Research Article Open Access
Impairment of the Posterior Part of the Mirror Neurons System in Alzheimer's Disease: Evidence from EEG Biomarkers
Moretti DV1*, Paternicò D1, Binetti G1, Zanetti O1 and Frisoni GB1,2
1IRCCS S, Giovanni di Dio Fatebenefratelli, Brescia, Italy
2Memory Clinic and LANVIE - Laboratory of Neuroimaging of Aging, University Hospitals and University of Geneva, Geneva, USA
- Corresponding Author:
- Moretti Davide
Vito IRCCS S. Giovanni di Dio
Fatebenefratelli via Pilastroni
4 25125 Brescia, Italy
Tel: +393924737701
E-mail: davide.moretti@afar.it
Received date: March 10, 2014; Accepted date: April 30, 2014; Published date: May 30, 2014
Citation:Moretti DV, Paternicò D, Binetti G, Zanetti O, Frisoni GB (2014) Impairment of the Posterior Part of the Mirror Neurons System in Alzheimer’s Disease: Evidence from EEG Biomarkers. J Alzheimers Dis Parkinsonism 4:153. doi: 10.4172/2161-0460.1000153
Copyright: © 2014 Moretti DV, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Mirror neurons have been localized in several locations, including the inferior parietal lobule (IPL). Increase of EEG alpha3/alpha2 frequency ratio has been detected in mild cognitive impairment (MCI) subjects who will convert in Alzheimer’s disease (AD). We investigated of the association of alpha3/alpha2 frequency ratio with cortical thickness in IPL in MCI. 74 adult subjects with MCI underwent EEG recording and high resolution MRI. Alpha3/alpha2 power ratio as well as cortical thickness was computed for each subject. Three MCI groups were obtained according to increasing tertile values of alpha3/alpha2 ratio. Difference of cortical thickness among the groups was estimated. High a3/2 group had wider cortical thinning than other groups, mapped on the IPL, Supramarginal and Precuneus bilaterally. High EEG alpha3/alpha2 frequency power ratio was associated with atrophy of IPL areas in MCI subjects. A link between AD and disrupture of mirror neurons system could be hypothesized.
Keywords
Neurons; Inferior parietal lobule; Cognitive impairment; Alzheimer’s disease
Introduction
‘‘Mirror neurons’’ were first reported in the premotor cortex of macaque monkeys [1]. These neurons fire, both when a monkey performs a specific action, but also when the monkey simply watches another monkey carry out the same action. This was the first description of a neural mechanism that allowed a ‘‘direct matching between the visual description of an action and its execution’’ [2]. There is now evidence, from functional imaging studies, to suggest that mirror neurons not only exist in man but are present in several locations, including the inferior parietal lobule (IPL) [3,4]. The inferior parietal lobule (IPL), even more so than the rest of the cortex, underwent an accelerated enlargement in the phylogenetic line leading to the great apes and hominids- splitting into the supramarginal and angular [5,6].
The Inferior parietal cortex (IPC), including the intraparietal sulcus (IPS), angular gyrus (AG), and supramarginal gyrus (SG), plays an important role in episodic memory, and is considered to be one of the specific neuroimaging markers in predicting the conversion of mild cognitive impairment (MCI) to Alzheimer’s disease (AD). With fMRI at the resting state, SG displayed decreased connectivity with a distribution of regions including the frontal and parietal regions [7].
Increasing thinning of temporo-parietal cortical areas is peculiar of AD and it could predicts conversion from MCI state to AD dementia [8]. Moreover, a PET study has demostrated that hypoperfusion in SG and AG could differentiate ADA from fronto-temporal dementia (FTD) [9]. It is widely accepted that the cerebral EEG rhythms reflect the underlying brain network activity [10]. As a consequence, modifications in EEG rhythms could be an early sign of disease associated with ADrelated structural and functional networks. Furthermore, the increase in alpha3/alpha2 power ratio has been demonstrated predictive of conversion of patients with MCI in AD, but not in non-AD dementia [11]. As a consequence, the working hypothesis of the present study is that an increase in alpha3/alpha2 EEG power ratio would like to be associated with brain atrophy in temporo-parietal brain networks, in particular with the IPL. If the case, a link between disrupture of mirror system neurons and AD should be hypothesized.
Results show that subjects with higher alpha3/alpha2 frequency power ratio when compared to subjects with lower alpha3/alpha2 frequency power ratio showed significant and wide thinning both of global cortical volume and specific brain areas, like the Supramarginal gyrus and Precuneus bilaterally. Other smaller regions of cortical thinning were localized on the right hemisphere in the Insula, Parietal and Temporal cortex. Results were discussed in the view of the possible disruption of the mirror neuron system in AD.
Materials and Methods
Subjects
74 Patients were selected from a prospective study on the natural history of cognitive impairment (the translational outpatient memory clinic-TOMC study) carried out in the outpatient facility and memory clinic of the National Institute for the Research and Care of Alzheimer’s Disease (IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy), aimed to study the natural history of persons without dementia with apparently primary cognitive deficits, i.e. not due to psychic or physical conditions, in the absence of functional impairment. All experimental protocols had been approved by the local ethics committee. Informed consent was obtained from all participants or their caregivers, according to the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Diagnostic criteria
The selection criteria has the aim to include as much as possible primary prodromal dementia due to neurodegenerative disorders. Demographic and cognitive features of the subjects in study are summarized in Table 1.
The Mini-Mental State Examination (MMSE), the Clinical Dementia Rating Scale (CDRS), the Hachinski Ischemic Scale (HIS) and the Instrumental and Basic Activities of Daily Living (IADL, BADL) have been used to evaluate the patients [12-15]. Furthermore, magnetic resonance imaging, (MRI) and laboratory testing were performed to rule out cognitive impairment due to non-degenerative origins. Inclusion criteria, based on previous studies [16-18], were all of the following: (i) memory or other cognitive disturbances reported by the patient, by a relative or by the general practitioner; (ii) MMSE score of 24–27/30, or MMSE of 28/30 and higher associated to low performance (score of 2–6 or higher) on the clock drawing test; (iii) no evidence of functional impairment in instrumental and basic activities of daily living. Exclusion criteria were: (i) patients aged 90 years and older (no minimum age to participate in the study); (ii) psychiatric diseases history (from mild to moderate or major depression; juvenileonset psychosis); (iii) present or anamnestic neurological signs of major stroke; (iv) epilepsy, drug addiction, alcohol dependence; (v) use of any psychotropic drugs, that could enhance the brain cognitive functions or bias the EEG activity (including acetylcholinesterase inhibitors); and (vi) uncontrolled systemic diseases (including diabetes mellitus); (vii) traumatic brain injuries. All subjects were right-handed. A geriatrician or neurologist performed: (i) semi-structured interview with the relatives (usually, the patient’s spouse or a child of the patient) or the patients themselves; (ii) physical and neurological examinations; (iii) tests of, gait, balance and physical function; (iv) full neuropsychological evaluation assessing verbal and non-verbal memory, attention and executive functions, abstract reasoning thinking, frontal functions, language, and apraxia and visuo-constructional abilities [19]; (v) assessment of presence and severity of depressive syndrome [20]. All the neuropsychological tests were standardized on Italian population, thus scores were compared to normative values with age, education and gender corrections in an Italian population. The subjects in study did not take psychotropic nor anti-seizure drugs.
EEG recordings
| Alpha3/Alpha2 power ratio | |||
|---|---|---|---|
| High | Low | p | |
| Number of subjects | 18 | 56 | --- |
| Age, years | 70.4 ±6.7 [60-85] |
71.4 ±4.2 [62-83] |
.45 |
| Education, years | 6.6 ±3.6 [4-18] |
6.8 ±5.1 [3-17] |
.36 |
| Mini Mental State Exam | 27.2 ±1.7 [23-29] |
27.5 ±2.3 [24-30] |
.55 |
| WMHs (mm3) | 2.78 ±2.58 | 4.54 ±3.80 | .22 |
| Alpha3/alpha2 | 1.29 ±0.1 [1.17-1.52] |
1.01±0.3 [1-1.16] |
.000 |
Table 1: Demographic and cognitive characteristics in the whole sample, disaggregated for increased levels of Alpha3/ Alpha2 (high >1, 17, low <1,17). Numbers denote mean ± standard deviation, number and [range]. p denotes significance on ANOVA.
Electrodes set in an elastic cap (Electro-Cap International, Inc.), positioned according to the 10–20 international systems, was used to record the EEG activity from 19 sites. Fpz was the site for the ground electrode whereas the left and right mastoids was used as reference for all electrodes (Figure 1). All recordings were obtained in the morning with subjects resting comfortably. The level of vigilance was maintained costant by an operator who checked on-line both the subject and the EEG traces. The subject was alerted if there were signs of behavioural and/or EEG drowsiness. Off-line re-reference of the scalp recordings to the common average was done prior to perform the detection and analysis of the EEG artifacts. A band-pass filter of 0.3–70 Hz was used to record EEG data, and a sampling rate of 250 Hz was used for digitizing (BrainAmp, BrainProducts, Germany). Impedance of the electrodes was set below 5 khz. The electrooculogram (EOG) was recorded to detect horizontal and vertical eye movements. The EEG recording lasted 5 min, with subjects resting with closed eyes. The variability of the data would be reduced with longer recordings. Anyway, the lenghthening of the recordings would also have increased the possibility to cause the slowing of EEG oscillations due to reduced vigilance and arousal. EEG data were fragmented off-line in consecutive epochs of 2 s, with a frequency resolution of 0.5 Hz then analyzed. The epochs analyzed were, on average, 140 ranging from 130 to 150. At first, a computerized automatic procedure preliminary identified the EEG epochs with ocular, muscular and other types of artifact [21-29]. Afterwards, the automatic selection was manually confirmed by two expert electroencephalographists. The epochs with ocular, muscular and other types of artifacts were no longer considered.
Analysis of individual frequency bands
The power density of EEG rhythms with a 0.5 Hz frequency resolution – ranging from 2 to 45 Hz – was computed by a digital power spectrum analysis based on the Fourier Fast Transform (FFT; Welch technique, Hanning windowing function, no phase shift). Power spectra were averaged across all recording electrodes and the theta/ alpha transition frequency (TF) and the individual alpha frequency (IAF) peak were selected as anchor frequencies, according to the literature guidelines [30, 31]. Since our EEG recordings were performed at rest, the TF, representing an estimate of the frequency at which the theta and alpha spectra intersect, was computed as the minimum power value in the alpha frequency range. On turn, the IAF frequency power represents the power value of higher peak within the extended alpha range (5–14 Hz). Based on TF and IAF, the frequency band range for each subject, was estimated as follows: delta from TF-4 to TF- 2, theta from TF-2 to TF, low alpha band (alpha1 and alpha2) from TF to IAF, and high alpha band (or alpha3) from IAF to IAF + 2. The alpha1 and alpha2 bands were computed for each subject as follows: alpha1 from TF to the middle point of the TF-IAF range, and alpha2 from such middle point to the IAF peak [21-29]. The mean frequency range computed in the whole group of MCI subjects are: delta 2.9–4.9 Hz; theta 4.9–6.9 Hz; alpha1 6.9–8.9 Hz; alpha2 8.9–10.9 Hz; alpha3 10.9–12.9 Hz. Finally, the relative power spectra was computed for each subject in the individualdetermined frequency bands. The ratio between the absolute power and the mean power spectra from 2 to 45 Hz engenders the relative power density for each frequency band. In particular, the relative band power at each band was defined as the mean of the relative band power for each frequency bin within that band. Increasing tertiles values of alpha3/alpha2 frequency power ratio were estimated in all subjects and 2 groups were thus formed: low tertile (a3/a2<1.17;) and high tertile (a3/a2>1.17). The two groups of MCI has been demostrated in previous studies to be different in nature. In particular, the high alpha3/alpha 2 EEG power ratio MCI group is at major risk to convert to Alzheimer’s disease, as well as to have different pattern of hippocampal atrophy, regional blood perfusion and basal ganglia and thalamus gray matter lesions as compared to the low alpha3/alpha2 power ratio MCI groups [23,32].
MRI scans
For each subject, a high-resolution sagittal T1 weighted volumetric MR scan was acquired at the Neuroradiology Unit of the ‘Citta` di Brescia’ Hospital, Brescia, by using a 1.0 T Philips Gyroscan scanner, with a gradient echo 3D technique: TR = 20 ms, TE = 5 ms, flip angle = 30, field of view = 220 mm, acquisition matrix 256 · 256, slice thickness 1.3 mm.
Cortical thickness estimation steps
Cortical thickness measurements for 74 MCI patients were made using a fully automated magnetic resonance imaging-based analysis technique: FreeSurfer, a set of software tools for the study of cortical and subcortical anatomy. Briefly, in the cortical surface stream, the models of the boundary between white matter and cortical gray matter as well as the pial surface were constructed. Once these surfaces are known, an array of anatomical measures becomes possible, including: cortical thickness, surface area, curvature, and surface normal at each point on the cortex. In addition, a cortical surface-based atlas has been defined based on average folding patterns mapped to a sphere and surfaces from individuals can be aligned with this atlas with a high-dimensional nonlinear registration algorithm. The surface-based pipeline consists of several stages previous described in [33,34].
Single subject analysis: For each subjects the T1-weighted, anatomical 3-D MRI dataset were converted from Dicom format into .mgz format, then intensity variations are corrected and a normalized intensity image is created. The volume is registered with the Talairach atlas through an affine registration. Next, the skull is stripped using a deformable template model [35] and extracerebral voxels are removed. The intensity normalized, skull-stripped image is then operated on by a segmentation procedure based on the geometric structure of the gray–white interface. Voxels are classified as white or grey matter, cutting planes are chosen to separate the hemispheres from each other. A white matter surface is then generated for each hemisphere by tiling the outside of the white matter mass for that hemisphere. This initial surface is then refined to follow the intensity gradients between the white and gray matter. The white surface is then nudged to follow the intensity gradients between the gray matter and CSF, obtaining the pial surface. Cortical thickness measurements were obtained by calculating the distance between those surfaces (white and pial surface) at each of approximately 160,000 points per hemisphere across the cortical mantle [36].
Group analysis: In order to relate and compare anatomical features across subjects, it is necessary to establish a mapping that specifies a unique correspondence between each location in one brain and the corresponding location in another. Thus, the pial surface of an individual subject is inflated to determine the large-scale folding patterns of the cortex and subsequently transformed into a sphere to minimize metric distortion. The folding patterns of the individual are then aligned with an average folding pattern using a high-resolution surface-based averaging. Thickness measures were mapped to the inflated surface of each participant’s brain reconstruction allowing visualization of data across the entire cortical surface. Finally, cortical thickness was smoothed with a 20-mm full width at half height Gaussian kernel to reduce local variations in the measurements for further analysis.
Statistical analysis
Differences between groups in sociodemographic and neuropsychological features were analyzed using SPSS version 13.0 (SPSS, Chicago, IL) performing an analysis of variance (ANOVA) for continuous variables and paired χ2 test for dichotomous variables. For continuous variables, post-hoc pairwise comparisons among groups were performed with the Games-Howell or Bonferroni tests depending on homogeneity of variance tested with Levene’s test.
Concerning the neuroimaging analysis, the Qdec interface in Freesurfer software was used: a vertex-by-vertex analysis was carried out performing a general linear model to analyse whether any difference in mean cortical thickness existed between groups (low: a3/a2<1.17 μV2; high: a3/a2>1.17 μV2). Age, sex, education, global cognitive level (MMSE score) and WMHs were introduced as covariates in the analysis to avoid confounding factors. Our results did not survived at p < 0.05 corrected, so we choose to apply an uncorrected but more restrictive significance threshold than 0.05 (p < .001) and we considered as significant only the clusters which also were wide equal or major to 30 mm2. Finally a surface map was generated to display the results on an average brain. For illustrative purpose significance was set to a P-value of < 0.01 uncorrected for multiple comparisons
Results
Table 1 shows the sociodemographic and neuropsychological characteristics of MCI subgroups defined by the values of alpha3/alpha2 frequency power ratio. The ANOVA analysis showed that there was not statistically significant differences between groups which resulted well paired for age, sex, white matter hyperintensities (WMHs) burden, education and global cognitive level. Anyway, age, sex, education, global cognitive level (MMSE score) and WMHs were introduced as covariates in the subsequent analysis to avoid confounding factors. Alpha3/alpha2 ratio levels were statistically (t-test) significant (p = 0.000).
Pattern of cortical thickness between groups
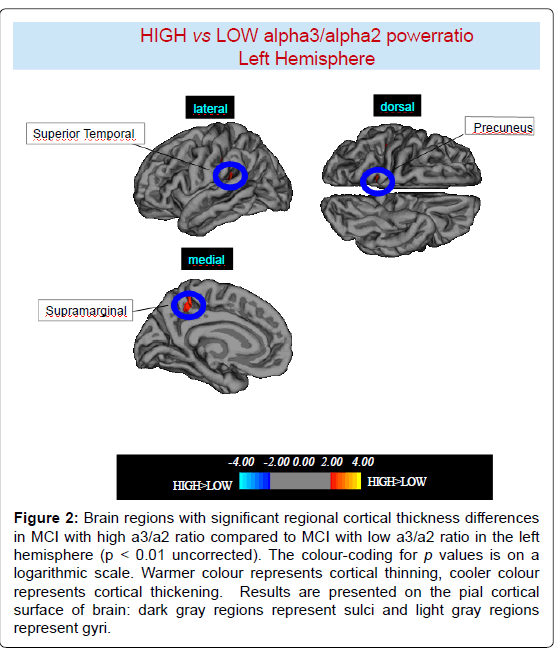
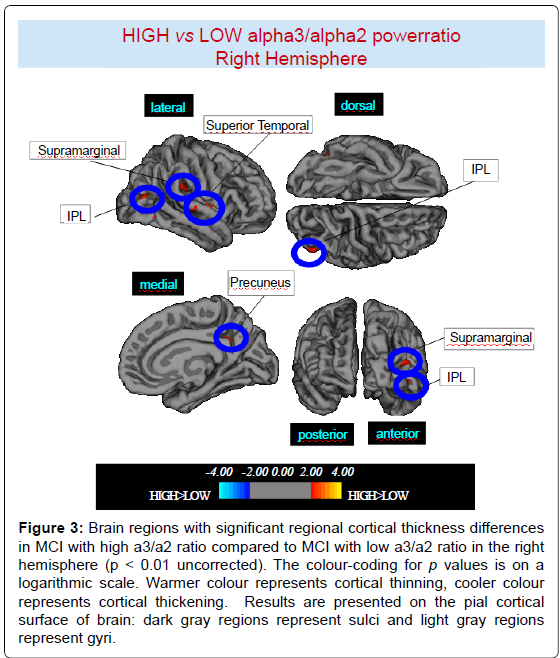
High vs Low: When compared to subjects with low a3/a2 ratios, patients with high a3/a2 ratio show thinning in the bilateral SuperioTemporal, Supramarginal and Precuneus cortex, in the right Inferior Parietal and Insula, in the left Supramarginal gyrus, and Precuneus. The total CGM reduction in High a3/2 group than Low a3/ a2 group was 471 mm2 (Figures 2, 3 and Table 2).
Discussion
Our results show a specific atrophy of cortical regions belonging to the posterior mirror neurons netework, namely the IPL. In particular, the SG, a large subdivsion of the IPL, is significanlty more atrophic in MCI subjects at major risk to develope AD. These results confirm the outcomes of a recent fMRI study, showing that, when specifically taxing cognitive skills related to the mirror neurons system, the posterior part is specifically impaired as compared to the relatively spared anterior part, located in the frontal lobe [37-42]. Moreover, two recent studies have demonstrated that the EEG high alpha3/alpha2 frequency power ratio group had a total cortical grey matter volume reduction of 471 mm2, greater than that both of the EEG low and middle alpha3/alpha2 frequency power ratio group (p < 0.001). This cortical atrophy was mapped in temporo-parietal brain areas and was correlated to memory performance, in particular in bilateral inferior parietal cortex [43-44].
The location of the IPL at the junction of the parietal, temporal and occipital lobes makes it ideally situated to perform the kinds of cross-modal abstraction (proprioception/ hearing/vision) required for motor praxis and mirror neuron-like computation. Recently it has been suggested that the mirror neuron system within the IPL (originally having evolved for the kind of cross-modal abstraction required for prehension and praxis) was subsequently exapted to also perform the more abstract types of re-conceptualisation, such as metaphor and arithmetic [45].
Figure 2:Brain regions with significant regional cortical thickness differences in MCI with high a3/a2 ratio compared to MCI with low a3/a2 ratio in the left hemisphere (p < 0.01 uncorrected). The colour-coding for p values is on a logarithmic scale. Warmer colour represents cortical thinning, cooler colour represents cortical thickening. Results are presented on the pial cortical surface of brain: dark gray regions represent sulci and light gray regions represent gyri.
Figure 3:Brain regions with significant regional cortical thickness differences in MCI with high a3/a2 ratio compared to MCI with low a3/a2 ratio in the right hemisphere (p < 0.01 uncorrected). The colour-coding for p values is on a logarithmic scale. Warmer colour represents cortical thinning, cooler colour represents cortical thickening. Results are presented on the pial cortical surface of brain: dark gray regions represent sulci and light gray regions represent gyri.
Multimodal functions of the IPL lobe play a critical role in the processing of visuo-spatial informations. Injury of angular and supramarginal gyri result in typical neuropsychological symptoms. The angular gyrus syndrome encompasses wide neuropsychological symptoms: anomie, alexia, acalculia, disgrafia, right-left disorientation, finger agnosia, ideomotor apraxia. The right parietal lobe syndrome includes visuospatial deficit, unilateral spatial neglect, constructional apraxia, aprosodia deficiency navigation and topographical disorientation. Moreover, in IPL takes place fundamental linguitic elaborations. In particular, the transition of graphemic symbols in phonological expression seems to be typical of the supramarginal gyrus. Impaired naming was associated with cortical thinning of the supramarginal gyrus (BA 40), [46].
| HIGH a3/a2 < LOW a3/a2 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cluster size (mm2) | REGION | SIDE | STEREOTAXIC COORDINATE | P | THICKNESS (mm2) | |||
| x | y | z | HIGH | LOW | ||||
| 61 | Superior Temporal | L | -47 | -36 | 9 | <0.0001 | 2.25 ± 0.15 | 2.00 ± 0.18 |
| 60 | Supramarginal | L | -40 | -36 | 18 | 0.0008 | 1.95 ± 0.18 | 2.08 ± 0.19 |
| 35 | Precuneus | L | -14 | -48 | 58 | 0.0002 | 1.87 ± 0.14 | 1.98 ± 0.19 |
| 58 | Superior temporal | R | 61 | -22 | -1 | <0.0001 | 2.18 ± 0.17 | 2.24 ± 0.24 |
| 59 | Supramarginal | R | 49 | -29 | 27 | <0.0001 | 1.94 ± 0.18 | 2.08 ± 0.20 |
| 52 | Precuneus | R | 11 | -49 | 30 | 0.0001 | 1.81 ± 0.17 | 1.93 ± 0.15 |
| 85 | Inferior parietal | R | 46 | -75 | 10 | 0.0001 | 1.94 ± 0.22 | 2.07 ± 0.23 |
| 61 | Insula | R | 38 | -2 | 3 | 0.0002 | 2.37 ± 0.28 | 2.57 ± 0.36 |
| 59 | Postcentral | L | -57 | -18 | 18 | 0.0002 | 1.51 ± 0.15 | 1.62 ± 0.17 |
| 71 | Supramarginal | L | -53 | -42 | 46 | 0.001 | 1.95 ± 0.18 | 2.11 ± 023 |
| 33 | Precuneus | L | -16 | -43 | 60 | 0.001 | 1.87 ± 0.14 | 1.94 ± 0.17 |
Table 2: Brain regions with significant regional cortical thickness differences in MCI with high a3/a2 ratio (> 1,17) compared to MCI with low a3/a2 ratio (<1,17). Cluster size represents the extension of contiguous significant voxels in the cluster obtained at p < 0.01 uncorrected (cluster size > 30 mm2). Stereotaxic coordinates reveal theposition of the most significant voxel of the cluster, and side denotes its localization on the left (L) or right (R) brain hemisphere. Thickness denotes the average cortical thickness and standard deviation values within the cluster in high and middle a3/a2 groups. P denotes the significance level of the differences in thickness between groups.
In AD both visuo-spatial and language skills are impaired. The presence of melokinetic apraxia is an early sign of AD, followed by typical disorientation or ideo-motor apraxia syndrome later in the course of disease [47]. This clinical manifestations are supported by morpho-structural and functional studies. For the discrimination of Alzheimer’s disease, the decresase of cortical thickness in supramarginal gyrus, together with ippocampal and entorinal cortex volume, has been demonstrated highly specific and sensible in identifiyng subjects with prodromal AD [48]. Furthermore, a resting PET study revealed hypoperfusion in bilateral inferior parietal lobule and cingulate scan as typical of AD covariance pattern [49]. Of note, the anomic aphasia can represent one of the initial symptoms of Alzheimer’s disease, recently coded as logopenic [50]. Recent studies have demonstrated that during the successful encoding of new items there is a desynchronization in the IPL language and visuo-spatial skills related networks whereas a synchronization prevent a successful semantic encoding [51,52]. It is of great interest that there is an overlapping between the brain regions associated with increase of EEG alpha3/alpha2 power ratio (hypersynchronization of high alpha) in our study and the IPL nodes in fMRI studies related to semantic encoding [53,54]. The deleterious role of synchronization has been recently demonstrated by an interesting study facing the intriguing reltationship between the functional and structural degeneration of temporo-parietal eteromodal associative regions in AD [55]. These regions were demonstrated to be selectively vulnerable in AD pathology, due to the damage of inhibitory interneurons providing a loss of inhibition at cellular level. According to the authors, the disinhibition provokes an increasing amount of neural activity at network level, giving as a final result an hypersynchronization of brain areas. Of note, this over activity is excitotoxic and determines cellular apoptosis and brain atrophy [55]. Also, Palop and Mucke emphasize the role of inhibitory interneuron dysfunction, leading to hyper synchronization [56]. Our results are in line with these previous influential studies. A possible integrative view of all the results could be as follows: 1) the higher neuronal activity in the hub regions starts from a disfunction of cellular inhibition; 2) the consequent disinhibition drives neural network to an over synchronization; 3) this over synchronization is peculiar of the hub regions of the IPL; 4) these over activated regions are prone ot degeneration and atrophy ; 5) a possible neurophsyiologic sign of this over synchronization is the increase of the alpha3/alpha 2 power ratio we have found in mirror system posterior regions.
Interestingly, the default mode network (DMN) is instrinsically connected with these networks. The disruption of the connectivity of these networks is observable during the resting state in asymptomatic individuals with early AD who have high amyloid burden both in the medial temporal lobe (MTL) system and the set of cortical regions collectively referred to as the default network (DMN) [51,52] . Specific regions of the default network, namely the precuneus and posterior cingulate, are selectively vulnerable to early amyloid deposition in AD pathology. These regions are also thought to play a key role in both memory encoding and retrieval, and are strongly functionally connected to the MTL. Recent studies have demonstrated that during the successful encoding of new items there is a de-synchronization in the temporo-parietal memory-related networks whereas asynchronization prevents a successful semantic encoding [55,57]
Moreover, the selective vulnerability of the MTL to amyloid deposition could explain that Alzheimer’s disease is associated with an increased risk of unprovoked seizures. Recent findings in have raised the possibility that aberrant excitatory activity with hyper synchronization of the neural network represents a primary upstream mechanism that may contribute to cognitive deficits AD patients [58]. A recent study demonstrated that amyloid fibrils induced significant membrane depolarization of pyramidal cells and increased the activity of excitatory cell populations, generating hyper synchronization in local field potentials. Such hyper synchronization of pyramidal cells in MTL culminates in epileptiform activity [59].
Conclusion
High EEG alpha3/alpha2 frequency power ratio in MCI subjects prone to convert in AD was associated with atrophy of IPL, where mirror neurons have been localized in humans. A link between AD and disrupture of mirror neurons system should be hypothesized.
References
- di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G (1992) Understanding motor events: a neurophysiological study. Exp Brain Res 91: 176-180.
- Rizzolatti G, Fogassi L, Gallese V (2001) Neurophysiological mechanisms underlying the understanding and imitation of action. Nat Rev Neurosci 2: 661-670.
- Parsons LM, Fox PT, Downs JH, Glass T, Hirsch TB, et al. (1995) Use of implicit motor imagery for visual shape discrimination as revealed by PET. Nature 375: 54-58.
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, et al. (2001) Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. Eur J Neurosci 13: 400-404.
- Hyvärinen J (1982) Posterior parietal lobe of the primate brain. Physiol Rev 62: 1060-1129.
- Ramachandran VS1, Oberman LM (2006) Broken mirrors: a theory of autism. Sci Am 295: 62-69.
- Liang P, Wang Z, Yang Y, Li K (2012) Three subsystems of the inferior parietal cortex are differently affected in mild cognitive impairment. J Alzheimers Dis 30: 475-487.
- Bakkour A, Morris JC, Dickerson BC (2009) The cortical signature of prodromal AD: regional thinning predicts mild AD dementia. Neurology 72: 1048-1055.
- Schroeter ML, Neumann J (2011) Combined Imaging Markers Dissociate Alzheimer's Disease and Frontotemporal Lobar Degeneration - An ALE Meta-Analysis. Front Aging Neurosci 3: 10.
- Steriade M (2006) Grouping of brain rhythms in corticothalamic systems. Neuroscience 137: 1087-106.
- Moretti DV, Frisoni GB, Fracassi C, Pievani M, Geroldi C, et al. (2011) MCI patients' EEGs show group differences between those who progress and those who do not progress to AD. Neurobiol Aging 32: 563-571.
- Folstein MF, Folstein SE, McHugh PR (1975) ‘Mini mental state’: a practical method for grading the cognitive state of patients for clinician. J Psychiatr Res 12: 189-98.
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL (1982) A new clinical scale for the staging of dementia. Br J Psychiatry 140: 566-572.
- Rosen WG, Terry RD, Fuld PA, Katzman R, Peck A (1980) Pathological verification of ischemic score in differentiation of dementias. Ann Neurol 7: 486-488.
- Lawton MP, Brody EM (1969) Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 9: 179-186.
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, et al. (2001) Current concepts in mild cognitive impairment. Arch Neurol 58: 1985-1992.
- Portet F, Ousset PJ, Visser PJ, Frisoni GB, Nobili F, et al. (2006) Mild cognitive impairment (MCI) in medical practice: a critical review of the concept and new diagnostic procedure. Report of the MCI Working Group of the European Consortium on Alzheimer’s Disease. J. Neurol. Neurosurg. Psychiatry 77: 714-718.
- Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P (2007) Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol 6: 734-746.
- Lezak M, Howieson D, Loring DW (2004) Neuropsychological Assessment, fourth edition. Oxford: University Press.
- Radloff LS (1977) The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement 1: 385-401.
- Moretti DV, Babiloni F, Carducci F, Cincotti F, Remondini E, et al. (2003) Computerized processing of EEG-EOG-EMG artifacts for multi-centric studies in EEG oscillations and event-related potentials. Int J Psychophysiol 47: 199-216.
- Moretti DV, Frisoni GB, Fracassi C, Pievani M, Geroldi C, et al. (2011) MCI patients' EEGs show group differences between those who progress and those who do not progress to AD. Neurobiol Aging 32: 563-571.
- Moretti DV, Paternicò D, Binetti G, Zanetti O, Frisoni GB (2012) EEG markers are associated to gray matter changes in thalamus and basal ganglia in subjects with mild cognitive impairment. Neuroimage 60: 489-96.
- Moretti DV, Pievani M, Geroldi C, Binetti G, Zanetti O, et al. (2009) Increasing hippocampal atrophy and cerebrovascular damage is differently associated with functional cortical coupling in MCI patients. Alzheimer Dis Assoc Disord 23: 323-332.
- Moretti DV, Pievani M, Fracassi C, Geroldi C, Calabria M, et al. (2008) Brain vascular damage of cholinergic pathways and EEG markers in mild cognitive impairment. J Alzheimers Dis 15: 357-372.
- Moretti DV, Prestia A, Fracassi C, Binetti G, Zanetti O, et al. (2012) Specific EEG changes associated with atrophy of hippocampus in subjects with mild cognitive impairment and Alzheimer's disease. Int J Alzheimers Dis 2012: 253153.
- Moretti DV, Prestia A, Fracassi C, Geroldi C, Binetti G, et al. (2011) Volumetric differences in mapped hippocampal regions correlate with increase of high alpha rhythm in Alzheimer's disease. Int J Alzheimers Dis 2011: 208218.
- Moretti DV, Babiloni C, Binetti G, Cassetta E, Dal Forno G, et al. (2004) Individual analysis of EEG frequency and band power in mild Alzheimer’s disease. Clin Neurophysiol 115: 299–308.
- Moretti DV, Miniussi C, Frisoni G, Zanetti O, Binetti G, et al. (2007) Vascular damage and EEG markers in subjects with mild cognitive impairment. Clin neurophysiology 118: 1866-1876.
- Klimesch W (1997) EEG-alpha rhythms and memory processes. Int J Psychophysiol 26: 319-340.
- Klimesch W (1999) EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Brain Res Rev 29: 169-195.
- Moretti DV, Pievani M, Fracassi C, Binetti G, Rosini S, et al. (2009) Increase of theta/gamma and alpha3/alpha2 ratio is associated with amygdalo-hippocampal complex atrophy. J Alzheimers Dis 17: 349-357.
- Fischl B, Sereno MI, Dale AM (1999) Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage 9: 195-207.
- Dale AM, Fischl B, Sereno MI (1999) Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 9: 179-194.
- Ségonne F, Dale AM, Busa E, Glessner M, Salat D, et al. (2004) A hybrid approach to the skull stripping problem in MRI. Neuroimage 22: 1060-1075.
- Fischl B, Dale AM (2000) Measuring the thickness of the human cerebral cortex using magnetic resonance images. PNAS 97: 11044-11049
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, et al. (2006) Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage 32: 180-194.
- Gronenschild EH, Habets P, Jacobs HI, Mengelers R, Rozendaal N, et al. (2012) The effects of FreeSurfer version, workstation type, and Macintosh operating system version on anatomical volume and cortical thickness measurements. PLoS One 7: e38234.
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, et al. (2003) Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry 60: 878-888.
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, et al. (2002) Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology 58: 695-701.
- DeCarli C, Fletcher E, Ramey V, Harvey D, Jagust WJ (2005) Anatomical mapping of white matter hyperintensities (WMH): exploring the relationships between periventricular WMH, deep WMH, and total WMH burden. Stroke 36: 50-55.
- Pennanen C, Testa C, Laasko MP, Hallikainen M, Helkala E L, et al. (2005) ;A voxel based morphometry study on mild cognitive impairment. J Neurol Neurosurg Psychiatry 76: 11–14.
- Moretti DV, Paternicò D, Binetti G, Zanetti O, Frisoni GB (2013) EEG upper/low alpha frequency power ratio relates to temporo-parietal brain atrophy and memory performances in mild cognitive impairment. Front Aging Neurosci 5: 63.
- Moretti DV, Paternicò D, Binetti G, Zanetti O, Frisoni GB (2014) Electroencephalographic Upper/Low Alpha Frequency Power Ratio Relates to Cortex Thinning in Mild Cognitive Impairment. Neurodegener Dis .
- McGeoch PD, Brang D, Ramachandran VS (2007) Apraxia, metaphor and mirror neurons. Med Hypotheses 69: 1165-1168.
- Leyton CE, Piguet O, Savage S, Burrell J, Hodges JR (2012) The neural basis of logopenic progressive aphasia. J Alzheimers Dis 32: 1051-1059.
- Ghika J (2008) Paleoneurology: neurodegenerative diseases are age-related diseases of specific brain regions recently developed by Homo sapiens. Med Hypotheses 71: 788-801.
- Devanand DP, Habeck CG, Tabert MH, Scarmeas N, Pelton GH, et al. (2006) PET network abnormalities and cognitive decline in patients with mild cognitive impairment. Neuropsychopharmacology 31: 1327-1334.
- Desikan RS, Cabral HJ, Hess CP, Dillon WP, Glastonbury CM, et al. (2009) Automated MRI measures identify individuals with mild cognitive impairment and Alzheimer's disease. Brain 132: 2048-2057.
- Gorno-Tempini ML, Brambati SM, Ginex V, Ogar J, Dronkers NF, et al. (2008) The logopenic/phonological variant of primary progressive aphasia. Neurology 71: 1227-1234.
- Sperling RA, Dickerson BC, Pihlajamaki M, Vannini P, LaViolette PS, et al. (2010) Functional alterations in memory networks in early Alzheimer's disease. Neuromolecular Med 12: 27-43.
- Chhatwal JP, Sperling RA (2012) Functional MRI of mnemonic networks across the spectrum of normal aging, mild cognitive impairment, and Alzheimer's disease. J Alzheimers Dis 31 Suppl 3: S155-167.
- Jones DT, Machulda MM, Vemuri P, McDade EM, Zeng G, et al. (2011) Age-related changes in the default mode network are more advanced in Alzheimer disease. Neurology 77: 1524-1531.
- Brier MR, Thomas JB, Snyder AZ, Benzinger TL, Zhang D, et al. (2012) Loss of intranetwork and internetwork resting state functional connections with Alzheimer's disease progression. J Neurosci 32: 8890-8899.
- de Haan W, Mott K, van Straaten EC, Scheltens P, Stam CJ (2012) Activity dependent degeneration explains hub vulnerability in Alzheimer's disease. PLoS Comput Biol 8: e1002582.
- Palop JJ, Mucke L (2010) Synaptic depression and aberrant excitatory network activity in Alzheimer's disease: two faces of the same coin? Neuromolecular Med 12: 48-55.
- Pievani M, de Haan W, Wu T, Seeley WW, Frisoni GB (2011) Functional network disruption in the degenerative dementias. Lancet Neurol 10: 829-843.
- Minkeviciene R, Rheims S, Dobszay MB, Zilberter M, Hartikainen J, et al. (2009) Amyloid beta-induced neuronal hyperexcitability triggers progressive epilepsy. J Neurosci 29: 3453-3462.
- Palop JJ, Mucke L (2009) Epilepsy and cognitive impairments in Alzheimer disease. Arch Neurol 66: 435-440.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 15087
- [From(publication date):
August-2014 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 10510
- PDF downloads : 4577