Impaired Response Inhibition in Psychosis
Received: 25-Jan-2023 / Manuscript No. CNOA-23-88689 / Editor assigned: 27-Jan-2023 / PreQC No. CNOA-23-88689(PQ) / Reviewed: 10-Feb-2023 / QC No. CNOA-23-88689 / Revised: 15-Feb-2023 / Manuscript No. CNOA-23-88689(R) / Accepted Date: 17-Feb-2023 / Published Date: 22-Feb-2023 DOI: 10.4172/cnoa.1000164
Abstract
Inhibitory Control or what is commonly known as Response Inhibition, as a neuropsychological construct is multifaceted and has been studied by a considerably large body of scientific literature. Various aspects of inhibitory control can be linked to different neurobiological, psychiatric, or behavioural implications both in the normal and pathological functioning of the brain. The psychotic spectrum consists of a group of conditions wherein research has revealed profound implications on inhibitory control. On one hand, there are various methodologies that can be successfully employed to study the various aspects of inhibitory control. On the other hand, researchers over the ages have used some classical paradigms to reveal the brain activities underlying this process. The paradigms, methods, and approaches differ from each other and have yielded results that are sometimes consistent with each other, and sometimes seem to contradict the findings in the existing literature. The differences in findings also take an attempt towards explaining the pathological brain on an anatomical, physiological as well as molecular level. The article tried to explore the existing body of literature and come up with a conceptual understanding of how the executive function of inhibitory control gets impaired in the brains of individuals suffering from disorders belonging to the psychotic spectrum and how it can be used as a parameter to separate the diagnosis of different psychotic disorders.
Keywords
Inhibitory control; Psychosis; Schizophrenia; Schizoaffective disorder; Bipolar disorder
Introduction
Psychotic disorders form a cluster of conditions that are, broadly speaking, characterized by delusions, hallucinations, disorganized thought or speech, and often accompanied by abnormal motor behavior. Most of these symptoms come under the common term of positive symptom cluster, which refers to bizarre additions to behavioural patterns. Another set of symptoms, commonly referred to as negative symptoms, refers to certain deficits in normal behavior like limited emotional expressibility or the inability to feel pleasure (anhedonia). Negative symptoms are responsible for a large amount of the morbidity associated with schizophrenia, but they are less common in other psychotic diseases [1].
Response Inhibition is one of the significantly important executive functions that refer to the cognitive ability to suppress actions that are not needed anymore by an individual or are inappropriate. In simpler words, response inhibition or inhibitory control means to “turn off” an existing thought, behavior, or action. Response inhibition can be explained on multiple levels like motor, attentional or behavioural. There are many experimental paradigms that have studied response inhibition but the most notable ones include Go/No-go and Stop- Signal Paradigms [2]. Experiments around response inhibition have been conducted and studied by various disciplines including abnormal psychology, cognitive neuroscience, and clinical neuroscience. Not just from various disciplines but researchers have also used a wide array of methodologies to dig deeper into the neural and behavioural underpinnings of response inhibition both in normal and healthy populations as well as patients suffering from psychiatric or neurological complications and these findings form the basis of a better treatment plan for patients. A study by Ye and others revealed that In comparison to control subjects, patients with Parkinson's disease had longer stop-signal reaction times, less stop-related activation in the right inferior frontal gyrus (IFG), and lower functional connectivity between the right IFG and the striatum. Similar results showing were found in patients with obsessive-compulsive disorder (OCD) in a study by Penade´s and colleagues. In the selective inhibition of motor responses, OCD patients performed much worse than controls [1].
Other studies have also confirmed deficits in response inhibition in ADHD, Autism Spectrum Disorder, etc.
This review is an attempt to focus particularly on the psychotic spectrum and how research has indicated the effect of these conditions on response inhibition. Within the psychotic spectrum, the maximum amount of research has been in the areas of schizophrenia and bipolar disorder, but fairly consistent results have also been revealed in the case of other psychotic conditions.
The Brain during Response Inhibition
The prefrontal cortex has been viewed as a major source of cognitive control and the capacity to suppress stimulus-evoked behavior since the earliest neuropsychological research. Human neuropsychology and cognitive neuroscience have discovered a network of cortical and sub-cortical regions that is particularly important for cancelling reactions seventy years after Holmes’ preliminary investigation. The role of certain brain areas like the Dorsolateral Prefrontal Cortex (DLPFC), Ventrolateral Prefrontal Cortex (VLPFC), and Anterior Cingulate Cortex (ACC) in the inhibition of response has also been indicated by the research literature. In most of these studies, the notable methodology involved event-related BOLD-fMRI signals, and the paradigms included mostly some variation of the Stop Signal or the Go/No-go task [3].
Liddle and others hypothesized that the activation in the bilateral prefrontal cortex and anterior cingulate cortex during No-go trials were statistically significant, and the results of the study confirm the same. It was also confirmed that the nature of activation in the ACC was similar to one during Go trials, which led the researchers to believe that during task performance the ACC was more responsible in doing the decision to initiate or inhibit the response.
Apart from these notable areas in the frontal cortex, other areas in the parietal cortex have also shown activation during No-go trials. The presence of reciprocal connections between the lateral frontal cortex and the parietal cortex, which have been well documented in rhesus monkeys, is compatible with the involvement of the parietal association cortex along with bilateral frontal lateral activation [4].
The Psychotic Spectrum
Schizophrenia has been associated with the frontal lobes and the idea of executive functioning since it was first theorized. Three types of study provide support for an executive function deficit in schizophrenia: neuropsychological research, neuroradiological research, and studies examining the relationship between structural and neuropsychological findings in schizophrenia patients [5]. In adult and adolescent schizophrenics, neuropsychological studies of cognitive impairment have shown a pattern of deficits suggestive of frontal system dysfunction, with impairments in delayed recall, conditional associative learning, word and design fluency, the WCST, and the SCWT, but no deficits in recognition memory, general language ability, or visuo-constructive tasks Research on prepotent response inhibition and interference control in the schizophrenia spectrum is crucial for some specific reasons.
• First, translational pathophysiology research has been made possible by the successful implementation of response inhibition tasks in animal models.
• Second, a number of inhibition tasks are used as biomarkers in the complex process of developing new drugs in the area of brain medicine, which calls for a thorough understanding of the pattern of impairment in schizophrenia.
• Third, end phenotypes for inhibitory impairments have been proposed by several researchers including Clementz and Hutton and Ettinger. End phenotypes, also known as intermediate phenotypes, identify a clinical disorder's genetic susceptibility, and they are investigated to learn more about the mechanistic implications of risk genes at various levels of study. The observation of an end phenotype in patients as well as in first-degree relatives of patients who are clinically unaffected is a crucial factor in the validation of an end phenotype.
Impairments in Schizophrenia
Symptoms of schizophrenia include abnormalities in thinking, feeling, perceiving, and acting. Numerous deficits are suggested by neurocognitive research, notably in the executive functions of patients suffering from schizophrenia [6].
Deficits in inhibition have frequently been linked to schizophrenia. These deficiencies are frequently linked to the prefrontal cortex and related networks' activity. Knowing how activities are planned and started, as well as the elements involved in stopping these actions, is necessary in order to comprehend purposeful inhibitory control. Even though schizophrenia is not the only psychopathological disorder with inadequate reaction inhibition, its issue is very distinct from that of other psychopathological groups. Considerable consideration is given to potential brain mechanisms behind the problems in initiating inhibitory responses and voluntary activities in schizophrenia.
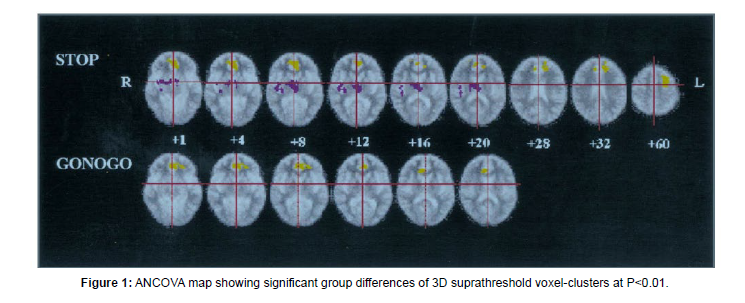
According to a large body of research, individuals with schizophrenia-spectrum illnesses display weak reaction inhibition when doing the Stop-Signal Task (SST). Apart from the areas of the Prefrontal Cortex (PFC), another notable area that has shown significant impairment is the Anterior Cingulate Cortex (ACC). In the study by Rubia, 1998 patients were made to do both go/no-go and stop signal tasks [3]. The differences between cortical and subcortical activation (or, deactivation) were highlighted (Figure 1). Patients exhibited decreased left anterior cingulate BOLD signal response throughout both inhibition tasks as well as decreased left rostral dorsolateral prefrontal and increased thalamus and putamen BOLD signal response during the execution of the stop signal task. Thus, when tested with motor response inhibition, patients with schizophrenia displayed aberrant neural network patterns of diminished left prefrontal activation and increased subcortical activity (Figure 1).
Other fMRI studies have reported similar findings. Clinically high-risk participants (CHR) and schizophrenia patients (SZ) showed reduced Right Inferior Frontal Gyrus and ACC NoGo activation compared to controls in a study conducted by Fryer. Based on their slow and inconsistent motor responses, decreased engagement of the right inferior frontal and dorsal ACC regions implicated in inhibitory control, and slow and variable motor responses, the CHR and SZ groups appear to have had difficulty developing strong and reliable prepotent responding. Only the control group displayed a functional connectivity relationship that was consistent with a higher response prepotency needing a stronger dissociation of inhibitory control regions from regions of the default mode network during response inhibition.
In some ERP studies, it was discovered that while the peak latencies of stop-signal N1 and P3 were delayed in patients, the Go stimulus and stop-signal ERP components (N1/P3) were lower in patients, indicating impairment early in stop-signal processing. In other studies reduced N1 and P3 amplitudes have also been reported [5].
Impairments in Schizoaffective disorder
The creation of a precise taxonomy for psychosis and mood illnesses is undoubtedly one of the most heated discussion points in psychiatry and neurosciences. There has always been a distinct and on-going debate over whether Schizoaffective Disorder (SA) should be classified separately from Schizophrenia (SZ) and mood disorders because of the mixed pathophysiological patterns displayed in patients suffering from the condition. In such a scenario a vital first step is the identification of biomarkers in mental illness. One such biomarker is response inhibition [6].
It is a well-established fact from event-related electrophysiological (EEG/ERP) studies that attenuated and delayed P300 has been a consistent finding in schizophrenia patients [7]. Certain studies have successfully made biological and neuropsychological distinctions between these conditions with respect to response inhibition. Mathalon found out that despite a considerable P300 drop in patients with schizophrenia, schizoaffective disorder patients did not show significantly reduced P300 amplitudes. This shows that whereas schizophrenia has compromised neurophysiological systems, schizoaffective disorder has intact mechanisms for allocating attentional resources to infrequent stimuli, whether they are task-relevant targets or task-irrelevant distractors.
In a study by Chun and others, the authors evaluated P300 as a possible biomarker to distinguish SZ, BD, and SA in the context of response inhibition. Even SZ, BD, and healthy controls may be physiologically distinctive from one another, according to findings of Sparse Logistic Regression based on specific aspects of P300, SA is still a group with less distinct demarcations.
Impairments in bipolar disorder
In all stages of the illness, impulsivity is a key feature of bipolar disorder. This increased impulsivity may be the result of abnormal response inhibition. Rapid-response impulsivity, characterized by the inability to fully process a stimulus before responding, and rewarddelay impulsivity, characterized by the inability to wait for a larger reward and associated with accelerated discounting of delayed reward, are two complementary mechanisms for response inhibition [8].
Studies have found both structural and functional abnormalities in Bipolar Disorder patients from neuroimaging and electrophysiological research. A study by Swann implied that bipolar disorder is characterized by impairments in attention and response inhibition, which are linked to a more severe course of the illness and may represent promising end phenotypes for bipolar disorder.
In the comparative study mentioned previously, P300 as an electrophysiological biomarker was successful in being able to distinguish Bipolar Disorder from Schizophrenia or Schizoaffective disorders. BD patients showed significantly longer P300 latencies compared to control participants [9]. The notable part is, when analyzing and delaying a response to NoGo stimuli, BD demonstrated normal P300 augmentation but delayed P300 latency. This suggested that BD is not related to diminished cognitive resources but rather to a distinct speed/accuracy trade-off. Particularly, latency separated BD from CT and fronto-central response inhibition-related P300 amplitude separated BD from SZ.
Findings related to P300 amplitudes are consistent, in a way, that SZ and BD are not really distinguishable based on that [2] and significantly reduced frontal N2 responses in bipolar patients, a hallmark of improper stop-signal processing, also demonstrated specific abnormalities in frontal response inhibition mechanisms. Therefore, abnormal and maybe compensatory cognitive control processes may need to be activated in order for there to be normative response inhibition in bipolar disease.
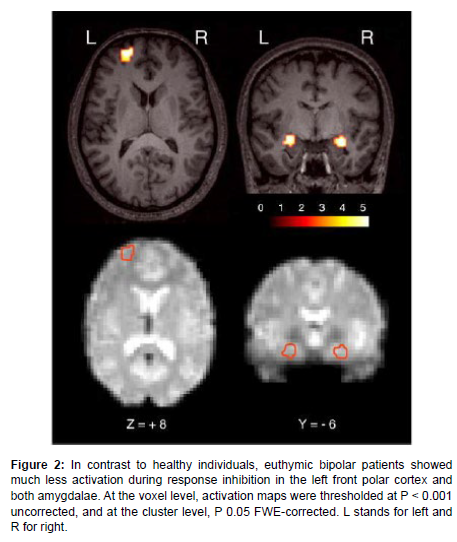
Neuroimaging results have clearly revealed the role of brain areas in BD like hypo activation in IFG, front polar cortex on the left, and dorsal amygdala on both sides (Figure 2).
Figure 2: In contrast to healthy individuals, euthymic bipolar patients showed much less activation during response inhibition in the left front polar cortex and both amygdalae. At the voxel level, activation maps were thresholded at P < 0.001 uncorrected, and at the cluster level, P 0.05 FWE-corrected. L stands for left and R for right.
Impairments in Schizotypy and related Personality types
A multidimensional personality organization known as "schizotypy" refers to a variety of personality traits that resemble the symptoms of schizophrenia, including the positive or psychotic-like dimension, the negative or deficit dimension, and the cognitivebehavioural disorganization dimension of schizophrenia. A variety of taxonomies of inhibitory control have been developed as inhibition is a heterogeneous concept [10]. A key component of executive control is the capacity to dismiss unimportant information and prevent undesirable responses. Ettinger analyzed clinically unaffected firstdegree relatives of schizophrenia patients and evaluated dimensions of schizotypy in both relatives and healthy controls to test the influence of hereditary variables and subclinical schizophrenia-like features and found associations between schizotypy and the ability to complete inhibitory tasks in relatives and controls, which are consistent with other research showing cognitive deficiencies in schizotypy.
Other studies have also reported that both at the behavioural and neural levels, schizotypy patients have problems with inhibiting responses [11]. The study examined both reactive and proactive inhibitory controls and found that compared to non-schizotypy people, those with schizotypy showed significantly higher N1 amplitude on all stop signal probability levels and higher P3 amplitudes in the case of proactive inhibition. On the other hand, during reactive inhibition, schizotypy patients showed significantly longer stop signal reaction times and smaller N2 amplitudes than non-schizotypy patients.
Certain other biomarkers like highly sensitive C-reactive protein (hsCRP) and childhood trauma have also come up in research to play a role in response inhibition in individuals with schizotypy. These findings concur with those of other research that examined the beneficial relationship between CRP levels and clinical aspects of schizophrenia. CRP level is associated with the degree of unpleasant symptoms, according to two research in particular. Not a lot of neuroimaging research has been conducted on schizophrenia proneness, oschizotypy, although one particular study did an fMRI study on prepulse inhibition (PPI) of the startle response. It refers to a decrease in reaction to a strong, startling stimulus (the pulse), when it is preceded by a non-startling stimulus of lower intensity, lasting between 30 and 500 milliseconds (the prepulse). Lower PPI and decreased activity in the inferior frontal gyrus, insula extending to putamen and thalamus, parahippocampal gyrus, inferior parietal, and middle temporal regions were linked to high psychosis propensity [12].
Discussion
The present review attempted to explore the patterns of neural mechanisms and how they differ from each other within the psychotic spectrum in terms of inhibitory control as a psychobiological marker of differentiation between the pathologies. In most of the studies, the common method for measuring response inhibition is the stop signal task, which is based on Logan's "race" model. It enables evaluation of the effectiveness of inducing an inhibitory response as well as the speed at which behavioral inhibitory (stopping) processes occur. Particularly in Psychosis, according to frontal dysfunction theories, the dorsal and ventral prefrontal areas are often involved in response inhibition [13].
In the case of Schizophrenia dysfunctions in the frontal and cingulate cortical areas became increasingly prominent while electrophysiology reflected delayed N1 and P3 ERP components with lower amplitudes.
Indeed, new research suggests that other areas like the right inferior frontal gyrus (rIFG) have a distinct response inhibition role, but the pathways implicated may vary between healthy people and those who have schizophrenia.
Schizoaffective disorder (SCA) is not well understood in relation to other psychiatric disorders. It has been proposed to be a subtype of either Bipolar Disorder (BPD) or Schizophrenia (SCZ), a heterogeneous combination of both, or the middle of a continuum with affective disease and SCZ at the two poles [14]. In the case of schizoaffective disorders, the P300 amplitudes did not take a drop like in schizophrenia which serves as a major biomarker for distinction and to some extent tries to solve the debate of classification.
Conclusion
These are consistent with the finding that Schizoaffective disorder's impairments in neurocognition and neuroimaging mimic schizophrenia more than bipolar illness. The fact that schizoaffective disorder is more skewed toward schizophrenia than bipolar disorder suggests that it is a subtype of schizophrenia or that it is a component of the continuum spectrum model of psychosis.
For Bipolar disorder, P300 has been successful in serving as a biomarker also separating the anatomy and pathophysiology of the diseases.
These findings are substantiated by electrophysiological and neuroimaging research as well and are consistently carried over to other psychiatric pathophysiology’s and even psychosis-propensity or schizotypy.
References
- Abrams R (1984) Genetic studies of the schizoaffective syndrome: a selective review. Schizophr Bull 10: 26-29.
- Aron AR (2007) The neural basis of inhibition in cognitive control. The neuroscientist 13: 214-228.
- Aron AR (2011) From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol psychiatry 69: e55-e68.
- Badcock JC, Michie PT, Johnson L, Combrinck J (2002) Acts of control in schizophrenia: dissociating the components of inhibition. Psychol Med 32: 287-297.
- Bannon S, Gonsalvez CJ, Croft RJ, Boyce PM (2002) Response inhibition deficits in obsessive–compulsive disorder. Psychiatry Res 110: 165-174.
- Bellgrove MA, Chambers CD, Vance A, Hall N, Karamitsios M, et al. (2006) Lateralized deficit of response inhibition in early-onset schizophrenia. Psychol Med 36: 495-505.
- Benes FM, Vincent SL, Alsterberg G, Bird ED, SanGiovanni JP (1992) Increased GABAA receptor binding in superficial layers of cingulate cortex in schizophrenics. J Neurosci 12: 924-929.
- Bestelmeyer PE, Phillips LH, Crombiz C, Benson P, Clair DS (2009) The P300 as a possible endophenotype for schizophrenia and bipolar disorder: Evidence from twin and patient studies. Psychiatry res 169: 212-219.
- Blasi G, Goldberg TE, Weickert T, Das S, Kohn P, et al. (2006) Brain regions underlying response inhibition and interference monitoring and suppression. Eur J Neurosci 23: 1658-1664.
- Bleuler E (1958) Dementia praecox or the group of schizophrenias, New York (International
- Carter CS, Barch DM (2007) Cognitive neuroscience-based approaches to measuring and improving treatment effects on cognition in schizophrenia: the CNTRICS initiative. Schizophr Bull 33: 1131-1137.
- Chambers CD, Bellgrove MA, Stokes MG, Henderson TR, Garavan H, et al. (2006) Executive “brake failure” following deactivation of human frontal lobe. J Cogn Neurosci 18: 444-455.
- Chun J, Karam ZN, Marzinzik F, Kamali M, O'Donnell L, et al. (2013) Can P300 distinguish among schizophrenia, schizoaffective and bipolar I disorders? An ERP study of response inhibition. Schizophr Res 151: 175-184.
- Clementz BA (1998) Psychophysiological measures of (dis) inhibition as liability indicators for schizophrenia. Psychophysiology 35: 648-668.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Universities Press) 1958. Google Scholar
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Citation: Pessoa B (2023) Impaired Response Inhibition in Psychosis. Clin Neuropsycho, 6: 164. DOI: 10.4172/cnoa.1000164
Copyright: © 2023 Pessoa B. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1966
- [From(publication date): 0-2023 - Nov 16, 2025]
- Breakdown by view type
- HTML page views: 1605
- PDF downloads: 361