Review Article Open Access
Impact of Symptomatic HIV- Related Neurocognitive Disorders in Survival of HIV- Infected Individuals: A Systematic Review and Meta-Analyses
Ahmad M Yakasai1*, Hamza Muhammad2, Aliyu Ibrahim3, Lukman F Owolabi3, Mahmood M Dalhat2, Zaiyad G. Habib4, Naseer A. Ishaq5, Aisha M. Nalado5, BabaMaiyaki M5, Muhammad S Mijinyawa5and Abdulrazaq G Habib2
1Public Health and Diagnostic Institute, College of Medical Sciences, Northwest University, Kano, Nigeria
2Infectious and Tropical Diseases Unit, Department of Medicine, Aminu Kano Teaching Hospita/Bayero University Kano, Kano, Nigeria
3Neurology Unit, Department of Medicine, Aminu Kano Teaching Hospital/ Bayero University Kano, Kano, Nigeria
4Department of Medicine University of Abuja Teaching Hospital, PMB 228 Gwagwalada Abuja, Nigeria
5Department of Medicine, Aminu Kano Teaching Hospital/ Bayero University Kano, Kano, Nigeria
- *Corresponding Author:
- Yakasai AM
Public Health and Diagnostic Institute
College of Medical Sciences
Northwest University, Kano, Nigeria
Tel: +234 806 541 9097
E-mail: mailto:ahmadmaifada@gmail.com
Received October 20, 2014; Accepted December 18, 2014; Published December 20, 2014
Citation: Yakasai AM, Muhammad H, Ibrahim A, Owolabi LF, Dalhat MM (2014) Impact of Symptomatic HIV- Related Neurocognitive Disorders in Survival of HIVInfected Individuals: A Systematic Review and Meta-Analyses. J Neuroinfect Dis 6:166. doi:10.4172/2314-7326.1000166
Copyright: ©2014 Yakasai AM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Neuroinfectious Diseases
Abstract
Background: HIV- related symptomatic neurocognitive disorders (SNCD) negatively influence the survival of affected patients. We conducted this meta-analysis to provide pooled estimates of mortality risk attributable to SNCD.
Methods: MEDLINE, Google scholar, Cochrane library PsycINFO and EMBASE were the data bases thoroughly searched up to April 2014. Two parallel meta-analyses were performed to derive hazard ratio (HR) and relative risk (RR) of mortality from 7 and 6 studies respectively. The level statistical heterogeneity in the included studies was assessed using I-squared (I2) statistic while metaregression and subgroup analyses mainly explored clinical and methodological heterogeneity. Other assessments were analyses for publication bias, small study effect, single study effect and study quality.
Results: Thirteen studies with satisfactory quality met the inclusion criteria. A total of 84 421 HIV+ individuals across 21 countries from Europe and America were involved. Subjects with SNCD have more than twice risk of death compared to subjects without SNCD: HR=2.1, 95% confidence interval (CI)=1.52-2.58; RR=2.46, 95% CI=1.63-3.69. The estimated HR translates in to 72% probability of subjects with SNCD dying earlier than subjects without SNCD. Risk of mortality is associated with declining CD4 cell count (p=0.038) and neurocognitive impairment in psychomotor and memory domains. In subgroup analyses, there was no significant difference in mortality risk with respect to HAART utilization, type of SNCD and availability of demographically adjusted normative scores. Despite limiting generalizability of findings to sub-Saharan Africa, inclusion of studies conducted in developed countries reduces confounding and increases the accuracy of defining pooled estimates.
Conclusion: HIV- related SNCD negatively influence survival in affected patients. Routine care of these patients should include neurocognitive screening preferably with a battery assessing domains that are predictive of mortality such as psychomotor and memory domains.
Keywords
HIV; Symptomatic neurocognitive disorders; Mortality; Survival; Systematic review; Meta-analysis
Abbreviations
AIDS: Acquired Immune Deficiency Syndrome; ADC: AIDS Dementia Complex; AAN: American Academy of Neurology; ANI: Asymptomatic Neurocognitive Impairment; ART: Antiretroviral Therapy; CNS: Central Nervous System; CPE: CNS Penetration Effectiveness; CSF: Cerebrospinal Fluid; COWAT: controlled oral word association test; CD4: Cluster of Differentiation; CI: Confidence Interval; ddi: Didanosine; ddC: Zalcitabine; d4T: Stavudine; FEM: Fixed Effect Model; HAART: Highly active Antiretroviral Therapy; HCV: Hepatitis C virus; HIV: Human Immunodeficiency Virus; HIV+-HIV Seropositive; HAND: HIV-associated Neurocognitive Disorders; HAD: HIV-associated Dementia; HIVD: HIV Dementia; HIVE: HIV Encephalopathy; HR: Hazard Ratio; NCI: Neurocognitive Impairment; MeSH: Medical Sub-heading; MND: Mild Neurocognitive Disorder; MCMD: Minor Cognitive Motor disorder; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-analysis; REM: Random Effects Model; RR: Relative Risk; SSA: Sub: Saharan Africa; SNCD: Symptomatic Neurocognitive Disorders; SE: Standard Error; 3TC: Lamivudine; WAIS: Weischler Adult Intelligence SCALE; WMS: Weischler Memory Scale; ZDV: Zidovudine
Introduction
Neurocognitive alterations in HIV-1 infection have been recognized since the beginning of HIV epidemic. Several terminologies were initially used to describe it: ADC, HIVE, subacute encephalitis, AIDSrelated dementia, HAND and HIVD. These conditions were clinically defined and represent the same disease with different severity. The AAN 1991 criteria was developed to provide a consensus nomenclature for both clinical and epidemiological purposes [1]. Subsequently it was reviewed in 2007 incorporating severity, functional impairment and effect of confounders to develop an algorithm for identifying HAND [2]. Most notable change was recognition of ANI.
Although there are variations in severity and diagnostic criteria, SNCD have an adverse effect on the lives of HIV+ patients especially increased mortality risk documented across several studies. [3-9]. A proposed ranking system of AIDS defining illnesses highlighted the importance of SNCD in relation to mortality [6]. In that classification ADC was found to have a moderate impact on mortality among HIV+ subjects. Despite efforts to grade HIV-associated neurocognitive syndromes and explore their impact on mortality, the magnitude and risk of mortality remains unclear due to variability in reported figures. In pre-HART era the RR of mortality from SNCD ranged from 1.47 to 6.40 while in post-HAART era the HR of mortality associated with SNCD ranged from 1.0 to 6.1 [3,5,7,10]. We conducted this meta-analysis to derive pooled estimates of the risk of mortality as a result of SNCD.
Methodology
Relevant English language publications on HIV- related SNCD and risk of mortality were searched for electronically in data bases. These included MEDLINE, Google scholar, the Cochrane data base, PsycINFO and EMBASE. Manual search of the references of relevant articles identified, systematic reviews and dissertations was also done. MeSH terms used in electronic search included ‘HIV’, ‘Neurocognitive disorders’, ‘HIV-associated Neurocognitive disorders’, ‘HIV-associated Dementia’, ‘Mild Neurocognitive Disorder, ‘Minor Cognitive Motor Disorder, ‘HIV Dementia’, ‘AIDS dementia complex’, ‘HIV-encephalopathy’, ‘Subacute encephalitis’, ‘Neurocognitive impairment’, ‘Dementia’, ‘Neurocognitive dysfunction’, ‘Neuropsychological impairment’, ‘Acquired Immune Deficiency Syndrome’, ‘mortality’, ‘death’ and ‘survival’. These MeSH terms were applied in different combinations to search for relevant publications up to April 2014. This metaanalysis adhered to the guidelines of PRISMA statements [11].
Inclusion and Exclusion Criteria
All the studies identified for possible inclusion in the meta-analysis were reviewed independently by two assessors. Whenever disagreement was encountered a third reviewer was consulted for clarification. Studies were included if they satisfied the following criteria.
1) HIV- related SNCD was reported.
2) Effect measure of mortality risk was reported or could be calculated from available data. For studies that provided risk of death for each neurocognitive domain without overall risk, data for psychomotor speed was extracted [5]. This is because psychomotor speed is commonly and consistently altered in HIV+ patients early in the disease and has been shown to predict development of dementia, AIDS and death [10]. Studies were excluded if they involved adolescents, had no required data or not meeting any of the inclusion criteria.
Extraction of Data
Information from relevant studies was collected on a Microsoft excel spread sheet. Subsequently relevant data was extracted for the meta-analysis.
Data Analysis
Hazard Ratios (HR) or Relative Risk (RR) of mortality among HIV+ subjects with SNCD were obtained from included studies. Log HR, log RR, SE of log HR and SE of log RR were computed for all the included studies. One study provided adjusted RR without confidence intervals hence unadjusted RR with 95% CI was calculated from the raw data available [10]. For studies that provided adjusted and unadjusted HR or RR, the adjusted effect measure is selected. Two parallel meta-analyses [12] were done to derive pooled estimates of HR and RR of mortality among patients with HIV-related SNCD. The probability of subjects with SNCD dying first as compared to those without SNCD was derived from the estimated HR using appropriate formula [13]. Effect measures derived from subgroup analyses were compared for statistical significance using test of interaction [11]. We assessed clinical and methodological heterogeneity via subgroup analyses while statistical heterogeneity was explored using Cochran’s Q test and I-squared (I2) statistic. Between-study heterogeneity was considered substantial when I2 is greater than 50%. Begg’s and Egger’s tests were employed to assess small study effect and publication bias [14,15]. Funnel plot derived from these tests and Galbraith plot were also used to visually assess publication bias. When heterogeneity is significant a REM is used to derive pooled estimates otherwise a FEM is used. The relationship between study-level covariates (CD4 count, age, proportion of female subjects, follow-up duration, sample size and proportion of subjects with AIDS and risk of death was analyzed using univariable weighted random effects meta-regression. Quality of included studies was also assessed. Statistical analysis was done with Stata version 10.0 (Stata Corp., College Station, TX, USA).
Characteristics of Included Studies
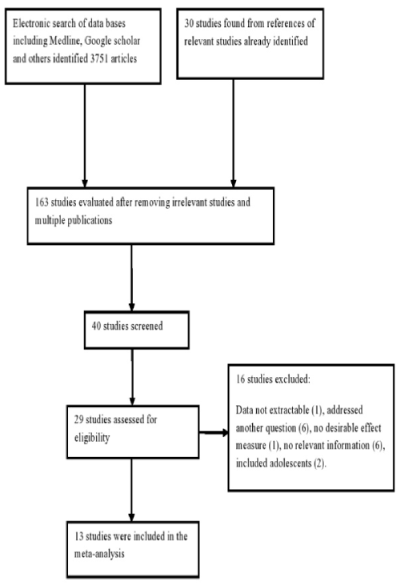
As shown in Figure 1, thirteen studies (including 2 sub-studies) met the inclusion criteria and their sociodemographic, clinical and neuropsychological characteristics are given in Tables 1 and 2 [3-10,13,14,16- 20]. They all had satisfactory quality as indicated in Table 3. One study provided data for subjects with and without virological failure [11] and another study provided data for pre- HAART and post- HAART era [19]. All the studies were conducted in Europe and America. Three studies reported mean viral load of 3.79 to 4.29 log copies/ml [3,7,8]. Prevalence of coinfection with HCV from two studies ranged from 24 to 44.6% [7,8]. The included studies excluded subjects with history of head injury, drug abuse, use of medications that could interfere with cognitive performance, CNS opportunistic infections (Toxoplasmosis, Progressive multifocal leucoencepalopathy, Cryptococcal meningitis, and Cytomegalovirus encephalitis), stroke, neuropsychiatric disorders and active psychosis. Five studies where conducted in post- HAART [3,6-8,19] while the other eight studies (and one sub-study) were conducted in pre-HAART era. [4,5,9,10,16-18,19,20] Antiretroviral drugs combinations prescribed to patients were reported in only one study (conducted in pre-HAART era): ddI + ZDV, ddC + ZDV, d4T + 3TC and ZDV alone [5].
| Authors/ publication year | Study design | Country | Follow up (yrs) | Time frame | Era | Sample size | Age * | CurrentCD4* | Confounders adjusted for |
|---|---|---|---|---|---|---|---|---|---|
| Vivithanaporn 2010[20] | PC | Canada | 7.6 | 1998-2008 | Post-HAART | 1651 | 33 (27-41) | 353 | Baseline/ nadir CD4 count, CD8 count, Viral Load, HCV, age, sex, mode of HIV transmission |
| Sevigny 2007[5] | PC | USA | >2 | 1998-2002 | Post-HAART | 329 | 41.9 | 138.6 | CD4 count, IQ score, Hb, ADI, age, race |
| Tozzi 2005(a) [19] | PC | Italy | 2.7 | From 1996 | Post-HAART | 182 | NR | NR | CD4 count, viral load, disease stage, HCV, age, sex |
| Tozzi 2005(b) [19] | PC | Italy | 2.7 | From 1996 | Post-HAART | 230 | NR | NR | Baseline CD4 count, viral load, disease stage, HCV, age, sex |
| Chaisson 1998[14] | PC | USA | 2.5 | 1989-1996 | Pre-HAART | 2081 | 35 (30-41) | 264 | Baseline CD4 count, opportunistic diseases |
| Conti 2000 (a) [15] | PC | Italy | <1 | 1990-1995 | Pre-HAART | 25737 | NR | NR | Baseline CD4 count, ADI, age, sex, region of Italy |
| Conti 2000 (b) [15] | PC | Italy | <1 | 1996-1998 | Post-HAART | 9581 | NR | NR | Baseline CD4 count, ADI, age, sex, region of Italy |
| Mocroft 1997[17] | RC | Europe b | 10 | 1979-1989 | Pre-HAART | 6548 | 34 | 86 | Baseline CD4 count, age, sex, region of Europe |
| ART-CC 2009† [4] | PC | Europe/ North America | 3.6 | 1998-2002 | Post-HAART | 31620 | 36 (31-44) | 256 | Baseline CD4 count, viral load, number/date of ART, age, sex, mode of HIV transmission |
| Ellis et al 1997[16] | PC | USA | 2.4 | 1987-1995 | Pre-HAART | 414 | 33 | 400 | CD4 count, Hb, β2 microglobulin, disease stage |
| Mayeux 1993[6] | PC | USA | 3 | 1992 | Pre-HAART | 111 | 42 (26-63) | 324.4 | CD4 count, red cell count, age, medical stage, motor symptoms |
| Sacktor 1996[9] | PC | USA | 9 | 1986-1994 | Pre-HAART | 291 | 38.1 | 543.5 | CD4 count, Hb, ARVs, number of attended visits |
| Wilkie 1998[7] | PC | USA | 3.5 | 1987-1991 | Pre-HAART | 119 | (21-58) | 234 | CD4 count, Hb, disease stage, ARVs, prophylactic drugs, age, sex |
| Hutchinson 1997 [21] | PC | Scotland | 6 | 1987-1995 | Pre-HAART | 248 | NR | 28 | CD4 count, ADI |
| Petruckevitch 1998[18] | RC | England | <1 | 1982-1995 | Pre-HAART | 2048 | NR | NR | CD4 count, disease stage, ADI, ZDV treatment, age, hospital attended |
ART-CC- Antiretroviral therapy Cohort Collaboration, ADI- AIDS defining illness, HCV- Hepatitis C virus, Hb- Hemoglobin, PC- prospective cohort, RC- retrospectively constructed cohort, NR- not reported, AAN- American Academy of Neurology criteria, ARVs-Antiretrovirals, ZDV- Zidovudine, HAART- Highly Active Antiretroviral Therapy,IQ- Intelligence Quotient. *mean or median, †17 European countries involved.
Table 1: Characteristics of included studies.
| Author/ publication year | Neurocognitive syndrome assessed | Criteria | Neurocognitive domains assessed | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SIP | Motor | Attention/ WM | Memory | Verbal fluency | Executive function | Learning | Visuospatial construction | |||
| Vivithanaporn 2010[20] | MCMD/ HAD | AAN 1991 | NA | NA | NA | NA | NA | NA | NA | - |
| Sevigny 2007[5] | HIV-D | AAN 2007 | + | + | - | + | + | - | - | - |
| Tozzi 2005 [19]a | MCMD/ HIV-D | AAN 1991 | + | - | + | - | + | + | - | - |
| Tozzi 2005 [19]b | MCMD/ HIV-D | AAN 1991 | + | - | + | - | + | + | - | - |
| Chaisson 1998[14] | ADC | NA | NA | NA | NA | NA | NA | NA | NA | - |
| Conti 2000 [15]a | HIVE | NA | NA | NA | NA | NA | NA | NA | NA | - |
| Conti 2000 [15]b | HIVE | NA | NA | NA | NA | NA | NA | NA | NA | - |
| Mocroft 1997[17] | ADC | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| ART-CC 2009[4] | ADC | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Ellis et al 1997[16] | MCMD | AAN 1991 | - | + | + | + | + | + | + | - |
| Mayeux 1993[6] | MCMD | AAN 1991 | + | + | + | + | + | + | + | + |
| Sacktor 1996[9] | NCI* | AAN 1991 | + | + | + | + | + | + | + | + |
| Wilkie 1998[7] | NCI* | NA | + | - | - | + | + | - | + | + |
| Hutchinson 1997 [21] | ADC | NA | + | - | - | + | - | - | + | - |
| Petruckevitch 1998[18] | ADC | NA | NAA | NA | NA | NA | NA | NA | NA | NA |
ART-CC- Antiretroviral therapy Cohort Collaboration, SIP- Speed of information processing, ADC- AIDS dementia complex, HIVE- HIV encephalopathy, MCMD- Minor cognitive motor disorder, HIV-D- HIV dementia, HAD- HIV-associated dementia, NA- not available, HR- Hazard Ratio, RR- Relative Risk, AAN- American Academy of Neurology criteria, WM- Working memory, NCI- Neurocognitive impairment, * NCI defined as sustained psychomotor slowing.
Table 2: Neuropsychological characteristics of included studies.
| Authors | Adequate sample size |
Adequate follow up duration | Reported baseline characteristics | Adjustment for confounders |
|---|---|---|---|---|
| Vivithanaporn [20] | Y | Y | Y | N |
| Sevigny [5] | Y | Y | Y | Y |
| Tozzi [19] | Y | Y | N | Y |
| ART-CC [4] | Y | Y | Y | Y |
| Chaisson [14] | Y | Y | Y | Y |
| Conti [15] | Y | N | N | Y |
| Mocroft [17] | Y | Y | Y | Y |
| Ellis et al [16] | Y | Y | Y | Y |
| Mayeux [6] | Y | Y | Y | Y |
| Sacktor [9] | Y | Y | Y | Y |
| Wilkie [7] | Y | Y | N | Y |
| Hutchinson [21] | Y | Y | N | Y |
| Petruckevitch [18] | Y | N | N | Y |
ART-CC- Antiretroviral therapy cohort collaboration. Y- Yes. N- NO.
Table 3: Quality assessment of included studies.
Meta-analyses
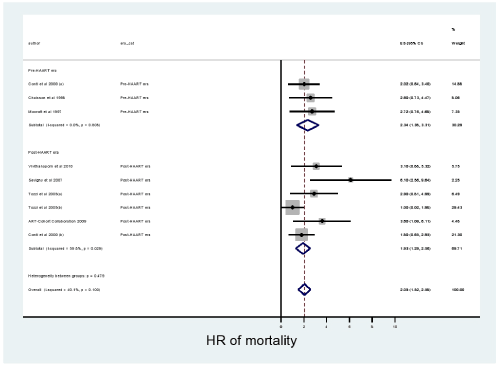
In all the statistical analyses performed there was no publication bias as p-values for both Begg’s and Egger’s tests were all non-significant. As shown in Figure 2, from 7 studies the pooled estimate of HR (95% CI) of mortality among HIV+ subjects with SNCD derived from FEM was 2.05 (1.52-2.58). Sensitivity analysis showed that the study by Mocroft et al. may influence the estimates [17]. Hence meta-analysis was repeated excluding that study and the FEM derived HR of mortality was 2.0 (1.45 -2.55). The probability of HIV+ subjects with SNCD dying earlier as compared to HIV+ subjects without SNCD was 72%.
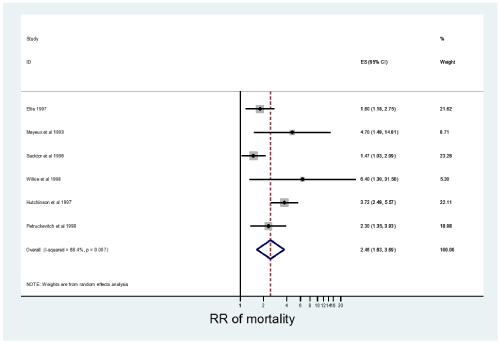
As shown in Figure 3, from 6 studies the pooled REM derived RR (95% CI) of mortality was 2.46 (1.63-3.69). Sensitivity analysis revealed that none of the studies had a profound influence on the estimates.
Subgroup analyses
Hazard ratio of mortality (95% CI) estimated from studies conducted in pre- HAART and post- HAART era were 2.34 (1.38-3.31) and 1.93 (1.29-2.56) respectively, with no significant difference between the two estimates (ratio of HR=1.2, 95% CI=0.696-2.114, p=0.248).
From 5 studies that assessed severe syndromes of SNCD (HIV-D, HIVE or ADC) the REM pooled estimate of HR (95% CI) of mortality among HIV+ subjects was 2.50 (2.07-3.03) [3,6,17,19,20]. The FEM estimate of HR (95% CI) of mortality pooled from 2 studies that assessed both mild and severe forms of SNCD (MCMD and HAD) was 2.83 (1.81-4.42) [7,8].
Studies that used age-, sex- and education-adjusted normative data to rate and classify neuropsychological test scores yielded mortality HR (95% CI) of 3.16 (2.09-4.78) [3,4,7,8]. Those studies that did not use demographically adjusted normative scores for establishing the diagnosis of SNCD yielded HR (95%) of mortality of 2.44 (2.02-2.94) [5,6,10,17- 20]. Comparison of these estimates via test of interaction yielded ratio of HR=1.3, 95% CI=0.675-1.670, p=0.887.
Meta-regression
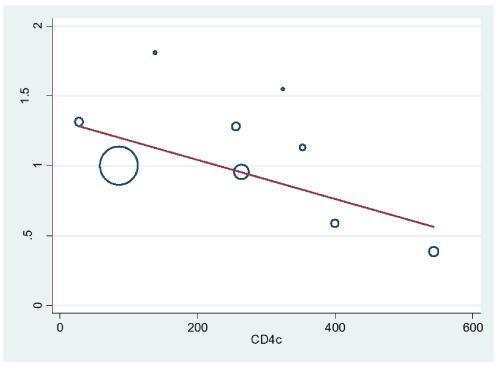
Figure 4 shows the significant relationship between risk of death and declining CD4 count. Other study-level parameters including proportion of female subjects, age, sample size and duration of follow-up were not associated with risk of mortality (respective p-values were 0.273, 0.445, 0.724 and 0.266).
Discussion
These meta-analyses involved 84 421 HIV+ individuals across 21 countries from Europe and America. In the absence of publication bias we found both HR and RR of mortality among HIV+ subjects with SNCD to be more than twice that of neurocognitively unimpaired HIV+ subjects. These two effect measures should be interpreted with caution as differences exist between them. A HR of 2.1 means that at any point in time HIV+ subjects with SNCD have more than twice chance of dying as compared to unimpaired HIV+ subjects. On the other hand RR of 2.46 indicates that among HIV+ subjects the risk of mortality among those with SNCD is more than twice that of subjects without SNCD. Nonetheless, a relationship does exist between HR and RR. The similarity or differences between these two effect measures is determined by a combination of 3 factors; duration of follow-up, risk of exposed group relative to unexposed group and rate of occurrence of desired event/outcome. When the follow-up duration is short and rate at which events occur is small, these two effect measures tend to approximate each other and their convergence increases with reducing product of the 3 determining factors [21].
The course of HIV- related neurocognitive disorders has been modified by HAART. Following the introduction of HAART in 1996 the incidence of ADC has reduced while its prevalence has increased due to prolonged survival after diagnosis. At CD4 count <100 cells/ ml in pre-HAART era subjects with ADC had 5 months median survival duration. However, at the same CD4 count in post- HAART era the median survival duration was 38 months [22]. In Australia 4 fold survival benefit was reported for ADC as against 2 fold for other NeuroAIDS diseases [23]. Despite the documented benefits of HAART in management of NeuroAIDS, patients with SNCD still had higher risk of mortality compared to those without SNCD possibly due to differences in viral dynamics. Intrathecal replication of HIV has been reported reported in patients with HAD, a severe form of SNCD [24]. Moreover, anti-retroviral drugs with low CPE could encourage viral replication in the CNS despite effective viral suppression in the peripheral blood. This enhances compartmentalization of HIV that contributes to development of neurological diseases and faster rate of disease progression.
These meta-analyses included studies conducted in Europe and America where clade B virus is predominant. Other regions of the world like SSA that is home to majority of people infected with HIV commonly have non-B virus as the predominant sub-type. AIDS-related morbidity and mortality could be influenced by viral clade diversity. Relative to sub-type A virus, sub-type D virus has been found to be associated with a higher mortality rate and a more rapid development of AIDS defining illnesses [25,26]. In patients with advanced disease and at high risk of developing neurocognitive disorders, frank dementia (HAD) is commoner among those infected with sub-type D than subtype A [27].This differential risk of dementia and death with respect to the viral sub-types may be related to the degree of neurovirulence of the various sub-types.
Meta-regression analysis in this study found significantly higher risk of mortality with declining CD4 cell count. Both the rate of decline and the rate of rise in CD4 count have been found to be associated with risk of mortality in HIV+ patients. A unit increment in CD4 cell count is associated with decreased risk of mortality in HIV+ patients with OIs [14]. On the other hand for each 100 cells/mm3 reduction in CD4 cells, NeuroAIDS is associated with 13.3% higher risk of mortality [8]. Although declining CD4 count is an important marker of immune dysfunction and a good predictor of mortality, SNCD have been found to predict mortality independent of CD4 cell count, HAART and other confounders [3,5,28].
Neurocognitive deficit in psychomotor speed [5,10] and memory [4,5] domains has been associated with increased risk of death. There has been conflicting reports with regard to deficits in language domain; Mayeux et al. found that impairment in language domain significantly predicted mortality whereas Wilkie et al. reported otherwise [4,5]. Possible explanation forwarded was that patients in the latter study were at more advanced stage of disease [5]. Sevigny et al. reported HIV dementia to be significantly associated with mortality. In that study subjects diagnosed with HIV dementia had the commonest cognitive impairment in test of verbal memory. This was followed by abnormalities in construction and motor speed in that order [3]. These conflicting reports highlighted the need for further studies to explore and char- acterize the pattern and magnitude of neurocognitive deficits in HIV+ patients with increased risk of mortality.
Our findings should be interpreted within the limits of the meta- analyses. The effect of clade diversity has been mentioned above. Among the included studies, there was none from Asia, Latin America and SSA. Two studies that failed to meet the inclusion criteria involved Sub-Saharan Africans: the first was the Swiss HIV cohort study that compared access to antiretroviral therapy, disease progression and survival between migrants from SSA and participants from Northwestern Europe in post-HAART era [29]; the second was conducted in rural Uganda and the study assessed disease progression to AIDS and death and relates these to CD4 count [30]. None of these studies assessed symptomatic neurocognitive disorders and were thus not included in the meta-analysis. This is a major concern because SSA is the region harboring the greatest burden of HIV, with poor resources, inadequate health care services, low ART coverage and high prevalence of other contributors of mortality (e.g. Tuberculosis). Hence generalizability of estimates meta-analytically pooled from studies conducted in industrialized countries may be limited.
Heterogeneity arising from differences in neuropsychological evaluation tools and criteria used is an important limitation worthy of note. Because majority of the studies were performed before the updated 2007 Frascati criteria, AAN 1991 algorithm was mainly used to classify most of the SNCD. This may not compromise the reliability of estimates in that ANI, the major difference between AAN 1991 and AAN 2007 criteria was only reported in one study that utilized the updated criteria [5]. Most importantly ANI was not included in this study owing to controversies surrounding it [31]. Lack of demographically adjusted normative scores in some of the studies could lead to under rating of impairments and in consequence misclassification of syndromes. This, however, may not have an impact on the reliability of pooled estimates since we found no significant difference between mortality HR estimates form studies with and without demographically adjusted normative neuropsychological test scores. The non-availability of viral load in majority of the included studies could not allow for assessment the effect of viral load on risk of mortality. Similarly, lack of adequate data concerning the antiretroviral drugs prescribed to study subjects precludes analysis on the impact of specific HAART drugs or regimen on improving survival.
In conclusion, HIV- related SNCD negatively influence survival in affected patients. Risk of mortality should therefore be considered when assessing the burden and impact SNCD on HIV+ patients, their families and the community at large. We recommended that clinicians and other healthcare givers should pay more attention to early detection of these conditions. Routine care of HIV+ patients should incorporate neurocognitive screening preferably with a battery assessing domains that are predictive of mortality such as psychomotor and memory.
References
- Janssen RS, Cornblath DR, Epstein LG (1991) Nomenclature and research case definitions for neurological manifestations of human immunodeficiency virus type-1 (HIV-1) infection. Report of a Working Group of the American Academy of Neurology AIDS Task Force. Neurology 41:778-785.
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, et al. (2007) Updated research nosology for HIV-associated neurocognitive disorders. Neurology 69: 1789-1799.
- Sevigny JJ1, Albert SM, McDermott MP, Schifitto G, McArthur JC, et al. (2007) An evaluation of neurocognitive status and markers of immune activation as predictors of time to death in advanced HIV infection. Arch Neurol 64: 97-102.
- Mayeux R, Stern Y, Tang MX, Todak G, Marder K, et al. (1993) Mortality risks in gay men with human immunodeficiency virus infection and cognitive impairment. Neurology 43: 176-182.
- Wilkie FL, Goodkin K, Eisdorfer C, Feaster D, Morgan R, et al. (1998) Mild cognitive impairment and risk of mortality in HIV-1 infection. J Neuropsychiatry ClinNeurosci 10: 125-132.
- Antiretroviral Therapy Cohort Collaboration (ART-CC), Mocroft A, Sterne JA, Egger M, May M, et al. (2009) Variable impact on mortality of AIDS-defining events diagnosed during combination antiretroviral therapy: not all AIDS-defining conditions are created equal. Clin Infect Dis 48: 1138-1151.
- Tozzi V, Balestra P, Serraino D, Bellagamba R, Corpolongo A, et al. (2005) Neurocognitive impairment and survival in a cohort of HIV-infected patients treated with HAART. AIDS Res Hum Retroviruses 21: 706-713.
- Vivithanaporn P, Heo G, Gamble J, Krentz HB, Hoke A, et al. (2010) Neurologic disease burden in treated HIV/AIDS predicts survival: a population-based study. Neurology 75: 1150-1158.
- Hutchinson SJ, Brettle RP, Gore SM (1997) Predicting survival in AIDS: refining the model. QJM 90: 685-692.
- Sacktor NC, Bacellar H, Hoover DR, Nance-Sproson TE, Selnes OA, et al. (1996) Psychomotor slowing in HIV infection: a predictor of dementia, AIDS and death. J Neurovirol 2: 404-410.
- Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6: e1000097.
- DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177-188.
- Symons MJ, Moore DT (2002) Hazard rate ratio and prospective epidemiological studies. J ClinEpidemiol 55: 893-899.
- Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50: 1088-1101.
- Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629-634.
- Ellis RJ, Deutsch R, Heaton RK, Marcotte TD, McCutchan JA, et al. (1997) Neurocognitive impairment is an independent risk factor for death in HIV infection. San Diego HIV Neurobehavioral Research Center Group. Arch Neurol 54: 416-424.
- Mocroft AJ, Lundgren JD, Monforte AD, Ledergerber B, Barton SE, et al. (1997) For the AIDS in Europe study group. Survival of AIDS patients according to type of AIDS-defining event. International Journal of Epidemiology 26: 400-407.
- Petruckevitch A, Del Amo J, Phillips AN, Johnson AM, Stephenson J, et al. (1998) Disease progression and survival following specific AIDS-defining conditions: a retrospective cohort study of 2048 HIV-infected persons in London. AIDS 12: 1007-1013.
- Conti S, Masocco M, Pezzoti P, Toccaceli V, Vichi M, et al. (2000) Differential impact of combined antiretroviral therapy on the survival of Italian patients with specific AIDS-defining illnesses. Journal of Acquired immune deficiency syndromes 25: 451-458.
- Chaisson RE, Gallant JE, Keruly JC, Moore RD (1998) Impact of opportunistic disease on survival in patients with HIV infection. AIDS 12: 29-33.
- Symons MJ, Moore DT (2002) Hazard rate ratio and prospective epidemiological studies. J ClinEpidemiol 55: 893-899.
- Dore GJ, McDonald A, Li Y, Kaldor JM, Brew BJ, for the national HIV surveillance committee. (2003) Marked improvement in survival following AIDS dementia complex in the era of highly active antiretroviral therapy. AIDS 17:1539 -1545.
- Dore GJ, Li Y, McDonald A, Ree H, Kaldo JM, for the national HIV surveillance committee. (2002) Impact of highly active antiretroviral therapy on individual AIDS-defining illness incidence and survival in Australia. J Acquir Immune DeficSyndr 29:388-95.
- Christo PP, Greco DB, Aleixo AW, Livramento JA. (2007) Factors influencing cerebrospinal fluid and plasma HIV-1 RNA detection rate in patients with and without opportunistic neurological disease during the HAART era. BMC Infectious Diseases 7:147.
- Baeten JM, Chohan B, Lavreys L, Chohan V, McClelland RS, et al. (2007) HIV-1 subtype D infection is associated with faster disease progression than subtype A in spite of similar plasma HIV-1 loads. J Infect Dis 195: 1177-1180.
- KiwanukaN, Laeyendecker O, Robb M, Kigozi G, Arroyo M, et al. (2008) Effect of human immunodeficiency virus Type 1 (HIV-1) subtype on disease progression in persons from Rakai, Uganda, with incident HIV-1 infection. J Infect Dis 197: 707-713.
- Sacktor N, Nakasujja N, Skolasky RL, Rezapour M, Robertson K, et al. (2009) HIV subtype D is associated with dementia, compared with subtype A, in immunosuppressed individuals at risk of cognitive impairment in Kampala, Uganda. Clin Infect Dis 49: 780-786.
- Price RW, Yiannoutsos CT, Clifford DB, Zaborski L, Tselis A, et al. (1999) Neurological outcomes in late HIV infection: adverse impact of neurological impairment on survival and protective effect of antiviral therapy. AIDS Clinical Trial Group and Neurological AIDS Research Consortium study team. AIDS 13: 1677-1685.
- Altman DG, Bland JM (2003) Interaction revisited: the difference between two estimates. BMJ 326: 219.
- Staehelin C, Rickenbach M, Low N, Egger M, Ledergerber B, et al. (2003) Migrants from Sub-Saharan Africa in the Swiss HIV Cohort Study: access to antiretroviral therapy, disease progression and survival. AIDS 17: 2237-2244.
- Morgan D, Mahe C, Mayanja B, Okongo JM, Lubega R, et al. (2002) HIV-1 infection in rural Africa: is there a difference in median time to AIDS and survival compared with that in industrialized countries? AIDS 16: 597-603.
Relevant Topics
- Bacteria Induced Neuropathies
- Blood-brain barrier
- Brain Infection
- Cerebral Spinal Fluid
- Encephalitis
- Fungal Infection
- Infectious Disease in Children
- Neuro-HIV and Bacterial Infection
- Neuro-Infections Induced Autoimmune Disorders
- Neurocystercercosis
- Neurocysticercosis
- Neuroepidemiology
- Neuroinfectious Agents
- Neuroinflammation
- Neurosyphilis
- Neurotropic viruses
- Neurovirology
- Rare Infectious Disease
- Toxoplasmosis
- Viral Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 14768
- [From(publication date):
February-2015 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 10127
- PDF downloads : 4641