Review Article Open Access
Impact of MRI Characterization of the Carotid Artery in the Understanding of Stroke
Creagh S1*, Ortiz A2, Fumero J3 and Paydar A41Ponce Health Sciences University, Ponce, Puerto Rico
2Diagnostic Radiologist, Aventura Hospital and Medical Center, FL, USA
3Diagnostic Radiologist, Hospital Episcopal San Lucas, Ponce, Puerto Rico
4Diagnostic Radiologist, University of Central Florida and Florida State University, Florida Hospital, USA
- *Corresponding Author:
- Creagh S
Ponce Health Sciences University
Ponce, Puerto Rico
Tel: 787-361-3988
E-mail: scarmencr@yahoo.com
Received date: February 23, 2017; Accepted date: March 02, 2017; Published date: March 06, 2017
Citation: Creagh S, Ortiz A, Fumero J, Paydar A (2017) Impact of MRI Characterization of the Carotid Artery in the Understanding of Stroke. OMICS J Rad 6:255. doi: 10.4172/2167-7964.1000255
Copyright: © 2017 Creagh S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Radiology
Abstract
Carotid artery stenosis accounts for approximately 10% of ischemic strokes. Improved methods of diagnosis of cerebrovascular atherosclerosis would result in significant improvement in quality of life and major savings in health care costs. Imaging plays a critical role evaluating patients suspected of acute stroke. Magnetic Resonance Imaging (MRI's) significant advantages over DUS in carotids imaging can pay off its cost by means of improved patient outcome. Although DUS is frequently performed to evaluate carotid disease, there is nonuniformity in practice among laboratories. MRI can detect and quantify major compositional features of the carotid plaque and comprehensively evaluate its complications. A high level of agreement exists between high-resolution in vivo MRI, gross, and histological findings on the thickness and sites of potential rupture of the fibrous cap in advanced carotid artery atherosclerosis. MRI is capable of classifying and distinguishing different stages of atherosclerotic lesions. It can also examine the mechanisms of regression, progression, and endothelial dysfunction of the carotid plaque. Carotid MRI is a histologically validated, non-invasive imaging method that can track atherosclerotic disease progression and regression. It can quantitatively evaluate parameters associated with plaque morphology and composition. MRI is able to quantify high-grade carotid artery stenosis and occlusion with good accuracy and reproducibility and provides an opportunity to prospectively examine the relationship between plaque features and subsequent cerebrovascular events. The aim of this article is to examine the efficacy of MRI versus DUS in identifying vulnerable carotid lesions in the acute stroke patient that can account for stroke etiology or mechanism.
Keywords
Magnetic Resonance Imaging (MRI); Carotid artery stenosis; Atherosclerotic plaque; Stroke
Introduction
Stroke represents the third primary cause of mortality in the U.S., with an incidence of approximately 700,000 deaths per year, while 600,000 patients experience the morbidity of aphasia, blindness, or paralysis. Carotid artery stenosis accounts for approximately 10% of ischemic strokes. Better techniques of diagnosis and treatment of cerebrovascular disease could enhance the quality of life and reduce health care expenses. Imaging plays a critical role in evaluating patients suspected of acute stroke and transient ischemic attack. A primary goal of carotid imaging is early identification of carotid atherosclerotic disease as the underlying stroke etiology, which is critical to treatment decisions and long-term management [1]. The significant advantages of MRI over Doppler ultrasound in carotids imaging of the acute stroke patient can, in turn, pay off the increased imaging costs by means of improved patient outcome and shortened need for institutional care [2].
Ultrasound delivers a quick, non-invasive method to evaluate the carotid arteries. DUS assesses carotid stenosis by the indirect NASCET criteria, which uses velocity measurements to indirectly measure the grade of luminal narrowing. This technique offers measurements of wall thickness, local diameter and flow parameters such as peak velocities, gradients and waveform. Yet, DUS is conditioned on its operator and has a restricted field of view. Although DUS is the most widely used tool implemented for the identification of carotid disease, a broad variation of practice outlines among vascular laboratories still exists.
Despite technological advances in sonographic equipment, improvements in operator expertise, and accrediting bodies to increase the quality of carotid sonographic examination, there is still a lack of agreement in how DUS is performed and understood among diverse laboratories and even among different operators in the same center. In order for a carotid sonogram to provide reliable and reproducible material, standardized protocols are highly advised.
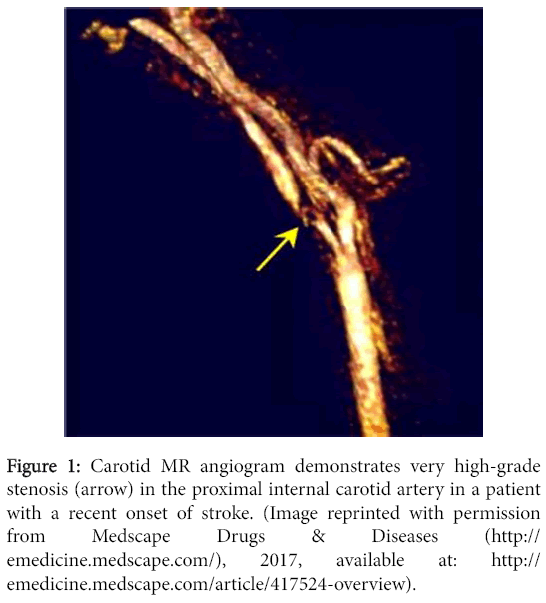
A major relevance of MRA in our current clinical setting is its accuracy in depicting the degree of carotid artery stenosis (Figure 1) as established by the direct NASCET method. The direct NASCET criteria utilizes direct measurements of the vessel luminal diameter at the point of stenosis and compares it to a non-diseased segment more dismally in order to quantify degree of stenosis. This is what MRA conventionally is used for today.
Figure 1: Carotid MR angiogram demonstrates very high-grade stenosis (arrow) in the proximal internal carotid artery in a patient with a recent onset of stroke. (Image reprinted with permission from Medscape Drugs & Diseases (http://emedicine.medscape.com/), 2017, available at: http://emedicine.medscape.com/article/417524-overview).
MRA has good sensitivity and accuracy at creating a reproducible three-dimensional look of the carotid bifurcation for the recognition of high-grade stenosis of the carotid artery and occlusion. The sensitivity of MRA technique for the identification of carotid artery occlusion or severe stenosis ranges from 91 to 99%, while specificities range from 88 to 99% [3].
In addition, conventional MRI techniques have shown great promise in characterizing carotid atherosclerotic plaque composition and morphology. This tool has significant capacity in assessing plaque load and plaque vulnerability. MRI is ideal to image carotid plaques since it is non-invasive, has exceptional contrast resolution, does not include ionizing radiation, allows imaging of the vessel wall and lumen, and can be redone consecutively to monitor evolution or regression, and the results are more reproducible than those of ultrasound. MRI can determine and assess the main elements of the carotid plaque such as fibrous tissue, lipid and calcium, which can be recognized based on changes on signal intensity from distinctive weightings. In particular, MRI has been proven to comprehensively evaluate the in vivo complications of the carotid plaque, such as rupture of a fibrous cap, haemorrhage/thrombus, and calcified nodules. Furthermore, MRI reproducibly characterizes the American Heart Association Lesion Type (AHA-LT) according to modified MRI criteria [4].
Objectives
To evaluate the efficacy of MRI, as compared to DUS in identifying vulnerable carotid lesions in the acute stroke patient that can account for the stroke etiology or mechanism.
Discussion
DUS is the most widely used imaging tool employed to enhance the identification of carotid disease. Thus, it is crucial that material offered by the DUS be reliable and reproducible. Yet, overall and despite progresses, carotid DUS is frequently executed with nonuniformity within a given center, and among different labs. Many settings have inconsistent interpretive standards and subsequent uncertainty on how to apply these criteria in diagnosing carotid stenosis.
In 2002, a consensus panel from the Society of Radiologists in Ultrasound recognized many irregularities in the carrying out and interpretation of carotid DUS. For instance, in our clinical setting there are errors in proper arranging of the Doppler gate and the Doppler angle. Given that Doppler velocities play a pivotal role in interpretative standards for carotid stenosis, errors in these variables will lead to major missteps in diagnosis. According to some panelists, keeping a fixed angle of insonation of 60 degree would offer a better consistency; yet others claimed that as long as the angle of insonation was less than or equal to 60, a proper consistency could be perfectly attained. Consequently, additional consideration on this issue is necessary [5].
Further issues in ICA evaluation involve inappropriate arranging of the sample volume, lacking sampling through a region of stenosis, and lack of representation of the distal end of a carotid plaque. Additionally, numerous mistakes can happen due to issues inherent to the patients (widespread calcification of plaque, significant ICA tortuosity, and tandem injuries [5]. There are considerable differences between equipment from different machines, manufacturers and between older and latest apparatus. This inconsistency in equipment could account for some of the disagreement in current literature regarding Doppler thresholds for the identification of carotid stenosis. Techniques by which the degree of ICA stenosis is informed differ from lab to lab and within some labs. Some give an approximation of the percent of stenosis, while others separate their estimations into five or six diagnostic groups or stages of stenosis. Some investigators have shown that the average increase in Doppler velocity has a direct correlation with the degree of stenosis as identified with angiography, yet there are very broad series of Doppler measurements comprising those means. Thus, it is not possible to categorize lesions into degrees as narrow as 10%. When considering whether DUS is capable of assessing the degree of stenosis by means of more extended strata the conclusions have been unsatisfactory. DUS is most effective at categorizing lesions as being over or under a single percentage of stenosis. Even though many centers rely on DUS to diagnose minor (<50%) degree of ICA narrowing, Doppler is imprecise for classifying stenosis less than 50%. Numerous imaging and Doppler guidelines are being applied at different centers for the estimation of ICA stenosis, such as ICA PSV, ICA EDV; yet the way these guidelines are implemented between different labs differ as well. There are many velocity thresholds in current literature to sort ICA stenosis, but there is significant discrepancy among these investigations in the way they determine specific Doppler criteria and in the thresholds suggested to establish ICA stenosis. The outlines used to report the final findings of DUS also differ among laboratories. Elaboration of internally approved Doppler thresholds may be complicated due to the limited correlative angiograms at the majority of centers. Traditionally, angiography has been seen as the “gold standard for determining Doppler thresholds for ICA stenosis, but currently angiography is barely done. The few that are actually performed only portray instances in which DUS results were erroneous or conflicting. Even when angiography has been employed to establish Doppler standards for ICA stenosis, variable methods have been employed for assessing ICA stenosis [5].
One of the most significant limitations of DUS in estimating the level of ICA stenosis is in the measurement of the flow velocity at the site of stenosis. When the aperture of a stenosis has diverging streamlines, the most accurate Doppler angle is not necessarily parallel to the axis of a vessel. Helical flow organization and disruptions as consequence of tortuosity represent additional factors making precise angle approximation challenging or not possible, even when colour flow has been employed as a reference.
When trying to translate Doppler shifts to velocities the chance of error increases as the Doppler angle is augmented as per the cosine function. Thus, the inconsistency in the approximation of velocity is greater in comparison with simple frequency recording.
In order to record Doppler signals with a linear probe, a sequence of transducer components are pulsed to create and orient the wave front. As a result, the spectrum that is documented is comprised of signals deriving from different insonation angles generating a wide range of spectrum. This can result in major velocity overestimation in the stenosis. This “error” varies considerably according to the particular insonation and flow. On the contrary, deficient gain or a low wall filter can lead to under approximation of the peak systolic velocity. Moreover, internal carotid artery PSV values are less reliable when there are changes in cardiovascular physiologic conditions such as complications in cardiac output, aortic disease, hypertension, and occlusive processes in both carotids [6].
When depicting plaques of reduced echogenicity or demonstrating lack of flow in the obstructed ICA, color Doppler is actually beneficial. However, it does not allow precise diameter measurements due to its low frame rate and a huge influence of the gain. Still, a few of the soft plaques that create low-level echoes can go unnoticed. Heavily calcified plaques may act as a barrier to the ultrasound waves and may cause posterior acoustic shadowing. Such shadow makes the implicated fragment unreachable by the Doppler analysis or gray scale. Ultrasound is unable to detect the whole vessel length since the last fragment of the carotid artery intersects the bone at the base of the skull. Another factor that makes it challenging to examine the vessel is the deposition of calcium in the carotid artery’s wall. In patients with stenosis high in the neck, unusually long lesions or arterial kinking, other diagnostic tests should be recommended [7]. The abovementioned limitations of DUS may bring into question its accuracy and reliability in the assessment of carotid artery stenosis and perhaps encourage the use of a more reliable imaging tool such as MRI for initial evaluation of the carotids in stroke patients.
To improve the knowledge about the correlation between fibrous cap splits and thromboembolic incidents and to enhance the recognition of high-risk lesions, an accurate and reproducible imaging tool of depicting the fibrous cap in vivo is necessary. High- resolution MRI is able to differentiate undamaged thick fibrous caps from undamaged thin and damaged caps in atherosclerotic human carotid arteries. This non-invasive tool has the ability to enhance analysis of the link between fibrous cap alterations and clinical implications and to allow trials that examine therapy meant to “stabilize” the fibrous cap.
MRI representation of a thin or rupture fibrous cap has been highly linked with a recent stroke or transient ischemic attack. This potential of MRI to noninvasively identify atherosclerotic plaques with a susceptibility to rupture before the onset of ischemic complications has remarkable clinical implications. Current prospective investigations will reveal how well fibrous cap features, as depicted by MRI, correlates with risks of ensuing ischemic complications [8]. MRI has great potential for serial studies of evolving atherosclerotic lesions because it is non-invasive and it is better than other imaging tools in differentiating soft tissue contrast. A number of studies have established that MRI can depict aspects such as fibrous tissues, calcium, lipid/necrotic core, thrombus and hemorrhage among other important morphological and compositional features of the carotid atherosclerotic plaque both in vitro and in vivo. There is high concordance between high resolution MRI, and histological data on the thickness and spots of impending rupture of the fibrous cap in progressive carotid artery atherosclerosis. Recently, it has been shown that the values of plaque composition provided by MRI have a statistical correlation with those of histology for Lipid Rich Necrotic Core (LR/NC), dense (fibrous) tissue and loose matrix (Table 1).
| Plaque composition (as percentage of the wall) for MRI vs. Histology | |||
|---|---|---|---|
| Plaque Component | MRI | Histology | p |
| Dense (fibrous) tissue | 66.3 | 64 | 0.4 |
| Lipid/necrotic core | 23.7 | 20.3 | 0.1 |
| Loose Matrix | 5.1 | 6.3 | 0.1 |
| Calcification | 5 | 9.4 | <0.001 |
Table 1: Plaque composition estimated as the percentage of the vessel wall area, assessed per artery, and then averaged across all arteries for MRI and histology.
The significant variation in calcification when estimated as a percentage of wall area (9.4 versus 5%; P<0.001) could be due to either less shrinkage of calcification when compared to other components during histological assessment, or MRI’s underestimation of regions displaying hypo intense signal due to the signal averaging of voxels with only moderate amount of calcification. For all tissue factors, the intra-reader and inter-reader reproducibility were good to exceptional [9].
MRI can have significant applications in natural history and clinical studies that evaluate the mechanism of cap thinning and disruption and could eventually deliver an accurate, non-invasive diagnostic and prognostic instrument for the care and prevention of the sequelae of an insidious condition such as stroke [10].
It is vital to determine a better way to predict the clinical events caused by carotid vulnerable lesions in symptomatic stroke patients. Ultrasound and MRI are recognized techniques of evaluating carotid plaque burden. However, unlike IMT ultrasound that by established approach measures posterior wall thickness, MRI enables quantification of plaque volume and is optimal for measuring eccentric plaques. The burden of carotid plaque originated from the posterior wall IMT steadily underestimates the volume of carotid plaque as provided by MRI, possibly because of the eccentric nature of carotid atherosclerosis. This implies non-uniform variations in regional plaque burden that might not be revealed by solely measuring the posterior wall. This finding highlights critical considerations when employing ultrasound in serial analysis of plaque burden and advocates the need for three-dimensional volumetric estimation of plaque burden [11]. Increasing evidence suggests that mechanisms governing advanced plaque progression may be different from those for early progression and require further investigation. Serial MRI data has shown that advanced plaque progression has an overall positive correlation with plaque wall stress and a negative correlation with flow shear stress at baseline [12].
The risk of rupture of atherosclerotic plaque may be shaped by susceptibility factors stimulating the development of atherosclerotic alterations. For instance, diabetes mellitus is a well-recognized leading factor for atherosclerosis that might represent a significant role in the remodeling of a stenotic plaque and in the progress of plaque vulnerability. Plaque imaging offered by MRI is capable of identifying possible variations in carotid plaque characteristics of patients with diabetes as compared with non-diabetic ones. A recent study found that MRI lesions categorized as high risk (types IV-V and VI) prevail in diabetic patients. Based on these differences, it has been proposed that those diabetic patients with MRI-detected high-risk lesion have an increased chance than non-diabetic patients with the same high-risk lesion category for the occurrence of cerebral ischemia following endarterectomy of carotid artery stenosis. MRI imaging of the carotid plaque signifies an innovative, non-invasive method to visualize these high-risk plaque aspects and, therefore, offers a new prospect for risk stratification in patients with diabetics [13].
High-resolution MRI is able to categorize and distinguish advanced from early and intermediate atherosclerotic plaques lesions in humans based on an adjusted AHA classification. This ability of lesion categorization may make MRI suitable to explore the means of regression and progression of the carotid plaque. The major worry regarding this lesion type categorization concerns the MRI resolution, which was not ideal to identify small structures or constituents such as surface defects, erosion, or small amounts of hemorrhage <0.25 mm in size. Motion artifacts, due to patient movement or arterial pulsation, also damage the quality of MRI.
Additional advances in imaging criteria, coil design and faster hardware are encouraged. The accuracy of MRI in plaque tissue classification could be further enhancing through comparison of preand post-contrast-enhanced T1W images. In addition, contrastenhanced MRI may represent the only reliable tool to identify neovasculature that could add to plaque instability. Such new advances will improve our capability to identify the components of the atherosclerotic plaque in vivo and permit us to attain better categorization abilities [14].
Multicontrast MRI can also identify and categorize carotid intraplaque hemorrhage with high sensitivity and moderate specificity. This instrument would allow in vivo evaluation of the involvement of intraplaque hemorrhage in the progress of ischemic complications. Furthermore, the recognition of such hemorrhage could raise awareness to the physicians about the risk of plaque vulnerability. Thus, an improved classification of the plaques may offer significant clinical material for patient management [14].
Studies have shown that endothelial dysfunction is associated with stroke. Although MRI can help assess various stiffness parameters such as vascular distensibility, flow measurements, and the pulse wave velocity by using true fast imaging with steady-state free precession and gradient-recalled-echo pulse sequences with a velocity-encoding gradient for phase-contrast MRI, only a few such investigations have been performed in this field. The reason for this is an increase availability of other imaging tools such as DUS, which also allows for evaluation of arterial stiffness.
However, a recent study showed that MRI offers insights not otherwise possible with regard to describing an age-related increase in pulse wave velocity in the proximal aorta relative to that in the distal aorta. In another MRI investigation in which central vascular distensibility was assessed, pulse wave velocity and flow-mediated dilatation showed global disruption in the vascular function of the brachial arteries, carotids, and aorta vascular function in young smokers [15].
Many non-stenotic lesions undergo expansive remodeling. Several authors have suggested that remodeling is a potential surrogate marker of plaque vulnerability. However, MRI is ideally suited for serial and non-invasive assessment of arterial remodeling, because MRI offers information regarding the anterior wall and lumen simultaneously. MRI-detected carotid plaque hemorrhage signal has also been connected with a higher risk of recurrent ischemic cerebrovascular events and could be an indicator of disease progression. According to a recent study, carotid plaques with hyperintense signal on an MRI sequence were associated with increased flow velocity, which is marker for stenosis progression [15].
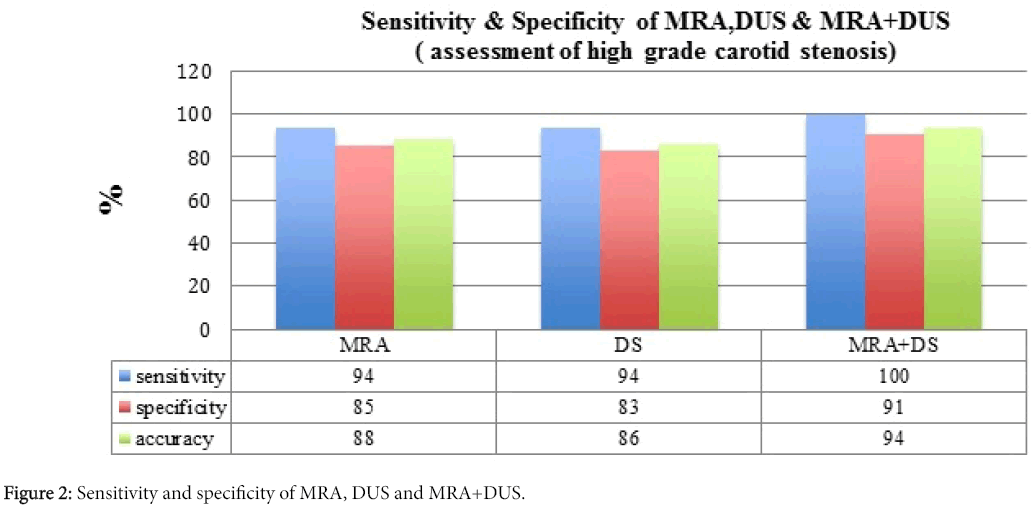
NASCET and ECST trials have proved the benefits of performing endarterectomy in those patients with symptomatic high-grade stenosis. Some studies are employed noninvasively and with rising frequency to evaluate the carotid bifurcation previous endarterectomy. Consequently, determination of their diagnostic accuracies is crucial for adequate patient management. MRA has 94% sensitivity, 85% specificity and 88% accuracy for the recognition of 70% to 99% stenosis while DUS has 94% sensitivity, 83% specificity, and 86% accuracy. Yet, combining data from MRA and DUS yields 100% sensitivity, 91% specificity, and 94% accuracy with no false-negative outcomes. Although MRA has better discriminatory power than DUS, combining DUS+MRA notably increases this overall accuracy, i.e., both sens+spec (Figure 2). Based on these results, the combined use of these two non-invasive studies is highly encouraged for a more efficient preoperative assessment of patients with carotid artery stenosis [16].
Conclusion
Carotid MRI and DUS might be capable of differentiating advanced and susceptible plaques from early, and/or intermediate atherosclerotic plaques that are more stable. Currently, Doppler is the most widely employed tool since MRI is more expensive and has limited accessibility. While low in price and broadly accessible, determination of plaque components by DUS does need skilled interpretation and high-quality carriers for their consistent use in clinical settings. MRI of the carotids is a non-invasive tool, histologically substantiated that can monitor atherosclerotic disease progression and regression, and offer a quantitative assessment a spectrum of values related to in vivo plaque morphology and structure.
MRI is able to quantify high-grade stenosis of the carotid artery and occlusion, as well as carotid plaque size and composition with good accuracy and reproducibility, and provides an opportunity to prospectively evaluate the association between plaque features and subsequent cerebrovascular events. MRI techniques able to image other significant features of carotid atherosclerotic disease in vivo such as inflammation, mechanical forces and neovascularization forces-are developing and may improve our notion of the pathophysiology of this multifactorial disease. Thus far, there is not a single MRI technique that can be employed to evaluate all the vulnerable plaque criteria, and it remains to be seen which imaging approach, if any, will be commonly used in clinical practice. Nevertheless, in current practice, the combination of MRA and DUS is probably sufficient for identifying patients with carotid artery severe stenosis and occlusion that are likely to benefit from endarterectomy [3].
References
- Schellinger PD (2005) The evolving role of advanced MR imaging as a management tool for adult ischemic stroke: a Western-European perspective. Neuroimaging Clin N Am 15: 245-258.
- Tatlisumak T (2002) Is CT or MRI the method of choice for imaging patients with acute stroke? Why should men divide if fate has united? Stroke 33: 2144-2145.
- Toole, James F, John EC (1994) Accurate measurement of carotid stenosis. J Neuroimaging 44: 222-230.
- Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, et al. (1995) A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. Circulation92: 1355-1374.
- Grant EG, Benson CB, Moneta GL, Alexandrov AV, Baker JD, et al. (2003) Carotid Artery Stenosis: Gray-Scale and Doppler US Diagnosis-Society of Radiologists in Ultrasound Consensus Conference. Radiology 229: 340-346.
- Arning C, Widder B, Von Reutern GM, Stiegler H, Görtler M (2010) Revision of DEGUM ultrasound criteria for grading internal carotid artery stenoses and transfer to NASCET measurement. Ultraschall in der Medizin 31: 251-257.
- Hames TK, Humphries KN, Ratliff DA, Birch SJ, Gazzard VM, et al. (1985) The validation of duplex scanning and continuous wave Doppler imaging: A comparison with conventional angiography. Ultrasound in medicine & biology 11: 827-834.
- Yuan C, Zhang SX, Polissar NL, Echelard D, Ortiz G, et al. (2002) Identification of fibrous cap rupture with magnetic resonance imaging is highly associated with recent transient ischemic attack or stroke. Circulation 105: 181-185.
- Saam T, Ferguson MS, Yarnykh VL, Takaya N, Xu D, et al. (2005) Quantitative evaluation of carotid plaque composition by in vivo MRI. Arteriosclerosis, thrombosis, and vascular biology 25: 234-239.
- Hatsukami TS, Ross R, Polissar NL, Yuan C (2000) Visualization of fibrous cap thickness and rupture in human atherosclerotic carotid plaque in vivo with high-resolution magnetic resonance imaging. Circulation 102: 959-964.
- Owers M, Harmann L, Prost R, Bright M, Doppalapudi A, et al. (2009) Underestimation of carotid plaque by ultrasound IMT and potential error in measurement of change in plaque burden: simultaneous comparison with 3 T MRI. J CardiovasMagnReson11: 171.
- Friedman MH, Bargeron CB, Deters OJ, Hutchins GM, Mark FF (1987) Correlation between wall shear and intimal thickness at a coronary artery branch. Atherosclerosis68: 27-33.
- Saam T, Hatsukami TS, Takaya N, Chu B, Underhill H, et al. (2007) The Vulnerable, or High-Risk, Atherosclerotic Plaque: Noninvasive MR Imaging for Characterization and Assessment. Radiology 244: 64-77.
- Chu B, Kampschulte A, Ferguson MS, Kerwin WS, Yarnykh VL, et al. (2004) Hemorrhage in the atherosclerotic carotid plaque: a high-resolution MRI study. Stroke 35: 1079-1084.
- Beaussier H, Naggara O, Calvet D, Joannides R, Guegan-Massardier E, et al. (2011) Mechanical and structural characteristics of carotid plaques by combined analysis with echotracking system and MR imaging. JACC:Cardiovas imaging 4: 468-477.
- Patel MR, Kuntz KM, Klufas RA, Kim D, Kramer J, et al. (1995) Preoperative assessment of the carotid bifurcation. Stroke 26: 1753-1758.
Relevant Topics
- Abdominal Radiology
- AI in Radiology
- Breast Imaging
- Cardiovascular Radiology
- Chest Radiology
- Clinical Radiology
- CT Imaging
- Diagnostic Radiology
- Emergency Radiology
- Fluoroscopy Radiology
- General Radiology
- Genitourinary Radiology
- Interventional Radiology Techniques
- Mammography
- Minimal Invasive surgery
- Musculoskeletal Radiology
- Neuroradiology
- Neuroradiology Advances
- Oral and Maxillofacial Radiology
- Radiography
- Radiology Imaging
- Surgical Radiology
- Tele Radiology
- Therapeutic Radiology
Recommended Journals
Article Tools
Article Usage
- Total views: 3481
- [From(publication date):
April-2017 - Mar 29, 2025] - Breakdown by view type
- HTML page views : 2626
- PDF downloads : 855