinazoline Derivatives Structurally Analogous to the Angostura Alkaloids Galiopine and Galipine. J Am ChemSoc 57: 729
- Cavalli A, Lizzi F, Bongarzone S, Brun R, LuiseKrauth-Siegel R, et al. (2009) Privileged structure-guided synthesis of quinazoline derivatives as inhibitors of trypanothionereductase. Bioorg Med ChemLett 19: 3031-3035.
- Akbari VK, Kahar RG, Patel KC (2013) Synthesis, characterization and antimicrobial studies of novel quinazoline derivatives of syndon 52B:1462-1467.
- Cao SL, Feng YP, Jiang YY, Liu SY, Ding GY, et al. (2005) Synthesis and in vitro antitumor activity of 4(3H)-quinazolinone derivatives with dithiocarbamate side chains. Bioorg Med ChemLett 15: 1915-1917.
- Giri RS, Thaker HM, Giordano T, Williams J, Rogers D, et al. (2009) Design, synthesis and characterization of novel 2-(2,4-disubstituted-thiazole-5-yl)-3-aryl-3H-quinazoline-4-one derivatives as inhibitors of NF-??B and AP-1 mediated transcription activation and as potential anti-inflammatory agents. Eur J MedChem 44: 2184–2189.
- Kadi AA, Azab AS, Alafeefy AM, Abdel SG (2006 ) Synthesis and biologicalscreening of some new substituted 2-mercapto-4(3H)quinazolinone analoguesas anticonvulsant agents.J PharmaSci 34: 147-158.
- Jatav V, Mishra P, Kashaw S (2008) CNS depressant and anticonvulsant activities of some novel 3-[5-substituted 1,3,4-thiadiazole-2-yl]-2-styryl quinazoline-4(3H)-ones. Eur J Med Chem 43: 1945-1951.
- Xia Y, Yang ZY, Hour MJ, Kuo SC, Xia P, et al. (2001) Antitumor agents. Part 204: synthesis and biological evaluation of substituted 2-aryl quinazolinones. Bioorg Med ChemLett 11: 1193-1196.
- Jessy EM, Sambanthan AT, Alex J, Sridevi CH, Srinivasan KK (2007) Indian J Pharm Sci 69: 476-478.
- Alagarsamy V, Thangathiruppathy A, Mandal SC, Rajasekaran S (2006) Pharmacological evaluation of 2-substituted (1,3,4) thiadiazoloquinazolines. Indian J Pharm Sci 68: 108-111.
- Sharma RN, Ravani R (2013) Synthesis and screening of 2-(2-(4-substituted piperazine-1-yl)-5-phenylthiazol-4-yl)-3-aryl quinazolinone derivatives as anticancer agents. Med Chem Res 22: 2788-2794.
- GuptaD, Kumar R, Roy RK, Sharma A, Ali I, Shamsuzzaman M, et al. (2013) Synthesisand biological evaluation of some new quinazolin-4(3H)ones derivatives as anticonvulsants. Med Chem Res 22: 3282-3288.
- Zheng Y, Bian M, Deng XQ, Wang SB, Quan ZS (2013) Synthesis and anticonvulsant activity evaluation of 5-phenyl-[1,2,4]triazolo[4,3-c]quinazolin-3-amines. Arch Pharm (Weinheim) 346: 119-126.
- El-Azab AS, Abdel-Hamide SG, Sayed-Ahmed MM, Hassan GS, El-Hadiyah TM, et al.(2013) Novel 4(3H)-quinazolinoneanalogs: synthesis and anticonvulsant activity. Medicinal Chemistry Research 22: 2815-2827.
- Malik S, Khan SA (2014) Design and evaluation of new hybrid pharmacophorequinazolino-tetrazoles as anticonvulsant strategy. Med Chem Res 23: 207-223.
- Zayed MF, Hassan MH (2014) Synthesis and biological evaluation studies of novel quinazolinone derivatives as antibacterial and anti-inflammatory agents. Saudi Pharm J 22: 157-162.
- Hussein MA (2013) Synthesis, anti-inflammatory, and structure antioxidant activity relationship of novel 4-quinazoline. Med Chem Res 22: 4641–4653.
- Eweas AF, El-Nezhawy AOH, Baiuomy AR, Awad MM (2013) Design, synthesis, anti-inflammatory, analgesic screening, and molecular docking of some novel 2-pyridyl (3H)-quinazolin-4-one derivatives. Med Chem Res 22: 1011-1020.
- Alagarsamy V, Saravanan G (2013) Synthesis and anticonvulsant activity of novel quinazolin-4(3H)-one derived pyrazoleanalogs. Med Chem Res 22: 1711-1722.
- Dempcy RO, Skibo EB (1991)Rational design of quinazoline-based irreversible inhibitors of human erythrocyte purine nucleoside phosphorylase. Biochemistry30: 8480-8487.
- Martin GJ, Moss J, AvakianS (1947) Folic acid activity of N-(4-(4-quinazoline)-benzoyl)glutamic acid. See comment in PubMed Commons below J BiolChem 167: 737-743.
- Davoll J, Johnson AM (1970) Quinazoline analogues of folic acid. J ChemSoc Perkin 8: 997-1002.
- Oatis JE Jr, Hynes JB (1977) Sycnthesis of quinazoline analogues of folic acid modified at postion 10. J Med Chem 20: 1393-1396.
- Scanlon KJ, Moroson BA, Bertino JR, Hynes JB (1979) Quinazoline analogues of folic acid as inhibitors of thymidylatesynthetase from bacterial and mammalian sources. MolPharmacol 16: 261-269.
- Nzila A (2006) The past, present and future of antifolates in the treatment of Plasmodium falciparum infection. J AntimicrobChemother 57: 1043-1054.
- Nanda AK, Ganguli S, Chakraborty R (2007) Antibacterial activity of some 3-(arylideneamino)-2-phenylquinazoline-4(3H)-ones: synthesis and preliminary QSAR studies. Molecules 12: 2413-2426.
- Bouley R, Kumarasiri M, Peng Z, Otero LH, Song W, et al. (2015) Discovery of antibiotic (E)-3-(3-carboxyphenyl)-2-(4-cyanostyryl)quinazolin-4(3H)-one. J Am ChemSoc 137: 1738-1741.
- Mhaske SB, Argade NP (2006) The chemistry of recently isolated naturally occurring quinazolinone alkaloids. Tetrahedron Letters 62: 9787–9826.
- Ochiai T, Ishida R (1982) Pharmacological studies on 6-amino-2-fluoromethyl-3-(O-tolyl)-4(3H)-quinazolinone (afloqualone), a new centrally acting muscle relaxant. (II) Effects on the spinal reflex potential and the rigidity. Jpn J Pharmacol 32:427-38.
- Chen K, Wang K, Kirichian AM,et al. (2006) Insilico design, synthesis, and biological evaluation of radioiodinatedquinazolinone derivatives for alkaline phosphatase-mediated cancer diagnosis and therapy. Mol Cancer Ther5: 3001-3013.
- Sorbera LA, Bartroli J, Castaner J (2003) Albaconazole antifungal. Drugs of the Future 28: 529-537.
- Henriksen K, Byrjalsen I, Nielsen RH, Madsen AN, Larsen LK, et al. (2009) A comparison of glycemic control, water retention, and musculoskeletal effects of balaglitazone and pioglitazone in diet-induced obese rats. Eur J Pharmacol 616: 340-345.
- Audeval B, Bouchacourt P, Rondier J (1988) Comparative study of diproqualone-ethenzamide versus glafenine for the treatment of rheumatic pain ofgonarthrosis and coxarthrosis. (French) Gazettemédicale de France 95: 70-72.
- Parmar SS, Kishor K, Seth PK, Arora RC (1969) Role of alkyl substitution in 2,3-disubstituted and 3-substituted 4-quinazolones on the inhibition of pyruvic acid oxidation. J Med Chem 12: 138-141.
- Mohing W, Suckert R, Lataste X (1981) Comparative study of fluproquazone in the management of post-operative pain. Arzneimittelforschung 31: 918-920.
- Wheatley D (1982) Analgesic properties of fluproquazone. RheumatolRehabil 21: 98-100.
- Sundrud MS, Koralov SB, Feuerer M, Calado DP, Kozhaya AE, et al. (2009) Halofuginone inhibits TH17 cell differentiation by activating the amino acid starvation response. Science 324: 1334-1338.
- Tan JH, Ou TM, Hou JQ, Lu YJ, Huang SL, et al. (2009) Isaindigotone derivatives: a new class of highly selective ligands for telomeric G-quadruplex DNA. J Med Chem 52: 2825-2835.
- Blagden SP, Molife LR, Seebaran A, Payne M, Reid AH, et al. (2008) A phase I trial of ispinesib, a kinesin spindle protein inhibitor, with docetaxel in patients with advanced solid tumours. Br J Cancer 98: 894-899.
- Smyth RD, Lee JK, Polk A, Chemburkar PB, Savacool AM (1973) Bioavailability of methaqualone. J ClinPharmacol 13: 391-400.
- JodrellDI ,Bowman A,Rye R,Byrne B, Boddy A(1999) A phase I study of the lipophilic thymidylate synthase inhibitor Thymitaq (nolatrexeddihydrochloride) given by 10-day oral administration.Br J Cancer 79: 915–920.
- Koe BK, Minor KW, Kondratas E, et al. (1986)Enhancement of benzodiazepine binding by methaqualone and related quinazolines.Drug Dev Res7: 255–268.
- Cohen E, Klarberg E, James VR Jr (1960) Quinazolinone sulfonamides. A new class of diuretic agentss. J Am ChemSoc 82: 2731–2735.
- Widemann BC, Balis FM, Godwin KS, McCully C, Adamson PC (1999) Theplasma pharmacokinetics and cerebrospinal fluid penetration of the thymidylatesynthase inhibitor raltitrexed (Tomudex) in a nonhuman primate model. Cancer ChemotherPharmacol44: 439–443.
- Welton AF, Dunton AW, McGhee B (1986) The pharmacological profile and initial clinical evaluation of tiacrilast (Ro 22-3747): a new antiallergic agent. Agents Actions 18: 313-317.
- Decker M (2005) Novel inhibitors of acetyl- and butyrylcholinesterase derived from the alkaloids dehydroevodiamine and rutaecarpine. Eur J Med Chem 40: 305-313.
- Mohri S (2001) Research and development of synthetic processes for pharmaceuticals: pursuit of rapid, inexpensive, and good processes. Journal of Synthetic Organic Chemistry 59: 514-515.
- Selvan TP, Kumar PV (2011) Quinazoline Marketed drugs - A Review. Res Pharm 1: 1-21.
- Hughes JP, Rees S, Kalindjian SB, Philpott KL (2011) Principles of early drug discovery. Br J Pharmacol 162: 1239-1249.
- Liao C, Sitzmann M, Pugliese A, Nicklaus MC (2011) Software and resources for computational medicinal chemistry. Future Med Chem 3: 1057-1085.
- Tian S, Wang J, Li Y, Li D, Xu L, et al. (2015) The application of in silico drug-likeness predictions in pharmaceutical research. Adv Drug Deliv Rev 86: 2-10.
- DiMasi JA, Hansen RW, Grabowski HG (2003) The price of innovation: new estimates of drug development costs. J Health Econ 22: 151-185.
- Darvas F, Keseru G, Papp A, Dorman G, Urge L, et al. (2002) In Silico and Ex silico ADME approaches for drug discovery. Curr Top Med Chem 2: 1287-1304.
- Hodgson J (2001) ADMETturning chemicals into drugs. Nat Biotechnol 19: 722-726.
- Cronin MTD (2003) Computer-assisted prediction of drug toxicity and metabolism in modern methods of drug discovery. In: Hilgenfeld R, Hillisch A (eds) Modern methods of drug discovery, Birkhäuser, Basel.
- Chowdary KP, Hymavathi (2004) Ind J Pharm Sci Res 63 (2) (2001) 150–154. [2] D. Corrigan, O. Corrigan, M. Healy, Int J Pharm 273: 171–182.
- Gardner CR, Walsh CT, Almarsson O (2004) Drugs as materials: valuing physical form in drug discovery. Nat Rev Drug Discov 3: 926-934.
Citation: Tiwary BK, Pradhan K, Nanda AK, Chakraborty R (2015) Implication of Quinazoline-4(3H)-ones in Medicinal Chemistry: A Brief Review. J Chem Biol Ther 1: 104. DOI: 10.4172/2572-0406.1000104
Copyright: © 2015 Tiwary BK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 26422
- [From(publication date): 1-2016 - Mar 28, 2025]
- Breakdown by view type
- HTML page views: 24120
- PDF downloads: 2302