Research Article Open Access
Impact of Open Cast Coal Mining on Fish and Fisheries of Simsang River, Meghalaya, India
Bandita Talukdar1, Jugabrat Das2, Himangshu Kr Kalita1, Sudem Basumatary1, Hrishikesh Choudhury1 and Dandadhar Sarma1*1Department of Zoology, Gauhati University, Assam, India
2Department of Zoology, Goalpara College, Assam, India
- *Corresponding Author:
- Dandadhar Sarma
Associate Professor, Department of Zoology
Gauhati University, Guwahati-781014, Assam, India
Tel: +91 94353 14768
E-mail: sarma_dandadhar@yahoo.com
Received date: October 21, 2016; Accepted date: November 26, 2016; Published date: December 06, 2016
Citation: Talukdar B, Das J, Kalita HK, Basumatary S, Choudhury H, et al. (2016) Impact of Open Cast Coal Mining on Fish and Fisheries of Simsang River, Meghalaya, India. J Marine Sci Res Dev 6: 214. doi: 10.4172/2155-9910.1000214
Copyright: © 2016 Talukdar B. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Marine Science: Research & Development
Abstract
Present paper deals with the impact of coal mining activities on the water quality and fish diversity of Simsang River from 2009 to 2015. Six sampling sites were selected on the basis of affected and unaffected areas of coal mining activities. Certain water quality attributes such as water colour, temperature, pH, dissolved oxygen, free CO2, chloride, total alkalinity, total dissolved solids, nitrate, ammonia, phosphate and sulphate were analysed along with the assessment of relative abundance of fishes. The estimated value of studied water quality parameters such as pH, dissolved oxygen and total alkalinity indicates degradation of water quality due to the effect of acid mine drainage (AMD) of coal mining. Polycyclic aromatic hydrocarbon (PAH) showed highest concentration of 4-ring PAH and Benzo[a]anthracene was the most important pollutant in the water collected from affected sites. Relative abundance of fish diversity was also estimated less in affected area of the River. A total of 64 fish species under 20 families were collected with highest number in cyprinidae family. The calculated value of diversity indices reflected the declining trend of fish diversity in the coal mining affected areas of the river due to degradation of water quality.
Keywords
Coal mining; Water quality; Fish diversity; Simsang river
Introduction
Coal mining activities in Garo Hills is posing severe threats to the aquatic biota of the Simsang River which is the longest river of Garo Hills, Meghalaya, India. In Garo Hills, two main reason is responsible for hazards to the biota of the river; firstly coal mine activities drains acid mine drainage (AMD) directly into the river and secondly dumping of coal for auction on its bank. As a result of excessive accumulation of AMD due to open cast coal mines practiced in the region, seasonally some area of the river devoid of any aquatic organism [1]. Common impact of coal mine activities include, low dissolved oxygen, higher sulphate content and turbidity which affect the aquatic life and reduce fish diversity to a great extent [2]. The primary cause of water quality degradation and the trend of biodiversity depletion in the water bodies of the coal mining areas is attributed mainly to the AMD, which makes water extremely acidic and loaded in heavy metal [3].
Though, there are many works were done on the different aspects of water and its alarming impact on river ecosystem, but there is a lack of information on impact of coal mines on a river ecosystem. Furthermore, it is worth mentioning that, in spite of the fact that Simsang River of Garo Hills is one of the important tourist marks, yet this river is under severe pollution threat. It is a matter of great concern that no systematic study on the coal mine pollution load of the river or its impact on aquatic species has been carried out so far. Therefore, the present study has been carried out to investigate the impact of coal mining on ecology and fishery potential of Simsang River, Meghalaya, India.
Materials and Methods
Study area
The present study was carried out from 2009 to 2015 in the Simsang River of Garo Hills District in Meghalaya (Figure 1), India covering an area of about 290 km2 of the entire stretch. Six study sites were selected on the basis of affected and unaffected areas of the River which are as follows; near Nokrek Biosphere Reserve (S1, longitude 90º23/59//E and latitude 25º31/21//N, free from coal mining activities), near Romagre, (S2, longitude 90º34/21//E and latitude 25º32/41//N, free from coal mining activities), Williamnagar (S3, longitude 90º39/43//E and latitude 25º27/36//N, coal dumping was found regularly at the bank of the river), Nangalbibra (S4, longitude 90º44/39//E and latitude 25º28/22//N, maximum coal mining activities are practiced in the hills of vicinity), Near Siju (S5, longitude 90º45/22//E and latitude 25º23/46//N, coal mining activities are practiced) and Baghmara (S6, longitude 90º37/9//E and latitude 25º12/1//N, transportation of coal through boats were found regularly).
Sample analysis
Water samples were collected seasonally [4]. Water Samples were collected randomly with five replicates from all the sites in the first quarter of every season. Fishes were collected from landing centre (twice a month) as well as by directly visiting the area where maximum fishing practices were being carried out. Experimental fishing was also carried out with the help of local fishermen employing cast net and gill net. The collected fishes were then preserved in 10% formaldehyde and fishes were identified using standard field guides [5,6].
Temperature (ºC), pH, dissolved oxygen (mg L-1), free CO2 (mg L-1), chloride (mg L-1), total alkalinity (mg L-1), TDS (ppm) and nitrate (mg L-1), phosphate (mg L-1), sulphate (mg L-1) and ammonia (mg L-1) were estimated following standard method of [7]; APHA [8]. For PAH analysis water samples (2.5 L) processing of water sample was done using liquid-liquid extraction (LLE) as described in APHA [8] and analyzed with Gas Chromatograph [9]. Saturated aliphatic hydrocarbons were eluted with 20 ml of n-hexane and then aromatic hydrocarbons were eluted with 30 ml of a mixture of hexane and dichloromethane (90:10) (v/v). The volume of the eluted fraction was reduced to 1 ml and then the aromatic hydrocarbon fraction was injected into a gas liquid chromatography equipped with a flam ionization detector (GC/ FID) [10]. Gas Chromatograph analysis was conducted on a fused silica capillary column of 60 m length, 0.25 mm id and 0.5 μm film thicknesses to detect 16 PAH components. The following PAHs were used for quantitation: naphthalene acenaphthylene, acenaphthene, fluorene, phenanthrene, anthracene, fluoranthene, pyrene, chrysene, benzo [a] anthracene, benzo [b] fluoranthene, benzo [k] fluoranthene, benzo [a] pyrene, dibenzo [a,h] anthracene, benzo [g,h,i] perylene, Indeno [1,2,3-cd] pyrene. Recoveries were carried out by the addition of PAHs standards mixture at the three levels of 1, 5 and 10 μg. All data were corrected according to the recovery percentage values. Ratio of phenanthrene to anthracene (Ph/An) and fluoranthene to pyrene (Fl/Py) have been widely used to distinguish petrogenic and pyrogenic (pyrolytic) sources of PAHs [11-13]. PAHs of petrogenic origin are generally characterized by Ph/An values >10, whereas combustion processes often result in low Ph/An ratios (<10). For the Fl/Py ratios, values greater than 1 have been used to indicate pyrolytic origins and values less than 1 are attributed to petrogenic source [14].
Statistical analysis
Mean values and Standard deviation (SD) of the water quality attributes were calculated using MS Excel computer program. Correlation matrix for mean values of all the variables was prepared based on Principal Component analysis. Principal component analysis (PCA) was carried out for water quality parameters and fish diversity in different sites using XLSTAT software version 2015.1.02. Principal Component Analysis (PCA) was calculated to measure the interrelationships among the water quality parameters and fish diversity of different sampling sites. The computer program Biodiversity Pro, Version 2 was used to calculate Shannon index; H’ [15], Berger-Parker dominance; 1/d [16], Simpson diversity; D [17], Hill’s Number; H1 [18] and MacIntosh Distance U [19].
Results and Discussion
Physico-chemical variables were found highly fluctuating in all the coal mine affected and unaffected areas of the River (Table 1). Water temperature in all the sites except site 4 is fairly similar and varies seasonally. It is comparatively higher (22.15 ± 4.7ºC) in the site 4, which is very close to the road side, where transporting and dumping of coal is carried out regularly. This is probably due to depth differences among the sites and also it can be related to seasonal pattern of air temperature in the region [20]. Lowest value of pH was observed in site 4 (5.03 ± 0.23) comparing with the other sites. Higher pH values were due to the flow of oxidized SO4–2 from the pyrite in the mining debris and also as a by-product of secondary reactions involving Fe in anaerobic conditions [21]. The dissolved oxygen (DO) content in the affected sites (Site 4 and 5) was very less than the other sites (Site 1, 2, 3 and 6). Low DO concentration in the coal affected sites was probably due to the consumption of oxygen in oxidation of pyrite, a process that results in dissolution of certain ions and metals such as SO4–2, Fe, Mn, Zn and Ni [22].
| *Parameters | Sampling sites | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | Site 6 | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Temperature | 11.7 | 3.9 | 15.725 | 4.2 | 16.525 | 3.8 | 22.15 | 4.7 | 15.325 | 4.8 | 14.4 | 4.7 |
| pH | 7.6 | 0.36 | 7.51 | 0.23 | 7.27 | 0.39 | 5.03 | 0.23 | 4.53 | 0.83 | 7.03 | 0.11 |
| DO | 9 | 0.41 | 8.8 | 0.58 | 8.86 | 0.59 | 4.77 | 0.22 | 4.75 | 0.29 | 6.27 | 0.43 |
| FCO2 | 6.2 | 1.59 | 6.37 | 1.84 | 6.75 | 2.44 | 15.75 | 8.41 | 19.95 | 16.12 | 6.4 | 2.56 |
| Chloride | 4.85 | 4.25 | 5.9 | 3.16 | 6.77 | 2.48 | 6.32 | 1.15 | 8.9 | 2.77 | 9.15 | 3.26 |
| TA | 18.35 | 9.43 | 21.4 | 8.81 | 21.22 | 11 | 6.3 | 2.58 | 5.07 | 2.62 | 17.67 | 12.89 |
| TDS | 22 | 1.83 | 21 | 1.41 | 21.5 | 1.91 | 84.75 | 22.07 | 145.75 | 116.86 | 23.5 | 1.73 |
| Nitrate | 1.45 | 0.34 | 1.42 | 0.17 | 1.55 | 0.72 | 3.8 | 0.22 | 3.57 | 0.43 | 2.17 | 0.54 |
| Ammonia | 0.01 | 0.006 | 0.02 | 0.009 | 0.04 | 0.014 | 0.07 | 0.018 | 0.08 | 0.016 | 0.1 | 0.013 |
| Phosphate | 0.035 | 0.013 | 0.042 | 0.009 | 0.045 | 0.026 | 0.202 | 0.049 | 0.207 | 0.015 | 0.712 | 0.16 |
| Sulphate | 8 | 1.83 | 8.52 | 2.72 | 8.75 | 2.36 | 29.5 | 14.48 | 31.75 | 21.41 | 3.37 | 1.25 |
*All parameters are expressed in mg L-1 except pH, temperature (°C) and total dissolved solids (ppm), SD-Standard deviation, Min-Minimum.
Table 1: Mean and standard deviation of certain physico-chemical parameters in different study sites of Simsang River, Meghalaya.
The concentration of free CO2 ranged from 4.0 to 40.0 mg L-1 throughout the sampling sites. Highest value of free CO2 was estimated in site 4 and 5. Higher concentration of free CO2 may cause potential damages to aquatic biota i.e., elimination of sensitive species and proliferation of tolerant species, direct acute effects and reduction in density, biomass and diversity of aquatic organisms etc. [23]. The mean chloride content of the study sites ranged from 4.8 to 9.1 mg L-1, which was found within the permissible limit for aquatic life. The highest chloride concentration observed in the site 4, 5 and 6 might be due to higher accumulation of AMD in the areas.
Total alkalinity (TA) of the mining affected sites was relatively low than that of the control sites. Singh [24] opined that moderate increase in the level of alkalinity favour increased aquatic biological activity. Thus, the present findings indicate the stress condition in the respective study sites of the aquatic animals. Total dissolved solids (TDS) indicated the presence of different materials both in colloidal and dissolved solids like Na, K, Ca and Mg in natural water [25]. In the present study, estimated TDS value ranges from 19 to 320 ppm, lowest was in site 2 and highest in the site 5. AMD water was characterized by high concentration (>50 mg/l) of total dissolved metals [26]. Nitrate generally originated from the explosives used to blast the coal in the mines. It accounts for about 85% of the total nitrogen released in mine drainage, while ammonia accounts for the rest. Higher amount of nitrate in the sites with highest coalmining activities (site 4 and 5) reveals the same property.
Ammonia is generally present in the aquatic ecosystem as the dissociated ammonium ion (NH4+). In the present study, ammonium concentration was in the increasing trend towards the downstream. It is ranged from 0.01 to 10.0 mg L-1, lowest was estimated in site 1 and highest recorded in the site 6. According to Banerjea [27], dissolved phosphorus below 0.05 ppm may be considered insufficient in terms of fish productivity. It has also been observed that phosphate concentration ranged from 0.02 to 0.9 mg L-1. Sulphate concentration is recorded higher in the most mining affected sites (Sites 4 and 5). Maximum 53.0 mg L-1 and 63 mg L-1 sulphate was observed in site 4 and 5 respectively. The high accumulation of sulphates is mainly due to presence of iron sulphide in coal and rocks and subsequently its reaction with water and oxygen [28].
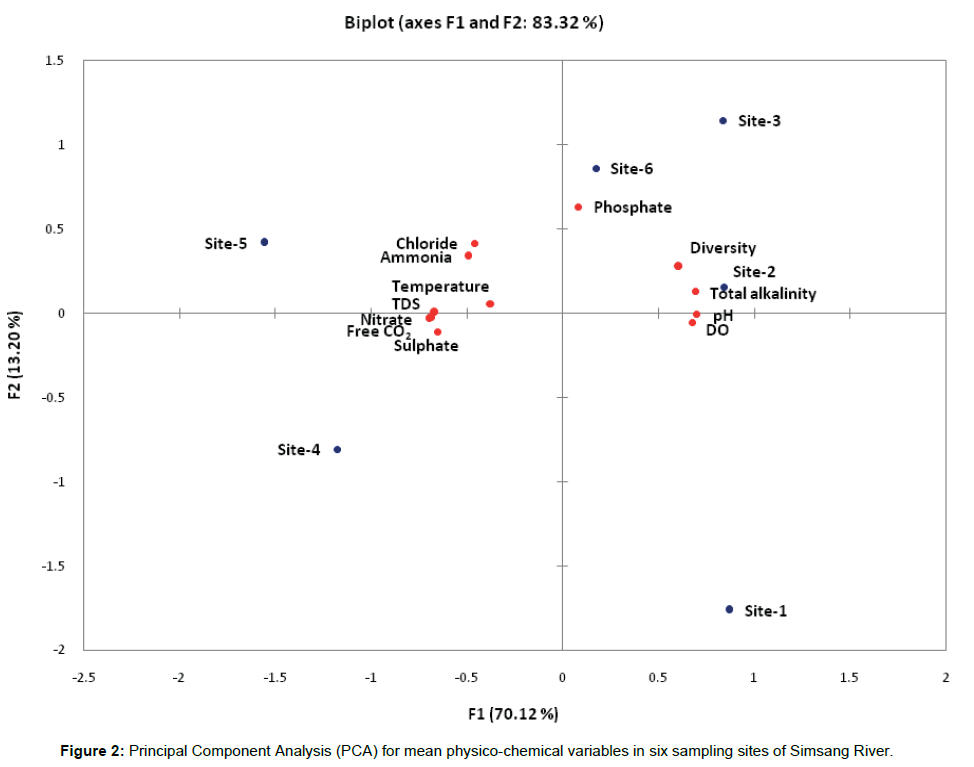
High degree of fluctuation in the water quality attributes has been observed collected from six sampling sites of Simsang River, Meghalaya. The correlations calculated amongst the variables (mean values) are presented through principal component analysis (Figure 2). The first two axis, account for 83.3% of the original matrix total variance, the first and second axis account for 70.1% and 13.2% respectively. The lowest factor loading in the first axis includes dissolved oxygen (DO) and pH in opposition to chloride, ammonia, temperature and total dissolved solids (TDS) concentration. Relevant factor loading for the second axis was highest consisting of phosphate, total alkalinity (TA) along with fish diversity, which is negatively correlated with nitrate, free CO2 and sulphate.
The correlation coefficient among the different variables also reflects the similar trend of correlation as presented in Table 2. The values are considered significant at the level 0.05 (α=0.05). Fish diversity has showed positive correlation with dissolved oxygen and total alkalinity and negative correlation with nitrate concentration. pH has showed positive significant correlation with dissolved oxygen and total alkalinity and negative correlation with free CO2, sulphate, nitrate and total dissolved solids (TDS). Dissolved oxygen (DO) has positive correlation with total alkalinity (TA), while negative correlation with free CO2, nitrate, total dissolved solids and ammonia. Free CO2 has positive correlation with sulphate, nitrate and total dissolved solids and negative correlation with total alkalinity. Total alkalinity has significant negative correlation with sulphate, nitrate and total dissolved solids (TDS), while chloride has significant positive correlation with ammonia. Sulphate has significant correlation with nitrate and total dissolved solids (TDS), while nitrate with only total dissolved solids (TDS).
| Variables | Diversity | Temperature | pH | DO | Free CO2 | Total alkalinity | Chloride | Sulphate | Nitrate | Phosphate | TDS | Ammonia |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diversity | 1 | -0.292 | 0.806 | 0.854 | -0.788 | 0.897 | -0.426 | -0.752 | -0.850 | 0.553 | -0.772 | -0.547 |
| Temperature | 1 | -0.562 | -0.537 | 0.479 | -0.501 | -0.023 | 0.588 | 0.641 | 0.252 | 0.340 | 0.270 | |
| pH | 1 | 0.917 | -0.985 | 0.976 | -0.612 | -0.956 | -0.974 | 0.054 | -0.957 | -0.622 | ||
| DO | 1 | -0.849 | 0.915 | -0.674 | -0.780 | -0.962 | 0.122 | -0.816 | -0.837 | |||
| Free CO2 | 1 | -0.962 | 0.594 | 0.975 | 0.928 | -0.067 | 0.987 | 0.521 | ||||
| Total alkalinity | 1 | -0.523 | -0.942 | -0.974 | 0.270 | -0.930 | -0.576 | |||||
| Chloride | 1 | 0.405 | 0.541 | 0.277 | 0.673 | 0.847 | ||||||
| Sulphate | 1 | 0.909 | -0.097 | 0.934 | 0.367 | |||||||
| Nitrate | 1 | -0.141 | 0.876 | 0.674 | ||||||||
| Phosphate | 1 | -0.062 | 0.126 | |||||||||
| TDS | 1 | 0.532 | ||||||||||
| Ammonia | 1 |
Values in bold are different from 0 with a significance level alpha=0.05.
Figure 2: Principal Component Analysis (PCA) for mean physico-chemical variables in six sampling sites of Simsang River.
The concentrations of the 16 detected PAHs in surface water of Simsang River are shown on Table 3. In terms of individual PAH composition in water, most compounds analyzed were detected in site 4. It has been observed that the concentration of low molecular weight (4-6 ring) polycyclic aromatic hydrocarbons (HPAHs) was maximum than that high molecular weight (2-3 ring) PAHs (LPAHs). The PAHs in water samples of site 4 and 5 originate from pyrolytic sources, while in other sites originate from both of pyrolytic and petrogenic sources (pyrolytic sources are more dominant). This was consistent with the results of the Langat River, Peninsular Malaysia [29] might be due to discharge of AMD in the river at these site.
| Components | Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | Site 6 |
|---|---|---|---|---|---|---|
| Naphthalene | 0.14 ± 0.01 | 1 ± 0.1 | 0.9 ± 0.52 | 8.8 ± 0.34 | 2.8 ± 0.07 | 1.54 ± 0.09 |
| Acenaphthylene | 0.02 ± 0.01 | 0.88 ± 0.34 | 0.9 ± 0.36 | 7.24 ± 0.21 | 2.5 ± 0.04 | 1 ± 0.21 |
| Acenaphthene | 0.05 ± 0.02 | 0.81 ± 0.27 | 0.88 ± 0.34 | 7.67 ± 0.37 | 2.9 ± 0.09 | 1.32 ± 0.02 |
| Fluorene | 0.004 ± 0.12 | 0.5 ± 0.03 | 0.46 ± 0.01 | 5.9 ± 0.45 | 1.88 ± 0.13 | 0.9 ± .5 |
| Phenanthrene | 0.003 ± 0.11 | 0.5 ± 0.02 | 0.3 ± 0.12 | 6.11 ± 0.28 | 2.4 ± 0.03 | 0.88 ± 0.34 |
| Anthracene | 0.09 ± 0.17 | 1.1 ± 0.09 | 0.92 ± .23 | 8.29 ± 0.37 | 3.1 ± 0.05 | 1 ± 0.83 |
| Total LPAH | 0.28 ± 0.57 | 4.7 ± 0.45 | 4.35 ± 0.9 | 44 ± 1.78 | 15.28 ± 0.66 | 6.56 ± 0.23 |
| Fluoranthene | 0.76 ± 0.13 | 1.8 ± 0.13 | 2.17 ± 0.05 | 19 ± 1.5 | 7.8 ± 0.19 | 2.4 ± 0.11 |
| Pyrene | 0.53 ± 0.09 | 1.6 ± 0.08 | 1.77 ± 0.02 | 12.33 ± 0.93 | 6.83 ± 0.17 | 2.1 ± 0.13 |
| Chrysene | 0.02 ± 0.01 | 0.9 ± 0.38 | 1.01 ± 0.11 | 15.8 ± 1.01 | 7.69 ± 0.21 | 1.6 ± 0.23 |
| Benzo[a]anthracene | 0.02 ± 0.1 | 1.8 ± 0.6 | 2.7 ± 0.17 | 22.78 ± 1.07 | 14.9 ± 0.75 | 1.8 ± 0.21 |
| Benzo[b]fluoranthene | 0.005 ± 0.23 | 0.3 ± 0.01 | 1.15 ± 0.09 | 20.3 ± 1.02 | 12.5 ± 0.35 | 1 ± 0.01 |
| Benzo[k]fluoranthene | 0.005 ± 0.11 | 0.2 ± 0.03 | 0.82 ± 0.25 | 13.9 ± 0.93 | 8.2 ± 0.34 | 0.9 ± 0.12 |
| Benzo[a]pyrene | 0.067 ± 0.13 | 0.33 ± 0.11 | 0.5 ± 0.32 | 17.45 ± 0.89 | 11.7 ± 0.23 | 0.89 ± 0.43 |
| Dibenzo[a,h]anthracene | 0.001 ± 0.1 | 0.078 ± 0.04 | 0.47 ± 0.13 | 19.67 ± 0.97 | 9.76 ± 0.19 | 0.82 ± 0.32 |
| Benzo[g,h,I]perylene | 0.003 ± 0.04 | 0.018 ± 0.02 | 0.26 ± 0.04 | 22.6 ± 1.7 | 14.3 ± 1.25 | 0.94 ± 1.45 |
| Indeno[1,2,3-cd]pyrene | 0.001 ± 0.1 | 0.035 ± 0.01 | 0.05 ± 0.03 | 6.72 ± 0.12 | 2.57 ± 0.15 | 0.6 ± 0.09 |
| Total HPAH | 1.5 ± 0.9 | 6.33 ± 0.23 | 12 ± 0.28 | 170.55 ± 23.85 | 96.25 ± 0.33 | 12.9 ± 2.87 |
| Total | 1.7 ± 0.17 | 11.1 ± 0.13 | 15.8 ± 0.57 | 214.58 ± 34.98 | 111.53 ± 0.4 | 19.5 ± 0.97 |
(All values are expressed in mean ± standard deviation)
Table 3: Concentrations of PAHs (ng L-1) in surface water of the Simsang River in different sampling sites.
A total 64 fish species have been collected from six sampling sites, out of which 30 were inhabitants of hills stream, 31 from plain water and 3 belongs to both hills and plain dweller species. Photoghraphs of some of the fishes collected from the river was given in Figure 3. Cyprinidae was the most abundant family, contributing 13 genera of fish followed by cobitidae (3 genera) and Sisoridae (3 genera) respectively (Table 4). Annual catching percentage of all the sampling sites indicates highest catching of Schistura reticulofasciata (16.02%).
| Family | Fish species | Occurrence | Annual catching (%) |
|---|---|---|---|
| Cyprinidae | Cabdio morar | S3 | 0.36 |
| Barilius barna | S3 | 0.97 | |
| Barilius bendelisis | S1, S2, S3, S6 | 6.97 | |
| Barilius vagra | S3 | 3.36 | |
| Chagunius chagunio | S3 | 1.27 | |
| Laubuca laubuca | S3 | 0.51 | |
| Cirrhinus mrigala | S6 | 2.09 | |
| Cirrhinus reba | S3 | 0.05 | |
| Danio dangila | S1, S2, S3 | 1.68 | |
| Danio rerio | S2, S3 | 1.42 | |
| Devario aequipinnatus | S1, S3, S4 | 3.76 | |
| Devario assamensis | S3 | 1.37 | |
| Garra annandalei | S1, S3 | 0.76 | |
| Garra kempi | S1, S2, S3, S6 | 0.31 | |
| Garra nasuta | S2, S3, S5 | 1.07 | |
| Puntius chola | S3 | 0.10 | |
| Pethia conchonius | S2, S3, S6 | 1.83 | |
| Puntius sophore | S1, S2, S3, S6 | 0.71 | |
| Puntius terio | S1, S2, S3 | 1.37 | |
| Raiamas bola | S3 | 0.66 | |
| Salmostoma bacaila | S1, S2 | 0.31 | |
| Tor putitora | S1, S2, S3 | 0.51 | |
| Tor tor | S1, S2, S3, S6 | 2.70 | |
| Psilorhynchidae | Psilorhynchus balitora | S1, S2, S3 | 1.93 |
| Balitoridae | Balitora brucei | S1, S2, S3 | 0.10 |
| Nemachilidae | Paracanthocobitis botia | S1, S3, S6 | 0.15 |
| Schistura fasciata | S1, S2, S3, S6 | 6.00 | |
| Schistura inglishi | S1, S2, S3, S6 | 3.66 | |
| Schistura reticulofasciata | S1, S2, S3 | 16.02 | |
| Schistura sijuensis | S1, S2, S3 | 0.51 | |
| Schistura sikmaiensis | S2, S5 | 0.81 | |
| Cobitidae | Botia dario | S3, S6 | 1.02 |
| Cantophrys gongota | S3 | 0.15 | |
| Lepidocephalichthys guntea | S2 | 0.46 | |
| Bagridae | Olyra kempi | S1, S2, S3, S6 | 2.64 |
| Schilbeidae | Ailia coila | S3, S6 | 0.41 |
| Clupisoma garua | S3 | 0.41 | |
| Sisoridae | Gagata cenia | S3 | 0.05 |
| Gagata gagata | S2 | 0.41 | |
| Glyptothorax cavia | S1, S2, S3 | 0.81 | |
| Glyptothorax telchitta | S1, S2, S3 | 1.63 | |
| Sisor rabdophorus | S2 | 0.31 | |
| Clariidae | Clarias gariepinus | S3 | 0.20 |
| Synbranchidae | Monopterus cuchia | S2, S3 | 0.51 |
| Gobiidae | Glossogobius giuris | S3, S5 | 0.41 |
| Glossogobius gutum | S3 | 0.51 | |
| Belontidae | Trichogaster fasciat | S2, S3 | 0.41 |
| Colisa lalia | S2 | 0.25 | |
| Xenentodon cancila | S3 | 0.51 | |
| Anabantidae | Anabas testudineus | S6 | 0.41 |
| Channidae | Channa gachua | S2, S3, S6 | 0.10 |
| Channa stewartti | S6 | 1.78 | |
| Channa punctate | S3, S6 | 0.25 | |
| Channa striata | S6 | 0.15 | |
| Mastacembelidae | Macrognathus aral | S3 | 0.10 |
| Macrognathus pancalus | S3, S6 | 1.22 | |
| Mastacembelus armatus | S3, S4, S6 | 2.70 | |
| Chandidae | Chanda nama | S2, S3 | 0.51 |
| Parambassis ranga | S2, S3 | 0.41 | |
| Badidae | Badis badis | S1, S2, S3, S6, | 10.17 |
| Nandidae | Nandus nandus | S2, S3 | 0.41 |
| Chaudhuriidae | Chaudhuria khajurial | S3 | 1.53 |
| Tetradontidae | Leiodon cutcutia | S6 | 0.25 |
Table 4: Occurrence and annual catching (%) of fish species in Simsang River.
Diversity indices of the fishes collected from Simsang River are presented in the Table 5. Shannon (H’) index of the collected fishes indicates maximum fish diversity in the site 3 (H’ 3.455) and lowest in the site 5 (H’ 1.06). Shannon’s index has a direct relationship with the species diversity [30], less diversity results minimum Shannon index value. Present study is also in conformity with the above. Berger- Parker Dominance (1/d) values which relate the species richness and abundance [31] were calculated 2.375 to 6.974. It has also follows similar trend of increase as in Shannon index, highest value calculated in site 3 and lowest in site 4 (2.44) and 5 (2.375). Simpson’s Index of Dominance (D) was calculated least in the site 1, 2, 3, 4 and 6, which might have resulted due to the equal distribution of species belongs to different families while higher (13.211) value was calculated in site 5 where species of few families were recorded. Similar observation was reported by Mylliemngap and Ramanujam (2011) in the coal Mining and adjacent non-coal Mining drainages of Jaintia Hills, India. Hill’s number of abundance (H1) shows the diversity richness of one site, the value of which increases with the increase of number of species. Reduction of fish diversity in the site 4 and 5 results less Hill’s number (6.911 and 6.659 respectively) and it increases to maximum in the site 3 (210.81) with highest number of fish species. The Mackintosh distance (U) index is ranged from minimum 0.337 (site 1) to maximum 1.021 (site 6). The values (U) indicate that the fish species in the study sites were not evenly distributed. In conclusion, coal mining activities has a strong impact on water quality and fish diversity of the Simsang River as evident from low pH, DO, sulphate concentration and least diversity indices [32].
| Index | Site-1 | Site-2 | Site-3 | Site-4 | Site-5 | Site-6 |
|---|---|---|---|---|---|---|
| Shannon H' (Log Base 2.718) | 2.607 | 2.932 | 3.455 | 1.086 | 1.06 | 2.656 |
| Berger-Parker Dominance (1/d) | 3.788 | 4.2 | 6.974 | 2.44 | 2.375 | 6.39 |
| Simpsons Diversity (D) | 0.068 | 0.049 | 0.023 | 0.021 | 13.211 | 0.028 |
| Hill's Number H1 | 62.075 | 99.074 | 210.81 | 6.911 | 6.659 | 66.543 |
| Mackintosh Distance (U) | 0.337 | 0.453 | 0.505 | 0.773 | 0.977 | 1.021 |
Table 5: Different fish diversity indices in six sampling sites of Simsang River, Meghalaya.
Conclusion
Coal mining also has socio-economic impacts. These includes displacement and unemployment, child labour, accidents, and theft. In Garo Hills, villagers are mostly the fisherman. Due to discharge of AMD to the river, fish production has reduced significantly. Decrease in fish diversity in the river, has resulted in high influxes of migrants in search of jobs. This, in turn, has resulted in increased changes to indigenous lifestyle, and increased competition among local residents for natural resources.
From this investigation it has been observed that some of the areas of Simsang River in Meghalaya is highly polluted due to AMD of coal mines and is gradually becoming unsuitable for fish and other aquatic biota.
Acknowledgement
Authors are thankful to Department of Biotechnology (BT/176/NE/TBP/2011), New Delhi, Government of India for providing financial assistance to carry out the investigation.
References
- Talukdar B, Basumatary S, Kalita HK, Baishya RA, Dutta A, et al. (2015) Histopathological alternations in liver and kidney of Tortor (Ham) inhabited in coal mining affected areas of Simsang River, Garohills; Meghalaya. Nat Aca Sc Lett 38: 321-324.
- Mylliemngap BK, Ramanujam SN (2011) Icthyodiversity in the Coal Mining and adjacent Non-Coal Mining Drainages of Jaintia Hills, India. Asian Fisheries Sc 24: 177-185.
- Pentreath RJ (1994) The Discharge of waters from Active and Abandoned mines. In: Hester, RE, and Harrison RM (eds) Mining and its Environmental Impacts,Royal Society of Chem, UK pp: 121-131.
- Barthakur M (1986) Weather and climate of North East India. North Eastern Geograph 18: 20-27.
- Talwar PK, Jhingran AG (1991) Inland fishes of India and adjacent countries. Vol 1 and II. Oxford and IBH Publ Co Pvt Ltd, New Delhi.
- Vishwanath W (2002) Fishes of North East India: A Field Guide to Species Identification. Department of Life Sciences, Manipur University, Imphal, India p: 198.
- Trivedi RK, Goal PK (1986) Chemical and Biological method for water pollution studies. 2nd edi. Environmental Publication. Karad, India.
- APHA (2005) Standard methods for the examination of water and wastewater, 21st Edition. Amer. Publ Heal Assoc, Amer. Water Works Assoc and Water Poll Contr Fed, Washington, DC.
- Siriwong W, Thirakhupt K, Siticharoenchai D, Rohitrattana J, Thongkongown P, et al. (2009) DDT and derivatives in indicator species of the aquatic food web of Rang sit agricultural area, Central Thailand. Ecological Indicators 9: 878-882.
- Nasr IN, Arief MH, Abdel-Aleem AH, Malhat FM (2010) Polycyclic aromatic hydrocarbons (PAHs) in aquatic environment at El Menofiya Governorate, Egypt. J Applied Sciences Res 6: 13-21.
- Magi E, Bianco R, Ianni C, Di Carro M (2002) Distribution of polycyclic aromatic hydrocarbons in the sediments of the Adriatic Sea. Env Poll 119: 91-98.
- Chen SJ, Luo XJ, Mai BX, Sheng GY, Fu JM, et al. (2006) Distribution and Mass Inventories of Polycyclic Aromatic Hydrocarbons and Organochlorine Pesticides in Sediments of the Pearl River Estuary and the Northern South China Sea. Envi Sci and Technol 40: 709-714.
- Yunker MB, Macdonald RW, Vingarzan R, Mitchell RH, Goyette D, et al. (2002) PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition. Organic Geochem 33: 489-515.
- Qiu YW, Zhang G, Liu GQ, Guo LL, Li XD, et al. (2009) Polycyclic aromatic hydrocarbons (PAHs) in the water column and sediment core of Deep Bay, South China. Estuarine, Coastal and Shelf Sci 83: 60-66.
- Shannon CE, Weaver W (1963) The mathematical theory of communication. University Illinois Press, Urbana, IL.
- Berger WH, Parker FL (1970) Diversity of planktonic foraminifera in deep-sea sediments. Science 168: 1345-1347.
- Simpson EH (1949) Measurement of diversity. Nature 163: 688.
- Hill MO (1973) Diversity and evenness: a unifying notation and its consequences. Ecol 54: 427-432.
- McIntosh RP (1967) An index of diversity and the relation of certain concepts to diversity. Ecol 48: 392-404.
- Carlos VM, Pompeo ML, Lobo FL, Meirelles ST (2011) Impact of coal mining on water quality of three artificial lakes in Morozini River Basin (Treviso, Santa Catarina State, Brazil). Acta Limno Brasili 23: 271-281.
- Waterloo B (2002) Hydrogeological evaluation and mathematical modeling. Waterloo Hydrogeologic, Inc Relatório.
- Blunden B, Indraratna B (2001) Pyrite Oxidation Model for Assessing Ground-Water Management Strategies in Acid Sulfate Soils. J of Geotech and Geoenvl Eng 127: 146-157.
- Potts WTW, McWilliams PG (1989) The effects of hydrogen and aluminum ions on fish gills. In: Morris R, et al. (eds), Acid toxicity and aquatic animals. Society for Experimental Biology, Seminar Series, Cambridge University Press p: 201-220.
- Singh G (1988) Impact of coal mining on mine water quality. Int J of Mine water qua 7: 49-59.
- Rashid HO, Hossain MS, Zannat U, Islam MS (2014) Environmental Impact of Coal Mining: A Case Study on the Barapukuria Coal Mining Industry, Dinajpur, Bangladesh. Middle-East J of Scientific Res 21: 268-274.
- Ziemkiewicz PF, Skousen JG, Brant DL, Sterner PL, Lovett RJ (1997) Acid mine drainage treatment with armored limestone in open limestone channels. J of Env Qua 26: 1017-1024.
- Banerjea SM (1971) Water quality and soil condition of fish ponds in some states of India in relation to fish production. Ind J Fish 14: 115-144.
- Swer S, Singh OP (2004) Status of water quality in coal mining areas of Meghalaya, India. In: Sinha IN, Ghose MK and Singh G (eds). Proceedings of the National Seminar on Environmental Engineering with special emphasis on Mining Environment (NSEEME 2004): 19-20.
- Riyahi BA, Zakaria MP, Yaziz MI, Hj Lajis MN, Bi X (2009) Polycyclic Aromatic Hydrocarbons and n-alkanes in Suspended Particulate Matter and Sediments from the Langat River, Peninsular Malaysia. Environment Asia 2: 1-10.
- Basavaraja D, Narayana J, Kiran BR, Puttaiah ET (2014) Fish diversity and abundance in relation to water quality of Anjanapura reservoir, Karnataka, India. Int J Curr Microbiol App Sci 3: 747-757.
- Olawusi PO, Ajibare AO (2014) Species richness, diversity and abundance of some Decapod Crustaceans in coastal waters of Ondo State, South West, Nigeria. Int J of Fauna and Bio Studies 1: 44-51.
- Directorate of Mineral resource (1992) Cottage coalmining in the state of Meghalya and its impact on the environment. In: Gupta, A and Dar DC (eds), Environment conservation and Wasteland development in Meghalaya. Meghalaya Science Society, Shillong, India.
Relevant Topics
- Algal Blooms
- Blue Carbon Sequestration
- Brackish Water
- Catfish
- Coral Bleaching
- Coral Reefs
- Deep Sea Fish
- Deep Sea Mining
- Ichthyoplankton
- Mangrove Ecosystem
- Marine Engineering
- Marine Fisheries
- Marine Mammal Research
- Marine Microbiome Analysis
- Marine Pollution
- Marine Reptiles
- Marine Science
- Ocean Currents
- Photoendosymbiosis
- Reef Biology
- Sea Food
- Sea Grass
- Sea Transportation
- Seaweed
Recommended Journals
Article Tools
Article Usage
- Total views: 7669
- [From(publication date):
December-2016 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 6571
- PDF downloads : 1098