Commentary Open Access
Impact of Seawater Salinity on Ultrastructure of Chloroplasts and Oleosomes in Relation to Fat Metabolism in Flag Leaf of Two Wheat Cultivars During Grain-filling
| Heshmat Soliman Aldesuquy* | |
| Botany Department, Faculty of Science, Mansoura University, Mansoura, Egypt | |
| Corresponding Author : | Heshmat Soliman Aldesuquy Botany Department Faculty of Science Mansoura University Mansoura, Egypt Tel: +2-050-2223786/201006573700 E-mail: heshmat-aldesuquy@hotmail.com |
| Received September 15, 2015; Accepted November 07, 2015; Published November 13, 2015 | |
| Citation: Aldesuquy HS (2015) Impact of Seawater Salinity on Ultrastructure of Chloroplasts and Oleosomes in Relation to Fat Metabolism in Flag Leaf of Two Wheat Cultivars During Grain-filling. Adv Crop Sci Tech 4:200. doi:10.4172/2329-8863.1000200 | |
| Copyright: © 2015 Aldesuquy HS. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Advances in Crop Science and Technology
Abstract
Salt stress enhances generation of Reactive Oxygen Species (ROS) which are usually produced within chloroplast and mitochondria because of sustained flow of electrons in these organelles, so that chloroplasts are one of the most sensitive systems to various stress factors. Effect of seawater salinity (10% and 25%) on ultrastructure of chloroplasts and oleosomes as well as oleosomes volume in relation to fat metabolism in flag leaf of two wheat cultivars, Gemmieza-9 (salt sensitive cultivar) and Sids-1 (salt tolerant cultivar) during grain-filling was investigated. Irrigation of wheat plants with 25% seawater induced dramatic changes in chloroplasts and oleosomes particularly after 21 days post-anthesis. The results showed that there were slight differences between the two cultivars in response to seawater at 10% and 14 days post-anthesis in terms of chloroplasts ultrastructure. Moreover, disorganized membrane system was identified with swollen thylakoids and many plastoglobuli were recognized in the chloroplasts in comparison to control plants. Changes in membrane structure are mainly due to the rapid oxidative damage evaluated as malondialdehyde, membrane leakage and membrane stability index. Numerous spherical oleosomes were observed as free in the vacuole of flag leaf cells of both untreated and seawater treated plants. Oleosomes appeared to have a sharply-defined osmiophilic interface and apparently lack a limiting membrane. Seawater irrigation induced a progressive increase in lipase activity and glycerol content in flag leaf of both cultivars during grain-filling. Sids-1 accumulated more glycerol and total saturated fatty acids percentage as well as more reduction in total unsaturated fatty acids percentage, mono-unsaturated fatty acids percentage and poly-unsaturated fatty acids percentage than sensitive one under salt-stress.
| Keywords |
| Chloroplasts; Fatty acids; Oleosomes; Seawater; Ultrastructure; Wheat |
| Introduction |
| To cope with the shortage fresh water for the possible development of agriculture, there is increasing awareness among agricultural scientists and planners in the utilization of seawater (at least diluted) for irrigation of wheat crop [1]. The effects of hyper salinity on leaf ultrastructure and physiology in the mangrove, Avicennia marina, were investigated [2]. |
| Oleosomes are a characteristic feature of leaf mesophyll cells (palisade and spongy parenchyma) in many angiosperms and they could occupy as much as an estimated 15% of mesophyll cells volume, depending on the species [3]. Furthermore, Oleosomes are storage of neutral lipids as an energy source and a source of components needed for membrane biogenesis or formation of other lipophilic components, like steroids. Also, it was thought possible that they might function as short-term storage of photosynthetic products where assimilates being stored in them during periods of peak photosynthesis and metabolized, for example, during the night [4]. Another possible function for oleosomes is adaptation to environmental stress such as salinity [5], cold temperature [6] and fungal infection [3]. |
| Salinity stress resulted in noticeable alterations and swelling of thylakoidal structures as reported in case of Nicotina bigelovii [7] and wheat [1,8] Geissler and others [9] showed that under saline conditions, the chloroplasts of Aster tripolium plants were partially horseshoe-shaped, their thylakoid membranes showed dilations, the spaces between the membranes looked swollen and un-dilated thylakoid areas were developed. In particular, the number of grana stacks was considerably reduced. |
| Lipid peroxidation rate was found to increase with increase of salt stress especially in sensitive cultivars [10] in this connection, Joshi et al. [11] stated that with increasing level of salinity stress, the malondialdehyde (MDA) content increased in four Brassica juncea varieties. The accumulation of glycerol is an adaptive response of plants and other organisms to the lack of water and salinity stress. In this connection, Torzilli [12] found that, salt stress increased the cellular concentrations of glycerol in Aureobasidium pullulans. Nichols et al. [13] found that in Shewanella gelidimarina, under hyper-osmotic and hypo-osmotic stress conditions, an increase in the proportion of saturated fatty acids was accompanied with increasing salinity level. |
| The shortage of fresh water is compelling researchers to investigate the use of saline water for irrigation [14]. Thus, the present work was undertaken to investigate the impact of seawater irrigation on ultrastructure of chloroplasts and oleosomes in relation to fat metabolism in flag leaves of two wheat cultivars, Gemmieza-9 (salt sensitive cultivar) and Sids-1 (salt tolerant cultivar) during grain-filling. |
| Materials and Methods |
| Plant material and growth conditions |
| Two wheat genotype, Gemmieza-9 (salt sensitive cultivar) and Sids 1 (salt tolerant cultivar) were selected. The sterilized grains from each cultivar were divided into two sets (≈ 500 g per set for each cultivar). The grains were drilled on 15th November 2009/2010 in plastic pots (25 cm in diameter) filled with 7 kg soil (clay: sand 2: 1v/v), where 15 grains were sown in each pot. The pots were then kept in a greenhouse at research area of Botany Department, Faculty of Science. The plants were subjected to natural day/night conditions (minimum/maximum air temperature and relative humidity were 15/25°C and 35/45% respectively) at mid-day during the experimental period. The plants were irrigated to field capacity by tap water. After two weeks from sowing, thinning was started so that five uniform seedlings were left in each pot for the subsequent studies. The plants from each cultivar were divided into three sets. The 1st set was still irrigated with normal tap water serving as control, whereas the 2nd or 3rd ones were irrigated with 10% and 25% seawater, receptively. Irrigation with seawater was applied after 30 days from sowing with a periodical soil washing (each two weeks) with tap water. The chemical analyses of the employed seawater, collected from Mediterranean Sea, revealed that it contains: Cl-, 21.6 kg m-3; Na+, 11.1 kg m-3; SO4-2, 2.85 kg m-3; K+, 0.49 kg m-3 and Phosphate, 16.6 μg dm-3. Its salinity was found to be 38.5 g kg-1; pH, 8.1 and EC, 47 mmhos cm-1. |
| After thinning and at heading, the plants of each cultivar received 36 kg N ha-1 as urea and 25 kg P ha-1 as superphosphate. For estimation of lipase activity, glycerol, fatty acids, lipid peroxidation, membrane stability index and membrane leakage, flag leaf samples were taken from each cultivar in triplicates and ten samples were taken for measurements of oleosomes volume from each treatment during grain-filling (14 & 21 days post-anthesis). Anthesis (begining of grain set) occurred after 85 days from sowing. |
| Electron microscopy |
| The flag leaf tissues were processed for transmission electron microscopy (TEM) according to Hayat [15]. Sections were examined and photographed by Jeol 1010 TEM manufactured by Jeol company, England. |
| Counting of chloroplasts number. |
| The number of chloroplasts in flag leaf mesophyll tissue of wheat plants was counted for each treatment in 1.0 μm thick sections cut parallel to the epidermis from Araldite-embedded material using ordinary light microscope. A computerized method was followed to count chloroplasts in semi-thin sections as those described by Aldesuquy and et al. [16]. |
| Measurement of oleosomes volume in semi-thin sections |
| A new technique developed using the image analysis for measuring the volume of oleosomes (in semi-thin sections from flag leaf mesophyll tissues was estimated to a leaf or cell unit volume for each treatment) was performed using the methods adopted by Aldesuquy et al. [16]. |
| Estimation of lipase activity |
| Lipase hydrolyses triglycerides to release free fatty acids and glycerol. The quantity of fatty acid released in unit time was measured by the quantity of NaOH required to maintain pH constant. The ml equivalent of alkali consumed was taken as a measure of the activity of the enzyme according to Jayaraman [17]. |
| Estimation of glycerol |
| Glycerol was estimated according to the method described and adopted by Borowitzka and Brown [18]. |
| Fatty acids analysis |
| The method of lipids extraction was adopted by Neumann [19] while the method used in methylation of fatty acids for gas-liquid chromatography Shimadzu (model: Shimadzu GC/MS-Qp5050A) analysis was essentially that adopted by Sink and others [20]. |
| Estimation of lipid peroxidation, membrane stability index and determination of membrane leakage (ML) |
| Estimation of lipid peroxidation was assayed spectrophotometrically using thiobarbituric acid-malondialdehyde assay (TBA-MDA) [21]. The membrane stability index (MSI) was determined according to Sairam et al. [22]. Membrane leakage was determined according Vahala et al. [23]. |
| Statistical analysis |
| A test for significant differences between means at p ≤ 0.05 was performed using Least Significant Difference (LSD) test Snedecor and Cochran [24]. |
| Results |
| Changes in flag leaf chloroplast ultrastructure |
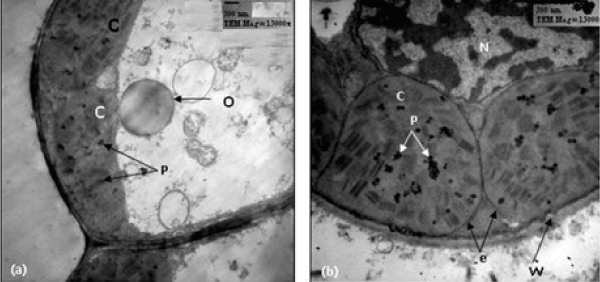
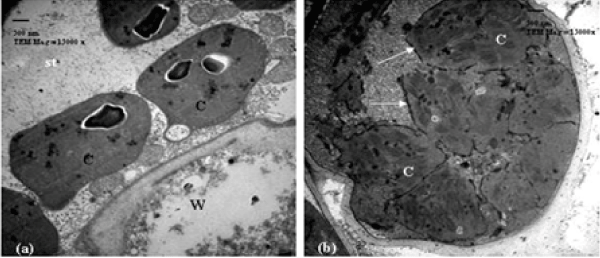
| Sensitive wheat cultivar (14 days post-anthesis): Regarding to untreated (control) sensitive cultivar, 14 days post-anthesis, the chloroplasts were normal and ellipsoidal in shape. They were closely associated with the cell wall and showed an organized membrane system of grana and stroma thylakoids with defined chloroplast envelope. They also contained few plastoglobuli (Figure 1). When this stage was treated with 10% seawater, little changes in the ultrastructure of chloroplasts were detected (Figure 2a). When this stage was treated with 25% seawater, the chloroplasts appeared to be spherical in shape and showed disorganized membrane system (Figure 2b). |
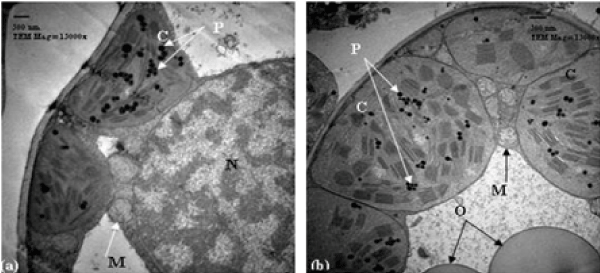
| Sensitive wheat cultivar (21 days post-anthesis): In relation to untreated (control) sensitive cultivar, 21 days post-anthesis, the chloroplasts were normal and ellipsoidal in shape. They were closely associated with the cell wall and showed an organized membrane system. They also contained starch grains and oleosomes. When this stage is treated with 10% seawater, the chloroplasts were spherical in shape and showed more damages. They showed disorganized membrane system with swollen thylakoids (Figure 3a). In case of 25% seawater, this stage showed chloroplasts no longer completely lining the cell walls. These chloroplasts showed signs of the onset of senescence with highly dilated grana and damaged envelope (Figure 3b). |
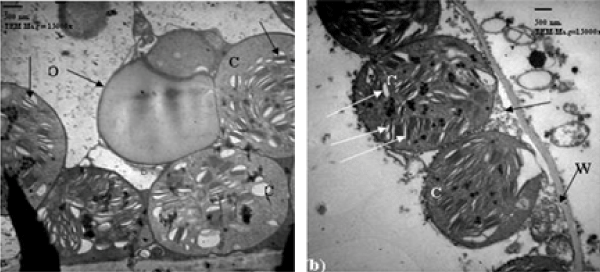
| Tolerant wheat cultivar (14 days post-anthesis): Regarding to untreated (control) resistant cultivar, 14 days post anthesis, the chloroplasts were closely associated with the cell wall and showed an organized membrane system of grana and stroma thylakoids with defined chloroplast envelope. At this stage, when the wheat cultivar was treated with 10% seawater, very slight changes in chloroplasts were observed. They were still closely associated with the cell wall with few exceptions and showed disorganized membrane system. The striking feature was the presence of small spherical bodies originated from the chloroplasts (Figure 4a). After the treatment by 25% seawater, many changes in chloroplasts were detected. The chloroplasts were abnormal and spherical in their shape and they moved away from the cell wall and showed disorganized membrane system of grana. They contained starch grains and numbers of plastoglobuli. Projections originated from the chloroplasts were also observed, in some cases, these projections enlarged and separated from the chloroplasts (Figure 4b). |
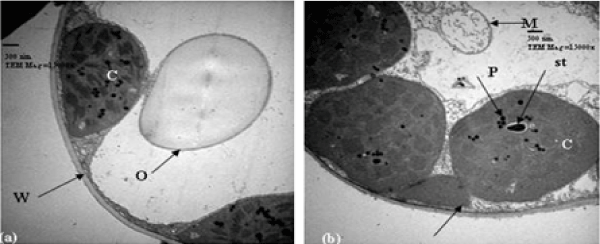
| Tolerant wheat cultivar (21 days post-anthesis): Regarding to untreated (control) tolerant cultivar, 21 days post-anthesis, the chloroplasts were normal and ellipsoidal in their shape. They were closely associated with the cell wall and plastoglobuli were found in the chloroplasts. At this stage, when the wheat cultivar was treated with 10% seawater, many changes in chloroplasts are observed. They were more or less spherical in their shape and moved away from the cell wall and showed disorganized membrane system of grana, but starch grains were present. A cytoplasmic inclusion attached to the chloroplast was rarely found (Figure 5a). In 25% seawater treatment, the damaged chloroplasts were observed inside mesophyll cells. They were spherical in shape and showed much disorganized membrane system and thicker envelope. Plastoglobuli were found in the chloroplasts. The chloroplast envelopes tended to contain an electron-dense body. The cell cytoplasm was very granulated and possessed electron-dense bodies (Figure 5b). |
| Changes in chloroplasts number: The data shown in Table 1 showed that, there was a marked decrease in chloroplasts number from 14 to 21 days post-anthesis in mesophyll cells of both wheat cultivars. In relation to control values, irrigation with seawater caused noticeable decreases (P ≤ 0.05) in chloroplasts number in mesophyll cells of wheat flag leaves in both cultivars during anthesis stage, except in 10% seawater which induced insignificant change in chloroplasts number in case of tolerant cultivar. The magnitude of reduction in chloroplasts number during grain filling was more pronounced with sensitive cultivar. |
| Changes in oleosomes volume: The data showed that, there was a tendency in control and seawater-stressed plants to increase the oleosomes volume from 14 to 21 days post-anthesis in both wheat cultivars. In relation to control values, concentrations of seawaterstress caused noticeable decrease (P ≤ 0.05) in oleosomes volume in mesophyll cells of wheat flag leaves in both cultivars during grainfilling. This decrease was insignificant at 10% in case of tolerant cultivar. Comparing both cultivars, under stress conditions, significantly larger oleosomes volumes was observed in salt tolerant cultivar than that of salt sensitive one during grain-filling (Table 1). |
| Changes in lipid peroxidation, membrane stability index (MSI) and membrane leakage (ML): As compared to the control values, all concentrations of seawater salinity induced a marked increase (P ≤ 0.05) in the values of lipid peroxidation of both wheat cultivars during anthesis stages. This effect was more conspicuous with the sensitive one. Furthermore, an obvious increase in MSI% and ML% in control and seawater-stressed plants from 14 to 21 days post-anthesis in both wheat cultivars. Moreover, the pattern of change in MSI% was opposite to that in ML%. As compared to control plants, the significant reduction (P ≤ 0.05) in MSI% due to seawater stress was accompanied with an increase in ML% of both wheat cultivars. The effect was more pronounced with the Gemmieza-9. Generally, the decrease in MSI% or the increase in lipid peroxidation and ML% was non-significant at 10% seawater in case of tolerant cultivar. Seawater stress reduced biomembranes stability through induction of lipid peroxidation that led to an increase in membrane leakage. The effect of seawater stress was more pronounced in Gemmieza-9 than Sids-1 particularly 25%. |
| Changes in lipase activity: The results showed that all the applied concentrations of seawater caused a noticeable increase (P ≤ 0.05) in lipase activity in flag leaf of both cultivars during grain-filling in relation to the control plants. This increase was insignificant at 10% in case of resistant cultivar. Salt sensitive cultivar showed higher lipase activity than salt tolerant one (Table 3). |
| Changes in glycerol: Seawater irrigation stimulated a progressive increase (P ≤ 0.05) in glycerol content of flag leaf in both cultivars during anthesis stages. The tolerant cultivar accumulated more glycerol than the sensitive one under salt-stress (Table 3). |
| Changes in fatty acids percentage |
| The pattern of changes in total saturated fatty acids (TSFA%) was opposite to that in total unsaturated fatty acids (TUFA%). As compared to control plants, the increase recorded in TSFA in response to seawater-stress was greatly accompanied by reduction in TUFA in flag leaves of both cultivars. As seawater concentrations increased, there was a simultaneous increase in the percentage of each saturated fatty acids and decrease in the percentage of each unsaturated fatty acids in flag leaves of both wheat cultivars during grain-filling (Tables 4 and 5). |
| Comparing cultivars, Sids-1 (salt tolerant) proved to be more tolerant than Gemmieza-9 (salt sensitive) where more increment in TSFA% and more reduction in total unsaturated fatty acids(TUFA %), monounsaturated (MUFA%) as well as PUFA% were observed in Sids-1.Wheat flag leaves in control plants were characterized by a high proportion of monounsaturated (MUFA) (60.18% and 64.13%) versus (1.62% and 2.48%) of polyunsaturated fatty acids (PUFA) and (37.82% and 33.38%) of saturated ones (SFA) for Gemmieza-9 and Sids-1 respectively. Oleic acid represented more than 57.20% and 58.62% of the total fatty acids (TFA), followed by palmitic acid (25.77% and 23.24%), stearic acid (4.32% in case of Gemmieza-9) and palmitoleic acid (5.51% in case of Sids-1), arachidic acid (3.98% and 4.88%), palmitoleic acid (2.98% in case of Gemmieza-9) and stearic acid (2.67% in case of Sids-1), linoleic acid (1.62% and 2.48%), pentadecanoic acid (1.45% and1.29%), behenic acid (1.42% and 1.00%), lauric acid (0.51% and 0.20%) and myristic acid (0.37% and 0.10%) in Gemmieza-9 and Sids-1 respectively. The most surprising results of the present study was that, 10% seawater salinity induced the appearance of new saturated fatty acids such as capric and tridecanoic acids while 25% seawater salinity induced the appearance of caprylic acid in the flag leaves of both wheat cultivar. |
| Discussion |
| There is increasing awareness among agricultural scientists and planners in the utilization of seawater (at least diluted) for irrigation of crops [25,26], but this application is limited due to the ill effect of seawater salinity. The effect of seawater salinity (10% and 25%) on ultrastructure of chloroplasts and oleosomes as well as oleosomes volume in relation to fat metabolism in flag leaf of two wheat cultivars during grain-filling was investigated. |
| Seawater irrigation altered the shape and ultrastructure of chloroplasts. Seawater caused an increase in the stroma and damaged of chloroplast membranes. The rounding of chloroplasts was attributed to the increase in the volume of the stroma and disorganization of the thylakoid membranes [13].The same observations were also reported by some authors (Liu et al. [26], Mitsuya et al. [27]). The concentrations of 10% or 25% seawater at 21 days post-anthesis in Gemmieza-9 caused pronounced swelling of the thylakoid membranes in chloroplasts. These results coincided with those obtained, in case of plants grown in high salinity treatments, such as Atriplex halimus [28] wheat [29] and Mesembryanthemum crystallinum [30]. |
| The appearance of vesicles and myelin-like structures in the vacuoles of wheat plants treated with seawater may be probably due to an increase of vesicles formation and myelin-like structures in the vacuoles of plants growing in high salinities and these structures are thought to be the pinocytotic invagination of tonoplast, which allows plants to translocate salt ions from the cytoplasm into the vacuoles [26]. Furthermore, it seems that a high concentration of Cl-ion might have penetrated the chloroplasts and caused the repulsion of negative charges on the surface of the thylakoid membranes [31]. |
| In salt-stressed cells, grana are disrupted and the thylakoids are loose and unevenly stacked. These changes in thylakoid structure may be due to the effect of seawater on bio-membranes stability through lipid peroxidation accompanied by an increase in membrane leakage where, Gemmieza-9 recorded maximum percentage of ML and minimum percentage of MSI. Furthermore, these changes in thylakoid structure are usually typical for oxidative stress damage and point to salinity-induced ROS production [32]. |
| The number of chloroplasts in both wheat cultivars was adversely influenced by the two concentrations of seawater with more reduction in case of Gemmieza-9. This observation is in accordance with the results obtained by Smethurst et al. [33]. Furthermore, this reduction may be a strategy of protection and/or acclimation, in which the reduction of energy waste, carbon skeletons and nutrients in pigment synthesis may favor other physiological processes. |
| Numerous functions may be play by oleosomes: (a) short-term energy reserves to release energy for plant growth and metabolism, (b) to provide the energy needed in the formation of grains, (c) adaptation to salt-stress and (d) supply fatty acids for membrane synthesis during growth. |
| Seawater irrigation increased the activity of lipase in both wheat cultivars with more activity in Gemmieza-9 cultivar. This result due to acceleration of salt stress to leaf senescence and hence the activity of lipase accelerate the conversion of storing fats into fatty acids and glycerol which can be utilized by plants for different metabolic processes as well as energy source. Furthermore, seawater irrigation caused a marked increase in glycerol content of flag leaf of both cultivars during anthesis stage with marked accumulation in Sids-1 than Gemmieza-9. These results are also in line with those obtained by Borowitzka and Brown [18], who reported that cells tended to accumulate high concentrations of glycerol with increasing the external salinity level in order to increase the internal cell osmotic pressure. Such increment would improve the performance of the cell at high salinity by preventing leakage of the compatible solute out of the cell and diffusion of potentially harmful ions into the cell [34]. Glycerol has been shown to be a compatible solute involves in osmotic adjustment in cells exposed to lowered water potential and the increases in the cellular concentrations of glycerol contribute to the acquisition of stress tolerance. Also, glycerol was required to balance the osmotic potential of Na+ and Cl– [12] Moreover, the increased concentration of NaCl within seawater resulted in the formation of glycerol as osmotic |
| agent to facilitate the retention of cellular water and maintain enzyme activity [18]. |
| The main roles of fatty acids in plants are related not only to cell membrane functions but also to metabolic processes. Seawater treatments induced marked changes in fatty acids composition of wheat flag leaf of both cultivars during anthesis stages. The data showed that seawater irrigation increased TSFA% and decreased TUFA %, MUFA% as well as PUFA% in flag leaf of both cultivars. By comparing the two cultivars, Sids-1 showed more increase in TSFA% and more reduction in TUFA%, MUFA% and PUFA%. These results are in accord with those obtained by Taarit et al. [35] in Salvia officinalis leaves under NaCl stress. Moreover, Xu and Beardall [36] stated that in a Dunaliella sp with increasing salinity level, the proportion of TSFA increased while PUFA decreased. It has been reported that chain length and saturation of fatty acids affect bilayer thickness and fluidity, which regulate different membrane functions. Moreover, the advantage of having saturated fatty acid is that the yield of ATP molecules during complete oxidation is higher than unsaturated fatty acids [37]. |
| The data showed that low (10%) and moderate (25%) seawater salinity levels decreased the degree of fatty acids unsaturation in flag leaf of both cultivars with greater reduction in Sids-1 than Gemmieza-9. This fact could be considered as one of the aspects of wheat adaptation to salinity since some plants could be protected against the oxidative effects of salt ions through restructuring membranes with less polyunsaturated fatty acids as indicated by Flower and Colmer [38], Francois and Kleiman [39] Also, this low unsaturation degree limited the membrane fluidity [37] and so restricted its permeability to Na+ and Cl- ions especially in Sids-1. |
| As seawater concentration increases, there was a simultaneous increase in the percentage of each saturated fatty acids and decrease in the percentage of each unsaturated fatty acids in flag leaf of both wheat cultivars during grain-filling. More interestingly, as a consequence of decreasing oleosomes volume, the flag leaf contents of glycerol and saturated fatty acids increased where unsaturated fatty acids decreased as cleared in seawater-stressed wheat plants [36]. |
| In the present study, we have confirmed that, there was a noticeable decline in chloroplast numbers in flag leaves of both wheat cultivars treated with seawater. This reduction could be attributed to the fact that salt stress often induces premature senescence. We have also confirmed that the observed decrease in the oleosome volume and fatty acids unsaturation level together with the increase in lipase activity and glycerol content in the flag leaves is correlated well with the wheat plant tolerance to seawater salinity. The constitutive accumulation of these metabolites, in addition to protection of cellular integrity may be a promising approach to creating salt tolerant crops. |
| Acknowledgement |
| The authors sincerely thank Prof. Zakaria Baka for technical support in preparing electron microscopic samples. |
References
- id="Reference_Titile_Link" value="1">Aldesuquy HS, Baka ZA, El-Shehaby OA, Ghanem HE (2013) Growth, Lipid peroxidation and antioxidant enzyme activities as a selection criterion for the salt tolerance of wheat cultivars irrigated by seawater. Phyton 53: 153-165.
- id="Reference_Titile_Link" value="2">Naidoo G, Hirral O, Naidoo Y (2011) Hypersalinity effects on ultrastructure and physiology in the mangroves. Flora 206: 184-211.
- id="Reference_Titile_Link" value="3">Parker ML1, Murphy GJ (1981) Oleosomes in flag leaves of wheat; their distribution, composition and fate during senesence and rust-infection.Planta 152: 36-43.
- id="Reference_Titile_Link" value="4">Murphy GJP, Parker ML (1984) Lipid composition and carbon turnover of wheat leaf oleosomes. J Exper Bot 35: 348-355.
- id="Reference_Titile_Link" value="5">Aldesuquy HS, Baka ZAM (1988) Interactive effects of seawater and plant hormones on the pigments content and chloroplast ultrastructure of wheat flag leaf. Proceedings, Sixth Egyptian Botanical Conference, Cairo University, Giza 1: 51-64.
- id="Reference_Titile_Link" value="6">Pihakaski K, Pihakaski S, Karunen P, Kallio P (1987) Seasonal changes in leaf lipids of Diapensialapponica, with special reference to storage lipid bodies. Nordic J Bot 7: 281-292.
- id="Reference_Titile_Link" value="7">Andrea B, Tani C (2009) Ultrastructural effects of salinity in Nicotianabigelovii callus cells and Allium cepa roots. Caryologia 62: 124-133.
- id="Reference_Titile_Link" value="8">Abdelkader AF, Aronsson H, KatalinSolymosi K, Boddi B, Sundqvist S (2007) High salt stress induces swollen prothylakoids in dark-grown wheat and alters both prolamellar body transformation and reformation after irradiation. J Expt Bot 58: 2553-2564.
- id="Reference_Titile_Link" value="9">Geissler NS, Hussin H, Koyro W (2009) Interactive effects of NaCl salinity and elevated atmospheric CO2 concentration on growth, photosynthesis, water relations and chemical composition of the potential cash crop halophyte Aster tripolium (L.). Environ and Expt Bot 65: 220-231.
- id="Reference_Titile_Link" value="10">Arora N, Bhardwaj R, Sharma P, Arora HK (2008) Homobrassinolide alleviates oxidative stress in salt-treated maize (Zea mays L.) plants. Brazilian J Plant Physiol 20: 153-157.
- id="Reference_Titile_Link" value="11">Joshi PK, Saxena SC, Arora S (2011) Characterization of Brassica juncea antioxidant potential under salinity stress. ActaPhysiol Plant 33: 811- 822.
- id="Reference_Titile_Link" value="12">Torzilli AP (1997) Tolerance to high temperature and salt stress by a salt marsh isolate of Aureobasidiumpullulans. Mycol 98: 786-792.
- id="Reference_Titile_Link" value="13">Nichols DS1, Olley J, Garda H, Brenner RR, McMeekin TA (2000) Effect of temperature and salinity stress on growth and lipid composition of Shewanellagelidimarina.Appl Environ Microbiol 66: 2422-2429.
- id="Reference_Titile_Link" value="14">Al-Busaidi A, Al-Rawahy S, Ahmed M (2009) Response of different tomato cultivars to diluted seawater salinity. Asian Journal of Crop Sci 1: 77-86.
- id="Reference_Titile_Link" value="15">Hayat MA (1989) Principles and Techniques of Electron Microscopy (Biological Applications), volume 1, The Macmillan Press Ltd., London.
- id="Reference_Titile_Link" value="16">Aldesuquy HS, Baka ZA, Mickky BM (2014) Kinetin and spermine mediated induction of salt tolerance in wheat plants: Leaf area, photosynthesis and chloroplast ultrastructure of flag leaf at ear emergence. Egyptian journal of basic and applied sciences.1:77-87.
- id="Reference_Titile_Link" value="17">Jayaraman J (1981) Laboratory Manual in Biochemistry. Wiley Eastern Limited, New Delhi, 132-133.
- id="Reference_Titile_Link" value="18">Borowitzka LJ, Brown AD (1974) the salt relations of marine and halophilic species of unicellular green algae. Dunalliella: The role of glycerol as a compatible solute. Archives of Microbiol 96: 37-52.
- id="Reference_Titile_Link" value="19">Neumann P (1995) UntersuchungenzurNahrungsqualitat von benthischem feinpartikularem Detritus fur Feinpartikelsammler, unter demAspekt seiner biochemischen Zusammensetzung im Breitenbach. Ph. D. Thesis, University of Marburg, German:135.
- id="Reference_Titile_Link" value="20">Sink JD, Walkins SL, Zeigler JH, Miller R C (1964) Analysis of fat by gas liquid chromatography. J Animal Sci 23: 111-121.
- id="Reference_Titile_Link" value="21">Hodges DM, DeLong, JM, Forney C, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207: 604-611.
- id="Reference_Titile_Link" value="22">Sairam RK, Rao KV, Srivastava GC (2002) Differential response of wheat genotypes to long-term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci 163: 1037- 1046.
- id="Reference_Titile_Link" value="23">Vahala J1, Ruonala R, Keinänen M, Tuominen H, Kangasjärvi J (2003) Ethylene insensitivity modulates ozone-induced cell death in birch.Plant Physiol 132: 185-195.
- id="Reference_Titile_Link" value="24">Snedecor GW, Cochran WG (1976) Statistical Methods, 6th ed. Oxoford IBH Publishing Co, New Delhi.
- id="Reference_Titile_Link" value="25">Aldesuquy HS, Baka ZAM, El-Shehaby OA, Ghanem HE (2012) Efficacy of seawater salinity on osmotic adjustment and solutes allocation in wheat (Triticumaestivum) flag leaf during grain filling. Inter J Plant Physiol and Biochem 4: 33-45.
- id="Reference_Titile_Link" value="26">Liu J, Wu X, He T, Zhang W (2004) Study of ultrastructure of Phragmitescommunis mesophyll cell under salt stress. ActaBotanicaBoreali-OccidentaliaSinica 24: 1035-1040.
- id="Reference_Titile_Link" value="27">Mitsuya S, Kawasaki M, Taniguchi M, Miyake HH (2003) Relationship between salinity-induced damages and aging in rice leaf tissues. Plant Proceeding Sci 6: 213-218.
- id="Reference_Titile_Link" value="28">Blumenthal-Goldschmidt S, Poljakoff-MayberA (1968) Effect of substrate salinity on growth and on submicroscopic structure of leaf cells of Atriplexhalimus (L.). Aust J Bot 16: 469-478.
- id="Reference_Titile_Link" value="29">Salama S, Trivedi S, Busheva M, Arafa AA, Garab G,Erdei L (1994) Effects of NaCl on growth, cation accumulation, chloroplast structure and function in wheat cultivars differing in salt tolerance. J Plant Physiol 144: 241-247.
- id="Reference_Titile_Link" value="30">V on Willert DJ1, Kramer D (1972) Ultrastructure and crassulacean acid metabolism in Mesembryanthemumcrystallinum leaves during normal and NaCl-induced ageing.Planta 107: 227-237.
- id="Reference_Titile_Link" value="31">Vijaranakul U, Jayaswal RK, Nadakavukaren MJ (2001) Alteration in chloroplast ultrastructure of suspension cultured Nicotianatabaccum cells by cadmium. Asian J Sci 27: 227-231.
- id="Reference_Titile_Link" value="32">Bondada BR, Oosterhuis DM (1988) Decline in photosynthesis as related to alterations in chloroplast ultrastructure of a cotton leaf during ontogeny. Photosynthica 35: 467-471.
- id="Reference_Titile_Link" value="33">Smethurst CF1, Gill WM, Shabala S (2009) Using excised leaves to screen lucerne for salt tolerance: physiological and cytological evidence.Plant Signal Behav 4: 39-41.
- id="Reference_Titile_Link" value="34">Elenkov L, Stefanov K, Dimitrove-Konaklieva S, Popov S (1996) Effect of salinity on lipid composition of Cladophoravagabunda. Phytochem 42: 39-44.
- id="Reference_Titile_Link" value="35">Taarit MB, Msaada K, Hosni K, Marzouk B (2010) Changes in fatty acid and essential oil composition of sage Salvia officinalis (L.) leaves under NaCl stress. Food Chem 119: 951-956.
- id="Reference_Titile_Link" value="36">Xu X, Beardall J (1997) Effect of salinity on fatty acid composition of a green microalga from an antarctic hyper-saline lake. Phytochem 45: 655-658.
- id="Reference_Titile_Link" value="37">Kerkeb L1, Donaire JP, Rodríguez-Rosales MP (2001) Plasma membrane H-ATPase activity is involved in adaptation of tomato calli to NaCl.Physiol Plant 111: 483-490.
- id="Reference_Titile_Link" value="38">Flowers TJ1, Colmer TD (2008) Salinity tolerance in halophytes.New Phytol 179: 945-963.
- id="Reference_Titile_Link" value="39">Francois LE, Kleiman R (1990) Salinity effects on vegetative growth, seed yield and fatty acid composition of crambe. Agricult J 82: 1110-1114.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 | Table 5 |
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
Relevant Topics
- Agricultural science
- Agronomy
- Climate impact on crops
- Crop Productivity
- Crop Sciences
- Crop Technology
- Field Crops Research
- Hybrid Seed Technology
- Irrigation Technology
- Organic Cover Crops
- Organic Crops
- Pest Management
- Plant Genetics
- Plant Breeding
- Plant Nutrition
- Seed Production
- Seed Science and Technology
- Soil Fertility
- Weed Control
Recommended Journals
Article Tools
Article Usage
- Total views: 12108
- [From(publication date):
February-2016 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 11052
- PDF downloads : 1056
Peer Reviewed Journals
Make the best use of Scientific Research and information from our 700 + peer reviewed, Open Access Journals
