Research Article Open Access
Impact of Different Colors of Artificial Light on Pigmentation and Growth Performance of Hybrid Red Tilapia (Oreochromis mosambicus × O. hornorum) Reared in Saline Well Water
Hadir A Aly, Mohamed M Abdel-Rahim*, Basem S Abdelaty, and Ayman M LotfyFish Rearing Lab, Aquaculture division, National Institute of Oceanography and Fisheries (NIOF), Alexandria, Egypt
- *Corresponding Author:
- Mohamed M Abdel-Rahim
Fish Rearing Lab, Aquaculture Division
National Institute of Oceanography and Fisheries (NIOF)
Alexandria, Egypt
Tel: +20 100 692 9599
E-mail: mohamed_m_ar@yahoo.com
Received date: May 01, 2017; Accepted date: May 22, 2017; Published date: May 29, 2017
Citation: Aly HA, Abdel-Rahim MM, Abdelaty BS, Lotfy AM (2017) Impact of Different Colors of Artificial Light on Pigmentation and Growth Performance of Hybrid Red Tilapia (Oreochromis mosambicus × O. hornorum) Reared in Saline Well Water. J Marine Sci Res Dev 7:229. doi: 10.4172/2155-9910.1000229
Copyright: © 2017 Aly HA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Marine Science: Research & Development
Abstract
Four light colors (red, white, green and blue) were tested to evaluate their effects on both body color enhancement and growth performance of Florida red tilapia (Oreochromis mosambicus Ã�? O. hornorum). Fish were stocked in 12 fiberglass tanks, (each of 2 m3 water volume), at a stocking rate of 100 fish per tank with average (p â�?¤ 0.05) are observed in the whole body chemical composition between treatments. Fingerlings initial weight of 2.66 g/fish, three replicates for each treatment. Fish were fed on a commercial diet containing 25% protein, two meals per day with a daily feeding rate of 7% in the first two weeks, then reduced to 5% until the end of the experimental period (6 weeks). The results of this study reveals that there are no significant differences (p>0.05) in growth performance indexes between treatments. However, fish exposed to red color showed highest growth values. With the same trend, results of survival percent and condition factor showed no significant differences between treatments. Significant differences that are exposed to blue color has the highest value of dry matter content, while, the lowest value is observed in fingerlings exposed to red color. Feed utilization (FI, FCR and PER) do not significantly affect the lighting color, while protein and energy utilization (PPV and EU) are significantly affected. Concerning the red color accumulated in the fish body, the fingerlings which are exposed to the blue color achieved the best β-carotene value (211.25 IU/100 g fish) with highly significant differences compared with other treatments. The content of β-carotene in blue color treatment is 8, 9, and 16 folds comparing with green, white, and red colors, respectively. Also, Red tilapia fish with black spots in the blue treatment were 12%, compared with 29% in green, 56% in white and 52% in red colors. It could be concluded that cultivating Red tilapia in a full saline well water using the artificial blue color will significantly improve not only the pigmentation of Red tilapia, the quality of fish in terms of the content of dry matter, but also the protein and the energy utilization of the consumed feed.
Keywords
Red tilapia; Artificial light color; Pigmentation; B-Carotene; Growth; Feed utilization
Introduction
Tilapia is one of the most widely cultivated fishes in the world [1]. Nevertheless, under high salinity water, the culturists must face its sensitivity to handling, disease infections [2], remarked retardation in growth, increased feed conversion ratio, up to high mortality ratio [3,4]. Florida red tilapia strain was developed in the 1970s by crossing a normal colored O. hornorum female with a red-gold male O. mossambicus [5]. This strain was introduced to Egypt in 1993. Florida red tilapia strain grows well in high saline water [6] and can tolerate salinity up to 36.2%, and the optimum limit is 17.8% [7], while Nile tilapia does not tolerate salinities above 20 ppt [8]. Therefore, world is moving towards expanding the cultivation of Red tilapia, the most resistant strain to high salt water [3].
The red color of hybrid tilapia is one of the most favorite traits, in addition to its tolerance to salinity. There are many factors influencing the chromatic state of fish, including nutritional, environmental, genetic and neuro-hormonal factors [9]. Dietary carotenoids play an important role in regulating fish color [10]. Because fish, like other animals, are unable to synthesize carotenoids, the skin color in fish is highly dependent on carotenoids present in the diet [11]. Therefore, dietary sources of carotenoids (natural or artificial) should be enhanced the artificial diets for colorful fishes, such as Red tilapia. Otherwise, fish will suffer from pale colors. Dietary β-carotene suppressed skin β-carotene, and pigments of False Clownfish, Amphiprion ocellaris [10].
In addition, the environmental conditions, including background [12,13], light color, light intensity [14], external and/or internal stresses etc. [15,16]. can alter the coloration of cultivated fishes. This phenomenon causes technical, and commercial problems and may reduce the marketability of the cultured fish [15]. Changing light intensity or background color could temporarily change the coloration of Clownfish. According to the opinion of [10], manipulating environmental conditions is unlikely to permanently alter the pigmentation of fish. However, this rapid, simple and inexpensive intervention will improve the color of farmed fish and increase the profitability of aquatic organisms.
Many studies were interested in investigating the genetic system of body color inheritance in red tilapia, where it is reported to be single in complete dominance inheritance [17,18]. It has been stated that there are three different genetic mechanisms responsible for the body coloration system in tilapia [19]. A single gene with incomplete dominance for pink, red and black colors, two genes with epistatic interaction and two independent genes, one for producing melanin and the other for producing red pigmentation. In vertebrates, the skin color is organized by enzymes stimulation, but aquaculturists have to consider that environmental colors are determining factors of skin color intensity and pattern overall the fish body. One of the environmental characteristic that affects the fish physiology is environmental color, which modulate several parameters such as; feeding [20], growth [21], reproduction [22], sex determination [23], aggression [24], larval jaw malformation [25], and stress response [26]. Moreover, fish can alter their color in response to environmental conditions, physiological challenges, stressful stimuli [27] and cultural condition (such as background color in red porgy, Pagrus pagrus tanks) [28]. However, fish could adapt to the background color by changing the skin color [29,30] and this phenomenon causes commercial problems in areas where this species is traditionally fished [28] and leads to a reduced marketability of the cultured fish [30].
A common problem with the Florida red tilapia is the color of the skin, which can revert to a black coloration phenotype like those of the Mozambique tilapia. This change can subsequently reduce market acceptability and demand, but change of the light coloration may mitigate this. The aim of this study was to evaluate different light colors on the growth, feeding efficiencies, biochemical composition and skin colorations of red tilapia reared in saline well water.
Material and Methods
Fish origin
Hybrid red tilapia (O. mossambicus × O. hornorum) was obtained from a GAFRD governmental marine fish hatchery located in Maryout Company for Fish Farms, west Alexandria, K21, Egypt. The fish were placed in 4 m3 fiberglass tanks and acclimated for a week on marine well water with a salinity of 32%, by gradual increasing from 8% to 32%.
Experimental procedures
This experiment is performed in El-Max Research Station for Applied Research (NIOF). After fish acclimation and settlement, 1200 fish with an average weight of 2.66 g are randomly selected and transferred to 12 experimental tanks each of 2 m3 water volume at a stocking density of 50 fish/m3 . Four lightning colors; red, blue, green and white, were tested as an artificial source of lighting, with three tanks for each treatment (color) as shown in Figure 1. Black barriers were placed between each treatment to avoid color interference The fish are fed on a commercial diet purchased from Aller Aqua Egypt Company (www.alleraquaegypt.com), contains (25% Crude Protein, 5.6% Crude fat, 7.15% Crude fibers, 403 Kcal/100 gm DM feed Gross energy, and 62.03 N:C ratio (mg CP: Kcal)); 2 times daily with feeding rate 7% of fish biomass during the first two weeks, and then reduced to 5% starting from week 3 up to the end of the experiment (after 6 weeks). The onset of the experiment is actually registered after 3 days of stocking (acclimatization period), where dead fish are removed and replaced with the same number and weight (Supplementary Figures 1-4).
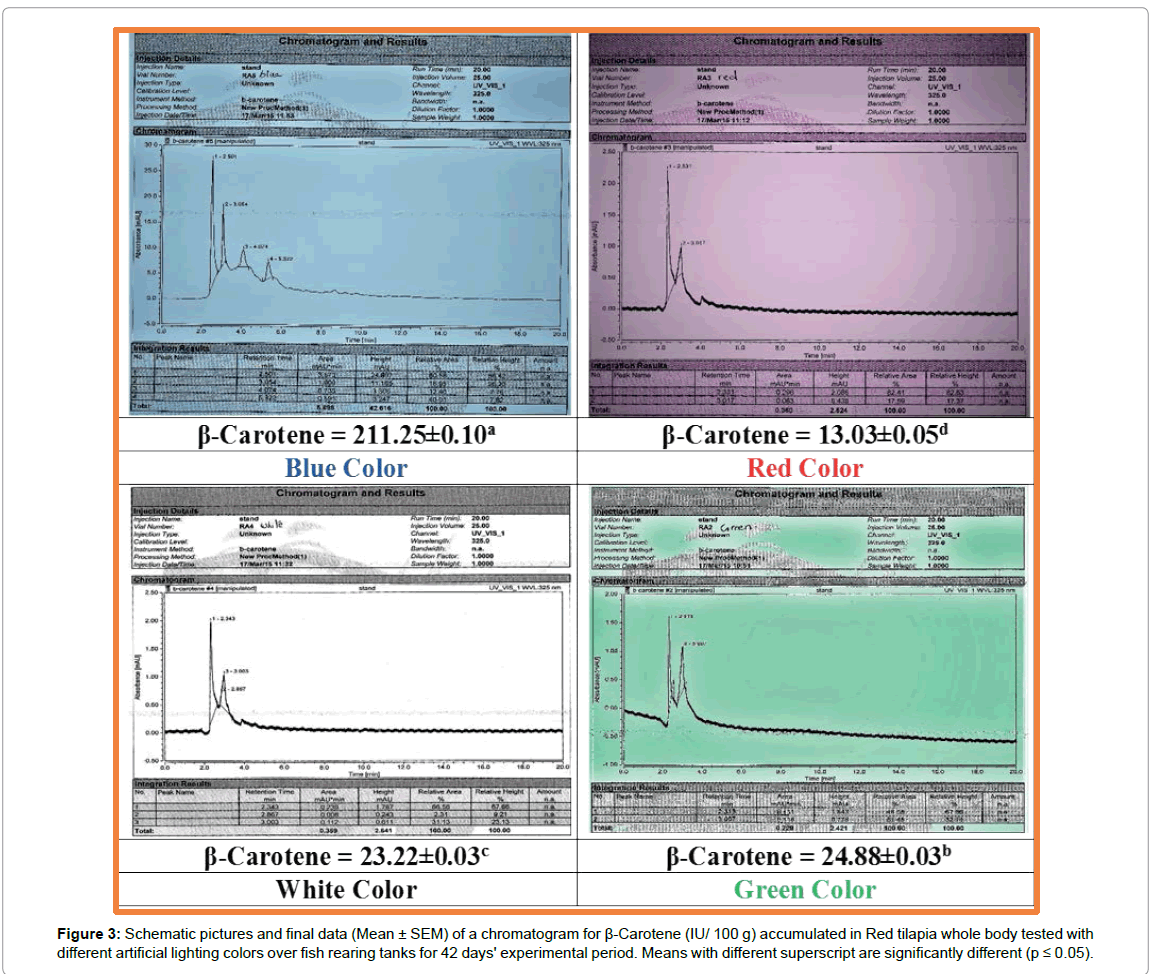
Figure 3: Schematic pictures and final data (Mean ± SEM) of a chromatogram for β-Carotene (IU/ 100 g) accumulated in Red tilapia whole body tested with different artificial lighting colors over fish rearing tanks for 42 days' experimental period. Means with different superscript are significantly different (p ≤ 0.05).
Studied Traits
Water quality analyses
Water temperature, salinity, pH, total ammonia-nitrogen (TAN), and un-ionized ammonia are monitored weekly throughout the experimental period. Temperature and pH were measured using portable pH Meter (pH-8424) (HANNA Instrument). Dissolved oxygen was measured by HI-9142 (HANNA Instrument). Water salinity is measured by a refractrometer (http://www.atago.net).The concentration of total ammonia nitrogen (TAN) was analyzed using YSI 9300 photometer and YSI Professional Plus. The concentration of un-ionized ammonia was calculated as a percentage of TAN according to U.S. Environmental Protection Agency [31].
Growth performance, condition factor, and survival
About 40 fish were randomly selected bi-weekly to estimate the gain in weight and length. The growth performance parameters are calculated according to the following equations:
Specific growth rate (%/day): SGR=100 × (ln Wt - ln W0)/ n [32]
Where: W0: initial mean weight of fish (grams).
Wt: final mean weight of fish (grams).
n: Experimental period (days).
ln: natural logarithm.
After 6 weeks, the fish in each tank are weighed individually to determine survival and final weight gain.
Survival (%)=100 × (final number of fish/ initial number of fish) [33,34].
Condition factor (K)=100 × (BW/ L3) [24].
Where: BW is fish body weight (gram); L is total fish length (cm)
Whole-body fish chemical analysis
At the beginning of the experiment, a representative fish sample is analyzed for body chemical composition (moisture, protein, lipid, ash). Also, at the end of the experimental period, ten fishes are randomly selected from each tank. The selected fishes are analyzed for body moisture, protein (Kjeldahl method), lipid (Soxhlet method), and ash (at 550°C for 16 hours) according to the AOAC (2000) [35].
Gross energy estimation (Kcal/100g)
Gross energy (GE) content of the experimental diet was estimated according to the following equation:
Gross energy (GE) was calculated as 5.64, 9.44 and 4.11 kcal GE/g for protein, lipid, and NFE, respectively.
Gross energy (GE) in Feed (kcal/100 g DM)=(protein content (%) × 5.64) + (Lipid content (%) × 9.44) + (carbohydrate content (%) × 4.1).
Gross energy (GE) in Fish Carcass (kcal/100g DM)=(protein content (%) × 5.64) + (Lipid content (%) × 9.44)).
Feed utilization measurements:
Feed utilization parameters are calculated according to the following equations [36]:
Feed Intake (FI, g/fish): This is the amount of feed given or supplied during the experimental period for each fish per gram.
Food conversion ratio (FCR)=feed intake (as DM in g)/body weight gain (g) [37].
Protein efficiency ratio (PER)=fish weight gain (g)/protein intake (g).
Protein productive value (PPV, %)=100 × (Pt –P0/protein intake (g)).
Where: P0: Protein content in fish carcass at the start.
Pt: Protein content in fish carcass at the end.
Energy gain, EG (Kcal): EG=Et-E0
Where: E0: energy content in fish carcass (Kcal) at the start
Et: energy content in fish carcass (Kcal) at the end
Energy utilization, EU (%): EU=100 × (Energy gain (Kcal)/ Energy intake (Kcal)].
Color measurement:
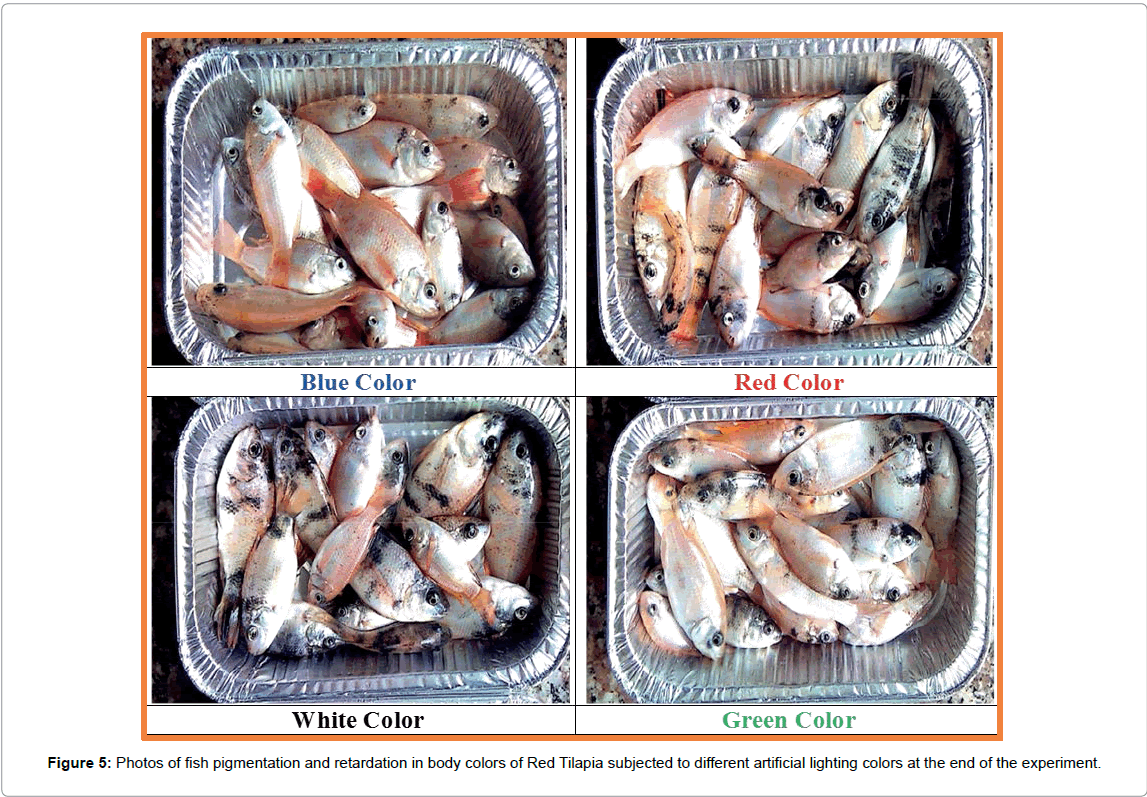
At the end of the experiment, a representative sample of fish from is collected and sent to a specialized laboratory to measure the total content of B-carotene. It was measured by Reversed-Phase Liquid Chromatography (LC) method according to [38]. Also, a sample from each treatment was photographed with a high-resolution camera as another way to gauge the clear differences in fish body color. Also, the percentage of Red tilapia fish with black spots (FBS) was measured as a percentage of the total number of fish at the end of this experiment. Any fish with remarked black spots was counted in this trait.
Statistical Analysis
Data are transferred to excel sheet to calculate the mean and standard error for final weight, weight gain, ADG, SGR, survival, condition factor, FCR, PER, PPV, EU, and b-carotene content. Then, these data are analyzed with a one-way analysis of variance (ANOVA) using SPSS 20 statistical package to evaluate the differences between the tested treatments. The differences within each experimental treatment are evaluated using Duncan’s Multiple Range Test at 0.05 Probabilities [39].
Results
Water quality, growth performance and survival
Average values of water quality parameters in the experimental tanks are; (temperature 18.6 ± 1.3°C; salinity 32.27 ± 0.76 ppt; pH 7.92 ± 0.34; dissolved oxygen 5.74 ± 0.63 ppm; total ammonia nitrogen (TAN) 0.186 ± 0.034 ppm, and 0.004 ± 0.00ppm un-ionized ammonia, NH3). No significant (p>0.05) differences are observed in water quality measurements between the tested artificial light colors.
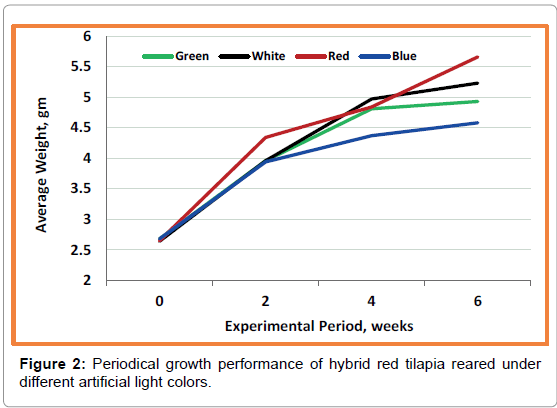
Results of bi-weekly growth performance of hybrid red tilapia indicate a clear tendency towards red and white colors (Figure 2). The final data of growth performance indexes (SGR) are presented in Table 1. The data reveal that fish exposed to red and white light attain the higher values of growth but without any significant (p>0.05) differences with the other tested colors. However, the fish exposed to blue light color have the lowest values. Also, condition factor (K), which is another consideration of evaluating fish growth, shows no significant (p>0.05) differences between the four tested treatments (Table 1). The obtained values of K ranged between 1.45-1.61. The results of survival (%) shows that all treatments had low percent varied between 59.33-61% without any significant differences (p>0.05) between treatments (Table 1).
| Treatments | Initial Weight, gm | Final Weight, gm | SGR,%/day | Condition factor (K) | Survival, % |
|---|---|---|---|---|---|
| Green | 2.68 ± 0.03 | 4.94 ± 0.41 | 1.45 ± 0.19 | 1.57 ± 0.18 | 59.33 ± 1.86 |
| White | 2.64 ± 0.01 | 5.23 ± 0.08 | 1.63 ± 0.04 | 1.61 ± 0.28 | 61.00 ± 1.16 |
| Red | 2.65 ± 0.01 | 5.66 ± 0.52 | 1.81 ± 0.21 | 1.53 ± 0.05 | 60.00 ± 3.06 |
| Blue | 2.68 ± 0.04 | 4.58 ± 0.50 | 1.28 ± 0.25 | 1.45 ± 0.12 | 59.33 ± 3.18 |
| Level of Significance | 0.541 | 0.365 | 0.277 | 0.935 | 0.956 |
Table 1: Mean ± SEM of growth performance, survival, condition factor and feed conversion ratio of Red tilapia fingerlings tested with different artificial light colors.
Whole-body proximate composition
Carcass composition of the Red tilapia at the end of this experiment is presented in Table 2. Moisture content is the highest in fingerlings which were tested with red light (68.91%), while the lowest value is obtained by fingerlings which were tested with the blue light (56.50%) with significant differences (p ≤ 0.05) between treatments. The dry matter content in blue light treatment is the highest (43.50%) with significant differences (p ≤ 0.05) when comparing with the other treatments.
| Treatments | Moisture% | Dry matter, % | CP, as %DM | EE, as %DM | Ash, as %DM | Carcass Energy (Kcal/100gm) |
|---|---|---|---|---|---|---|
| Green | 63.50 ± 0.18c | 36.50 ± 0.18b | 49.83 ± 0.13a | 32.77 ± 0.19c | 17.49 ± 0.13b | 590.44 ± 2.52b |
| White | 64.50 ± 0.09b | 35.50 ± 0.09c | 49.40 ± 0.14b | 32.60 ± 0.08c | 17.90 ± 0.06a | 586.36 ± 0.09b |
| Red | 68.91 ± 0.15a | 31.09 ± 0.15d | 46.93 ± 0.12d | 35.90 ± 0.08a | 16.60 ± 0.13c | 603.60 ± 0.47a |
| Blue | 56.50 ± 0.16d | 43.50 ± 0.16a | 47.33 ± 0.08c | 35.20 ± 0.10b | 16.80 ± 0.09c | 599.25 ± 0.98a |
| Level of Significance | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
Table 2: Mean ± SEM of carcass chemical composition of Red tilapia fingerlings tested with different artificial light colors.
The results of CP manifested that, the highest value is obtained with green treatment (49.83%), followed by the white one (49.4%) without any significant differences (p>0.05), while the poorest CP value is obtained by fingerlings which stocked in red light (46.93%) with significant differences (p ≤ 0.05). Concerning the EE values, the highest values are achieved by fingerlings which are stocked in red and blue color (35.9 and 35.26%, respectively). Whereas, the poorest values were achieved by fingerlings which were stocked in white color (32.77%) with significant differences (p ≤ 0.05). Regarding ash content, the lowest value is obtained by fingerlings which were stocked in red color followed by blue color (16.6 and 16.8% DM, respectively). Higher significant contents of carcass energy (Kcal/100 gm) were measured in tilapia fish treated with red and blue colors compared with green and white colors.
The results of CP manifested that, the highest value is obtained with green treatment (49.83%), followed by the white one (49.4%) without any significant differences (p>0.05), while the poorest CP value is obtained by fingerlings which stocked in red light (46.93%) with significant differences (p ≤ 0.05). Concerning the EE values, the highest values are achieved by fingerlings which are stocked in red and blue color (35.9 and 35.26%, respectively). Whereas, the poorest values were achieved by fingerlings which were stocked in white color (32.77%) with significant differences (p ≤ 0.05). Regarding ash content, the lowest value is obtained by fingerlings which were stocked in red color followed by blue color (16.6 and 16.8% DM, respectively). Higher significant contents of carcass energy (Kcal/100 gm) were measured in tilapia fish treated with red and blue colors compared with green and white colors.
Feed utilization
Feed utilization (FI, FCR, PER, PPV, EG, and EU) are presented in Table 3. The differences between treatments regarding FI, FCR, PER, and EG are insignificant (p>0.05). Feed intake (FI) was affected by the lighting color, with 35.9% higher in FI under Red color (6.51 g/ fish) compared with blue color (4.79 g/fish). The lowest value of FCR is obtained from fingerlings exposed to blue color (2.76), while the highest FCR (2.24) is achieved with red color treatment. Contrarily to the results of FCR, and PER, the PPV, and EU values are significantly in favor of blue color compared with the other tested colors. PPV (%) under blue color treatment is 26.9, 22.3 and 67.7% higher than green, white, and red color treatments, respectively. With the same trend, EU (%) under blue color treatment is 39.6, 39.6 and 60.9% higher than green, white, and red color treatments, respectively.
| Treatments | FI, gm/fish | FCR | PER, g | PPV, % | EG, kcal | EU, % |
|---|---|---|---|---|---|---|
| Green | 5.82 ± 0.99 | 2.61 ± 0.26 | 1.57 ± 0.19 | 37.66 ± 3.63b | 6.87 ± 0.84 | 30.00 ± 2.83b |
| White | 5.93 ± 0.10 | 2.29 ± 0.06 | 1.75 ± 0.06 | 37.85 ± 1.32b | 7.17 ± 0.19 | 30.01 ± 0.69b |
| Red | 6.51 ± 0.36 | 2.24 ± 0.24 | 1.84 ± 0.23 | 28.49 ± 2.99c | 6.89 ± 0.93 | 26.04 ± 2.14b |
| Blue | 4.79 ± 0.57 | 2.76 ± 0.49 | 1.53 ± 0.23 | 47.79 ± 2.33a | 8.24 ± 1.15 | 41.89 ± 1.45a |
| Level of Significance | 0.309 | 0.588 | 0.630 | 0.007 | 0.649 | 0.002 |
Table 3: Mean ± SEM of feed conversion ratio (FCR), Protein efficiency ratio (PER),protein productive value (PPV), energy gain (EG), and energy utilization (EU) of Redtilapia fingerlings tested with different artificial light colors.
β-Carotene content
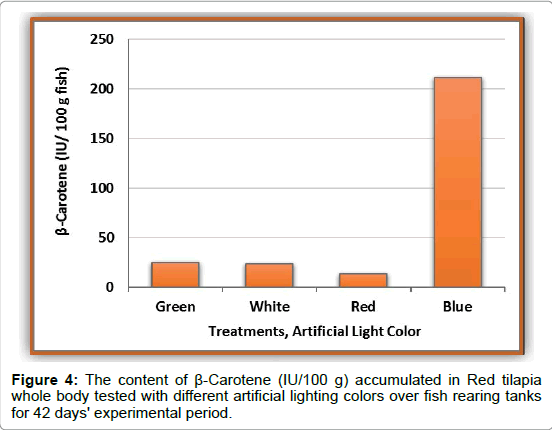
The data of β-carotene for Red tilapia fingerlings are presented in Figures 2-4. Schematic pictures and final data (Mean ± SEM) where β-carotene (IU/ 100 g) are clearly identified under wavelength 325 nm manifested the differences in body color between the tested treatments. Also, real photos for red tilapia at the end of the experiment can clearly confirm the results of the chemical analysis by the chromatograph as shown in Figure 5. The data illustrated that, the highest amount of carotene is achieved by fingerlings which were exposed to blue artificial light, the whereβ-carotene value is 211.25 IU/100g with highly significant differences (p ≤ 0.05) in comparing with the other treatments. The blue color is followed by the green color (24.88 IU/100 g), whereas, the lowest value (13.03 IU/100 g) is recorded by red tilapia fingerlings exposed to red artificial light. This means that the content of β-carotene in blue color treatment was 8, 9, and 16 folds comparing with green, white, and red colors, respectively. Figure 5 clearly shows that the Red tilapia fish with black spots (representing retardation in genetic traits) in the blue treatment were 12%, compared with 29% in green, 56% in white and 52% in red colors.
Discussion
Growth performance and feed utilization
Red tilapia has higher market value than Nile tilapia, mainly due to its beautiful color that needs continuous selection to retain and pass their red color from generation to generation [40]. The available data discussing the relationship between illumination color and growth performance still very few. The obtained results of the present study shows that the blue color represented the lowest growth performance and it may due to the reduced vision, which prevents fish to detect the feed well, while red color has better growth performance in spite of the insignificance between the tested colors. This result is congruent with [41] who found that red color stimulates feeding intake and improves growth performance more than yellow, green, blue, and white colors. This is attributed to that fish under stress environment such as red color is more aggressive and grew almost many times as much in the less stressful conditions (blue color) [26].
The effect of indirect or background color originated from the reflected color of fish rearing tank on fish growth has been investigated [12,13,42]. Steel et al. [39] notices that Summer flounder Paralichthys dentatus held in red tanks expresses the greatest weight increase. But, the fish held in dark blue tanks performed the poorest weight gain. However, no significant differences are observed between tank colors. Similar results are obtained by Karakatsouli et al. [43], who finds out that rainbow trout Oncorhynchus mykiss shows faster growth and improved physiological condition under red light, with reduced growth under blue light. The growth and skin color of Caspian kutum Rutilus frisii have been examined with five tank colors (white, red, blue, yellow and black) [12]. The results show that there are no significant differences in growth performance between the different experimental groups, but the final body weight tended to be higher in the yellow color tank. On the other hand, in the case of gilthead seabream Sparus aura, red light significantly increases brain dopaminergic activity, while a tendency towards reduced growth is also observed. Also, some other studies demonstrated that there are no significant differences in growth performance when comparing different environmental colors such as on Seahorses Hippocampus abdominal [44], on scaled carp Cyprinus carpio L. [45] and on Atlantic salmon Salmo salar L [46]. Nevertheless, in a study to evaluate the effect of tank wall color and light level, treatments affect the growth and survival of Eurasian perch larvae Perca fluviatilis L. [42]. The greatest growth in weight and length is observed in tanks with light gray and white walls, while the lowest growth is recorded in the tank with the black wall.
Condition factor is an indicator of the general fish condition. It is used to assess the status of the aquatic ecosystem in which fish live [47,48]. When the value of condition factor is higher, this means that the fish has reared in a better condition, such as less stress, better water quality, availability of well-balanced feeds, etc. Condition factor is influenced by both biotic and abiotic environmental conditions. In the present study, it seems that the effect of artificial light color on the condition factor was very limited. The values of condition factor ‘K’ recorded in the present study are of greater than one under different lighting colors, indicating a good heath status [42]. K values of Red tilapia are similar with the findings of Ayoade et al. [49] with values 1.63-165 and less than 3.4 for females and 3.3 for males of Red tilapia [50,51].
Results of the present study for Feed utilization (FI, FCR, and PER) show insignificant differences between treatments with high values of FCR in all groups. This might be attributed to low water temperature. Lighting color definitely affects feed utilization in the present study. The lower value of FCR and the higher values of FI for tilapia fish tested with Red lighting color agree with the findings of [41], who finds out that red light stimulates feeding motivation and improves feed conversion in Nile tilapia, but without extra feeding. This is most likely by affecting central control centers. Also, PER values reflect all the previous results of growth performance parameters.
The importance of light reflection of rearing tank color on feed utilization has been confirmed. Similar results of the present study [12], detected no significant differences in growth between the different tested tank color, but feeding rate (FR) in the yellow color was lower than that in the black color with no significant differences. Furthermore [52] observed that there was significantly lower feeding rate in Eurasian Perch Perca fluviatilis held in yellow tanks when compare with black tanks. Also [13] observed significantly lower FCR for Summer Flounder (Paralichthys dentatus) held in red tanks when compared against dark and light blue and green Aquaria. In the same study, the highest FCR (poorest) were observed in tilapia held in dark blue-colored aquaria.
In our experiment, PPV and EU values were significantly higher under blue color treatment, indicating that the fish health is much better than the other tested colors. The best health condition of tilapia fish under blue color is due to lack of secretion of cortisol associated with the inappropriate conditions or stressors [26,16] and other stress hormones like glucocorticoids [53,54].
Whole-body proximate composition
Based on the results of the present study, significant effects of the artificial light color on the chemical composition of Red tilapia can be clearly detected. There is an evident correlation between the blue light color and the quality of red tilapia. Volpato et al. [26] stated that blue light color can prevent or reduce stresses, and improves the welfare of the Nile tilapia, while red color has negative effects on fish welfare [51]. The positive effects of blue color on the quality of Red tilapia meat in the present study can be confirmed through the higher content of dry matter, and the lower content of ash. These results are incongruent with the findings of Sharaf, Volpato, Moharram, Zaki, et al. [3,26,55,56]. The main explanation is that plasma cortisol levels may be at low levels in fish submitted to a blue light as stated by Schierle [38], while one putative explanation is that fish exposing to red light color may have higher levels of plasma cortisol. There was also a positive correlation between ventilation rate [VR] resulted from social stressors, and plasma cortisol concentration in Red tilapia [16]. There is a positive correlation between stressors and plasma cortisol levels in Red tilapia [16]. Increasing Plasma cortisol might effect on body composition leading to higher levels of moisture and lower levels of the protein [57].
The darker lights results in higher EE values and lower protein and ash content than the lighter colors. A similar trend was observed by many authors. In the same context [58], reported that Nile tilapia longterm exposure to green, red and darkness colors showed a significant decrease in total protein compared to the yellow color (control). On the other hand, total protein shows an insignificant decrease in the group exposed to long-term blue light.
Different background colors may also influence the whole body proximate composition of fish. Also, McLean et al. [12] illustrated that total protein of the Caspian kutum Rtilus frisii is the highest in blackcolored tanks and the poorest value was in yellow color treatment, without any significant differences. Nevertheless, lipid content in the yellow tanks treatment was significantly higher than black, but the body moisture was inverse. McLean et al. [13] demonstrated that there are no significant differences in dry matter and moisture between groups (dark blue, light blue, black, green and red tank colors) on tilapia fillet. Furthermore, the highest fillet lipid level is in fish maintained in redcolored tanks and the lowest is in tilapia held in black-colored tanks, without significant differences. Similarly, fillet protein level didn’t vary across the various groups.
Β-Carotene content
The purity and strength of color in red tilapia is one of the most important characteristics that help to increase the demand and at the same time affect the selling price. The influence of light coloration on the fish color and beta-carotene content was profound of the present study. The explanation of these values might be attributed to the stressreleasing hormones. The positive effects of blue light color prevented or reduced the confinement-induced cortisol response [26]. Under blue color, the ability of red tilapia to deposit β-Carotene present in the offered feed with higher rates might be improved than other colors. The result of the present study is consistent with [59] demonstrated that a blue or green background color could give strength to the orange color, while a white color made fish less color saturated but brighter. In another viewpoint, under a stressful condition, fish are responding by the secretion of stress hormones especially glucocorticoids, which have a remarked positive correlation with the value of carotenoid coloration in fish [53]. Only fish with good health can tolerate high glucocorticoid levels, and thus advertise this ability through better deposition of carotenoids in its body [54]. Higher plasma cortisol levels, faster ventilation rate, and darker skin color were intensified more by Red tilapia fish subjected to confinement and social stresses [16]. There is no conflict between the results of the present experiment and both points of view. Based on Figure 5, there is no conflict between the results of the present experiment and both points of view. To clarify this point, the degree of Red color intensity and Black color retardation rate in the tested fish, showed that the fish that were exposed to a lower degree of light stress (blue color), displayed a lower percent of fish containing the black spots (12%) and higher content of carotene in its body. However, those fish exposed to higher levels of stress, especially red color showed a higher percent of fish containing black spots (more than 4-folds) and lower percent of carotene (less than 16-folds) compared with blue color.
Rapid color change in fishes is controlled by the brain through the pigment called chromatophore [60]. The hormonal control of color alteration in fishes involves two peptide hormones released from the pituitary gland, namely α-MSH and MCH. When aquatic organisms are placed on a stressful dark background, the MSH cells are activated and release a higher concentration of α-MSH into the blood, causing a dispersion of pigments in the dermal melanophores of the skin [12]. Also, the higher level of α-MSH induces cortisol release from the internal tissue as demonstrated for Oreochromis mossambicus [61].
The tank color has a clear and documented effect on fish coloration. McLean et al. [12] reported that tank color significantly affected on all body color parameters of Juvenile Caspian Kutum Rtilus frisii. The values of (lightness) and (whiteness) are significantly lower in black and red tank color than other color treatments. Redness and yellowness are higher in red and yellow, respectively. However, the blue and white colors do not significantly affect the body color. Also Yasir et al. [58] reported that, there are no changes in skin color of Nile Tilapia in different environmental color (blue, green, red, yellow and darkness). With the exception of darkness treatment, fish skin color changed from gray to dark, and finally to the black color when the darkness effect is removed. It seems that the effect of lighting color is species-specific. The effects of substrate color and light intensity on skin color change of the juvenile seahorse, Hippocampus Erectus were investigated [62]. The juveniles cultured with substrates of mixed colors (green and orange, or green and red) had higher color change rates and survival than those reared in a single color substrate (green, orange, red, or black). Also, increasing light intensity increases color change rate of the juveniles. In this context Yasir et al. [14] found that ambient light intensity could regulate fish color performance, but cannot change the pigment dominance by β - carotene.
The results of β-Carotene obtained in this experiment are conclusive and very important economically and technically. However, many studies need to be done to clarify the scientific explanation for this color change phenomenon in Red tilapia.
Conclusion
It could be concluded that cultivating Red tilapia under a full saline well water using artificial blue color will significantly improve the pigmentation of red tilapia, and the quality of fish in terms of the content of dry matter, but also protein and energy utilization of the consumed feed. Despite the positive effect of red light on growth, but it had negative effects on the content of carotene, the whole-body chemical composition and the increased percentage of fish with blackcolor spots. In conclusion, our results might be useful in improving fish coloration with simple, economic and sustainable way.
Acknowledgment
We would like to show our gratitude to the “anonymous” reviewer for his socalled constructive insights, careful and conscientious reading and sharing his pearls of wisdom with us. We are also immensely grateful to Prof. Dr. Mohamed Essa, Fish Rearing Lab, NIOF, for his earlier comments that greatly improved the manuscript and Prof. Dr. Elsayed Elebiary for assistance with his papers about Red tilapia and continuous support and encouragements.
References
- FAO (2014) The State of World Fisheries and Aquaculture 2014. Food and Agriculture Organization of the United Nations. Rome p: 223.
- Jaspe CJ, Caipang CMA (2011) Small-scale hatchery and larval rearing techniques for local strains of saline-tolerant tilapia, Oreochromis spp. ABAH Bioflux3: 71-77.
- Sharaf SM, Mohamed KA, Eldanasoury MA, Nafea RR (2013) Growth and Physiological Responses of Nile Tilapia Oreochromisniloticus and Hybrid Red Tilapia (O. mossambicus �?? X O. niloticus�??) by Changes in Salinity of Rearing Water. Journal of Animal, Poultry & Fish Production, Suez Canal University 1:23-31.
- Lawson EO, Anetekhai MA (2011) Salinity Tolerance and Preference of Hatchery Reared Nile Tilapia, Oreochromisniloticus (Linneaus 1758). Asian Journal of Agricultural Sciences 3: 104-110.
- Behrends LL, Nelson RG, Smitherman RO, Stone NM (1982) Breeding and Culture of Red-Gold Color Phase of Tilapia. J. World Maricul. Soc. 13: 210-220.
- Watanabe WO, Fitzimmons K, Yi Y (2006) Farming tilapia in saline waters. In: C. Lim, C.D. Webster (eds.). Tilapia : Biology, Culture, and Nutrition. Food ProductsPress/Haworth Press, New York, London, Oxford.pp 347-447.
- El-Sayed AM (2006) Tilapia Culture in Salt Water. EnvironmentalRequirements, Nutritional Implications and EconomicPotentials. In: Elizabeth Cruz SuárezL, Denis Ricque Marie, Mireya Tapia Sa lazar, Martha G NietoLópez, David AV illarrealCavazos, Ana C Puello Cruz y Armando García Ortega (eds.). Advances in Aquaculture Nutrition.VIII International Symposium on Aquaculture Nutrition. 15 - 17 November. AutonomousUniversity of Nuevo León, Monterrey, Nuevo León, Mexico ISBN 970-694-333-5.
- Baroiller JF, Clota F, Cotta HD, Derivaz M, Lazard J, Vergent A (2000) Sea water adaptability of two tilapia species (S. melanotheron and O. niloticus) and their reciprocal F1 hybrids. p: 303.
- Fujii R (1993) Coloration and chromatophores. In: Evans DH (ed.) The Physiology of Fishes, Boca Raton,pp: 535-562.
- Yasir I, Qin JG (2010) Effect of dietary carotenoids on skin color and pigments of false clownfish, Amphiprionocellaris, Cuvier. Journal of the World Aquaculture Society 41: 308-318.
- Torrissen OJ, Hardy RW, Shearer KD, Scott TM, Stone FE (1990) Effects of dietary canthaxanthin level and lipid level on apparent digestibility coefficients for canthaxanthin in rainbow trout (Oncorhynchusmykiss). Aquaculture 88: 351-362.
- Imanpoo MR, Abdollahi M (2011) Effects of Tank Color on Growth, Stress Response and Skin Color of JuvenileCaspianKutumRtilusfrisiiKutum. Global Veterinaria 6: 118-125.
- McLean E, Cotter P, Thain C, King N (2008) Tank color impacts performance of culturedfish. Ribarstvo 66: 43-54.
- Yasir I, Qin JG (2009a) Effect of Light Intensity on Color Performance of False Clownfish, AmphiprionocellarisCuvier. Journal of the World Aquaculture Society 40: 337-350.
- Han D, Xie SH, Lei W, Zhu X, Yang Y (2005) Effect of light intensity on growth, survival and skin color of juvenile Chinese longsnout Catfish (LeiocassislongirostrisGünther). Aquaculture 248: 299-306.
- Templonuevo RMC, Vera Cruz EM (2016) Responses of Red Nile Tilapia (Oreochromisniloticus L.) subjected to Social and Confinement Stresses. The CLSU International J of Sci& Tec 1: 7-14.
- Huang CM, Chang SL, Cheng HJ, Liao IC (1988) Single geneinheritance of red body coloration in Taiwanesered tilapia. Aquaculture 74: 227-232.
- Hulata G, Karplus I, Harpaz S (1995) Evaluation of somered tilapia strains for aquaculture : growth and colorsegregation in hybridprogeny, Aquaculture Res 26: 765-771.
- Tave D (1987) Genetics and breeding of tilapia: A review, Proc. of The Second International Symposium on Tilapia in Aquaculture, Bangkok, Thailand, p: 285- 294.
- Duray MN, Estudillo CB, Alpasan LG (1996) The effect of background color and rotiferdensity on rotiferintake, growth and survival of the grouper (Epinephelussuillus) larvae. Aquaculture 146: 217-224.
- Dowing G, Litvak MK (2000) The effect of photoperiod, tank colour and light intensity on growth of larval haddock. Aquaculture International 7: 369-382.
- Volpato GL, Duarte CRA, Luchiari AC (2004) Environmentalcolor affect Nile tilapia reproduction. Brazilian J. Medical and BiologicalRes37: 479-483.
- Turner PM (2008) Effects of light intensity and tank background color on sexdetermination in southernflounder (Paralichthyslethostigma). A thesissubmitted to the GraduatebFaculty of North Carolina State University In partial fulfillment of the requirements for the Degree of Master of Science Zoology Raleigh, North Carolina p: 71.
- Hoglund E, Balm PHM, Winberg S (2002) Behavioral and neuroendocrine effects of environmental background colour and social interaction in arctic char (Salvelinusalpinus). J. ExperimentalBiol. 205: 2535-2543.
- Cobcroft JM, Battaglene SC (2009) Jaw malformation in stripedtrumpeterLatrislineatalarvaelinked to wallingbehaviour and tank colour. Aquaculture 289: 247-282.
- Volpato GL, Barreto RE (2001) Environmentalblue light prevents stress in the fish Nile tilapia. Brazilian J. Medical and BiologicalRes34: 1041-1045.
- Szisch V, Van der Salm AL, WendelaarBonga SEM, Pavlidis M (2002) Physiologicalcolour changes in the redporgy, Pagruspagrus, following adaptation to bluelightingspectrum. Fish Physiology and Biochemistry27: 1-8.
- Rotllant J, Tort L, Montero D, Pavlidis M, Martinez M, WendelaarBonga SE Balm PHM (2003) Background colour influence on the stress response in culturedredporgyParguspargus. Aquaculture 223: 129-139.
- Fernandez PJ, Bagnara JT (1991) Effect of background color and lowtemperature on skin color and circulating alpha-MSH in twospecies of leopardfrog. General and Comparative Endocrinol. 83: 132-141.
- Han D, Xie SH, Lei W, Zhu X, Yang Y (2005) Effect of light intensity on growth, survival and skin color of juvenileChineselongsnoutcatfish (Leiocassislongirostris Günther). Aquaculture 248: 299-306.
- Environmental Protection Agency (1989) Ambient water quality criteria for ammonia (Saltwater). EPA 440/5-88-004, National Technical Information Service (NTIS), Springfield, VA.
- Castell JD, Tiews K (1980) Report on the EIFAC, IUNS and ICES working group on the standardization of methodology in fish nutrition research. Hamburg, FederalRepublic of Germany, EIFAC TechnicalPaper.
- Newman RM, Martin FB (1983) Estimation of fish production rates and associated variances. Canadian J of Fisheries and AquaticSci 40: 1729-1936.
- Ricker WE (1975) Computation and interpretation of biologicalstatistics of fish populations. FishResBoard Can Bull 191: 1-382.
- AOAC International (2000) Official Methods of Analysis of AOAC International, 17th ed. AOAC, Arlington, VA, USA.
- Brody S (1954) Bioenergetics and Growth. ReinholdPublishing, New York.
- Jhingran, VG (1991) Fish and Fisheries of India(2ndedn.) India,p : 666.
- Schierle J, Pietsch B, Ceresa A, Fizet C, Waysek EH (2004) Method for the Determination of β-Carotene in Supplements and RawMaterials by Reversed-Phase LiquidChromatography: Single Laboratory Validation. Journal of AOAC International 87: 1070-1082.
- Steel RGD, Torrie JH (1980) Principles and Procedures of Statistics: A BiometricalApproach (2nd edn.)McGraw Hill Book Co.: New York.
- Lovshin LL (2000) Criteria for Selecting Nile Tilapia and Red Tilapia for Culture. CRSP Research Report p: 157.
- Volpato GL, Bovi TS, de Freitas RHA, da Sliva DF, Delicio HC, et al. (2013) Red Light Stimulates Feeding Motivation in Fish but Does Not Improve Growth. PLoS ONE 8: e59134.
- Tamazouzt L, Béatrice C, Pascal F (2000) Tank wallcolour and light level affect growth and survival of Eurasianperchlarvae (Percafluviatilis L). Aquaculture 182: 85-90.
- Karakatsouli N, Papoutsoglou SE, Pizzonia G, Tsatsos G, Tsopelakos A, et al. (2007) Effects of light spectrum on growth and physiologicalstatus of giltheadseabreamSparusaurata and rainbowtroutOncorhynchusmykissrearedunderrecirculating system conditions. Aquacult. Eng 36: 302-309.
- Martinez-Cardenas L, Purser GJ (2007) Effect of tank colour on Artemia ingestion, growth and survival in culturedearlyjuvenile pot-belliedseahorses (Hippocampusabdominalis). Aquaculture 264: 92-100.
- Papoutsoglou SE, Mylonakis G, Miliou H, Karakatsouli NP, Chadio S (2000) Effects of background color on growth performances and physiologicalresponses of scaledcarp (Cyprinuscarpio L.) reared in a closedcirculated system. Aquaculture Engineering 22: 309-318.
- Stefansson SO, Hansen T (1989) Effects of tank colour on growth and smoltification of Atlantic salmon (Salmosalar L). Aquaculture 81: 379-386.
- Anene A. (2005) Condition Factor of Four Cichlid Species of a Man-made Lake in Imo State, Southeastern Nigeria. Turk J Fish AquatSci5: 43-47.
- Datta SN, Kaur VI, Dhawan A, Jassal G. (2013) Estimation of length-weight relationship and condition factor of spotted snakehead Channapunctata (Bloch) under different feeding regimes. Springer Plus. 2:436.
- Ayoade AA, Ikulala AOO (2007) Length weight relationship, condition factor and stomach contents of Hemichromisbimaculatus, Sarotherodonmelanotheron and Chromidotilapiaguentheri (Perciformes: Cichlidae) in Eleiyele Lake, Southwestern Nigeria. Revista de Biología Tropical 55: 969-977.
- Malik A, Abbas G, Soomro MA, Shah SSA, Asadullah A, Bhutto AH, Jamali GQ, Roonjho Z (2017) Length-weight Relationship and Condition Factor of Red Tilapia (Hybrid) Reared in Cemented Tanks of Sun-bright Red Tilapia and Ornamental Hatchery-Karachi, Sindh-Pakistan. Sindh Univ Res Jour 49:159-162.
- Volpato GL, Bovi TS, de Freitas RHA, da Sliva DF, Delicio HC, et al. (2013) Red Light Stimulates Feeding Motivation in Fish but Does Not Improve Growth. PLoS ONE 8: e59134.
- Strand A, Alanara A, Staffan F, Magnhagen C (2007) Effects of tank colour and light intensity on feedintake, growth rate and energyexpenditure of juvenileEurasianperch, Percafluviatilis L. Aquaculture 272: 312-318.
- Backström T, Brännäs E, Nilsson J, Magnhagen C (2014) Behaviour, physiology and carotenoid pigmentation in Arctic CharrSalvelinusalpinus. J. Fish Biol. 84: 1-9.
- Sefc KM, Brown AC, Clotfelter ED (2014) Carotenoid-based coloration in cichlid fishes- a review. Comparative Biochemistry and Physiology, Part A 173: 42-51.
- Moharram SG, El-Ebiary SHE (1999) Effect of dietary lipid level on puberty and oocyte development in Florida red tilapia Oreochromis species (family: Cichlidae) reared under different salinity levels. Bulletin of the National Institute of Oceanography and Fisheries 25: 253-270.
- Zaki MA, El-Ebiary EH (1997) The use of aquatic plant proteins in completed diets for Florida Red tilapia. Bull NatInstOceanogr and Fish 23: 459-472.
- Fatemeh A1, Sanaz G, Shahla J (2008) Plasma cortisol changes and body composition in Stizostedionlucioperca exposed to handling stress. Pak J Biol Sci. 11:623-7.
- Sabri DM, Elnwishy N, Nwonwu F (2012) Effect of EnvironmentalColor on the Behavioral and PhysiologicalResponse of Nile Tilapia, OreochromisNiloticuss. Global J Science FrontierResearchBiological Sciences 12:11-20.
- Yasir I, Qin JG (2009b) Impact of background on color performance of false clownfish, Amphiprionocellaris, Cuvier. The Journal of World Aquaculture Society 40:724-736.
- Skold HN, Aspengren S, Wallin M (2012) Rapid Color Change in Fish and Amphibians - Function, Regulation, and Emerging Applications. Pigment Cell Melanoma Research 26:29-38.
- Lamers AE, Flik G, Atsma W, WendelaarBonga SE (1992) A role for di-acetyl alpha-melanocyte stimulating-hormone in the control of cortisol release in the teleost Oreochromismossambicus. J Endocrinol. 135: 285-292.
- Lin Q, Lin J, Huang L (2009) Effects of substrate color, light intensity and temperature on survival and skin color change on juvenile seahorses, Hippocampus erectus Perry, 1810. Aquaculture 298: 157-161.
Relevant Topics
- Algal Blooms
- Blue Carbon Sequestration
- Brackish Water
- Catfish
- Coral Bleaching
- Coral Reefs
- Deep Sea Fish
- Deep Sea Mining
- Ichthyoplankton
- Mangrove Ecosystem
- Marine Engineering
- Marine Fisheries
- Marine Mammal Research
- Marine Microbiome Analysis
- Marine Pollution
- Marine Reptiles
- Marine Science
- Ocean Currents
- Photoendosymbiosis
- Reef Biology
- Sea Food
- Sea Grass
- Sea Transportation
- Seaweed
Recommended Journals
Article Tools
Article Usage
- Total views: 9370
- [From(publication date):
June-2017 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 8162
- PDF downloads : 1208